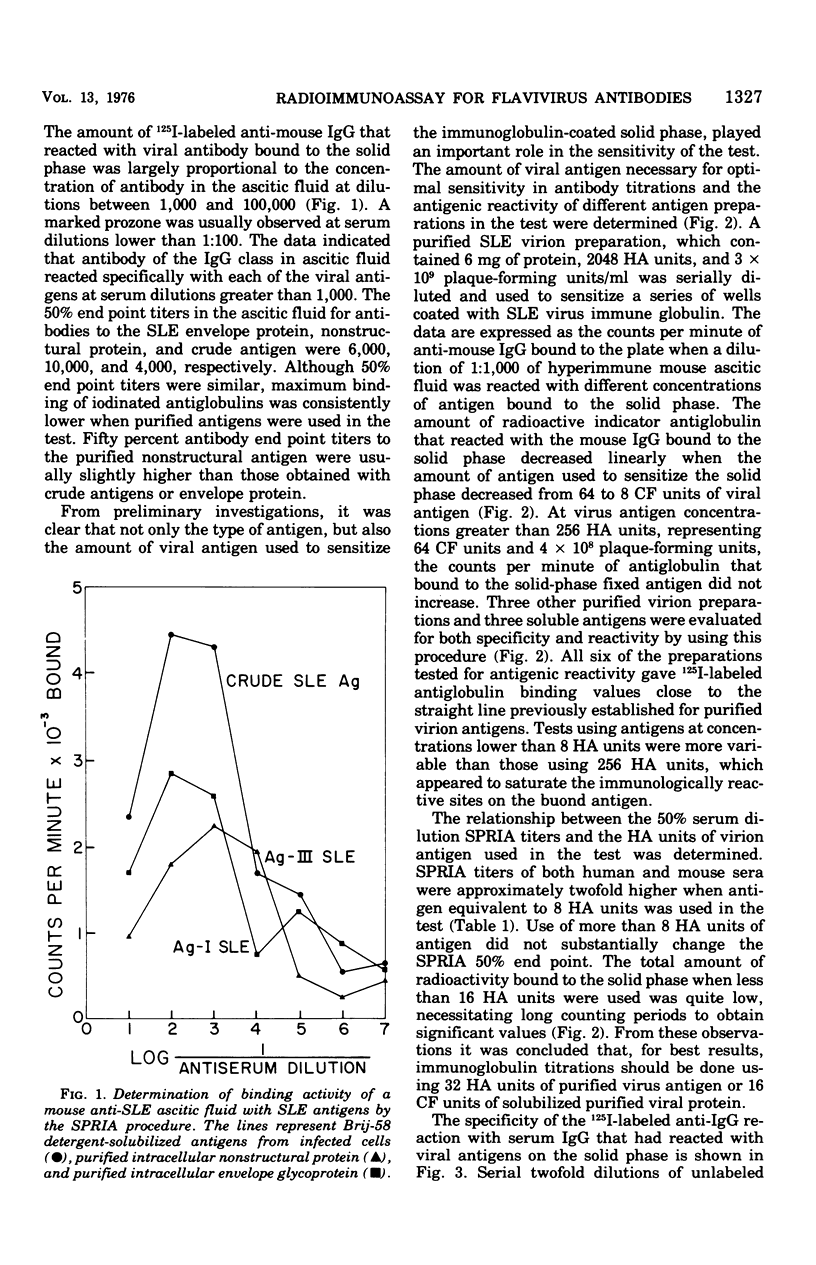
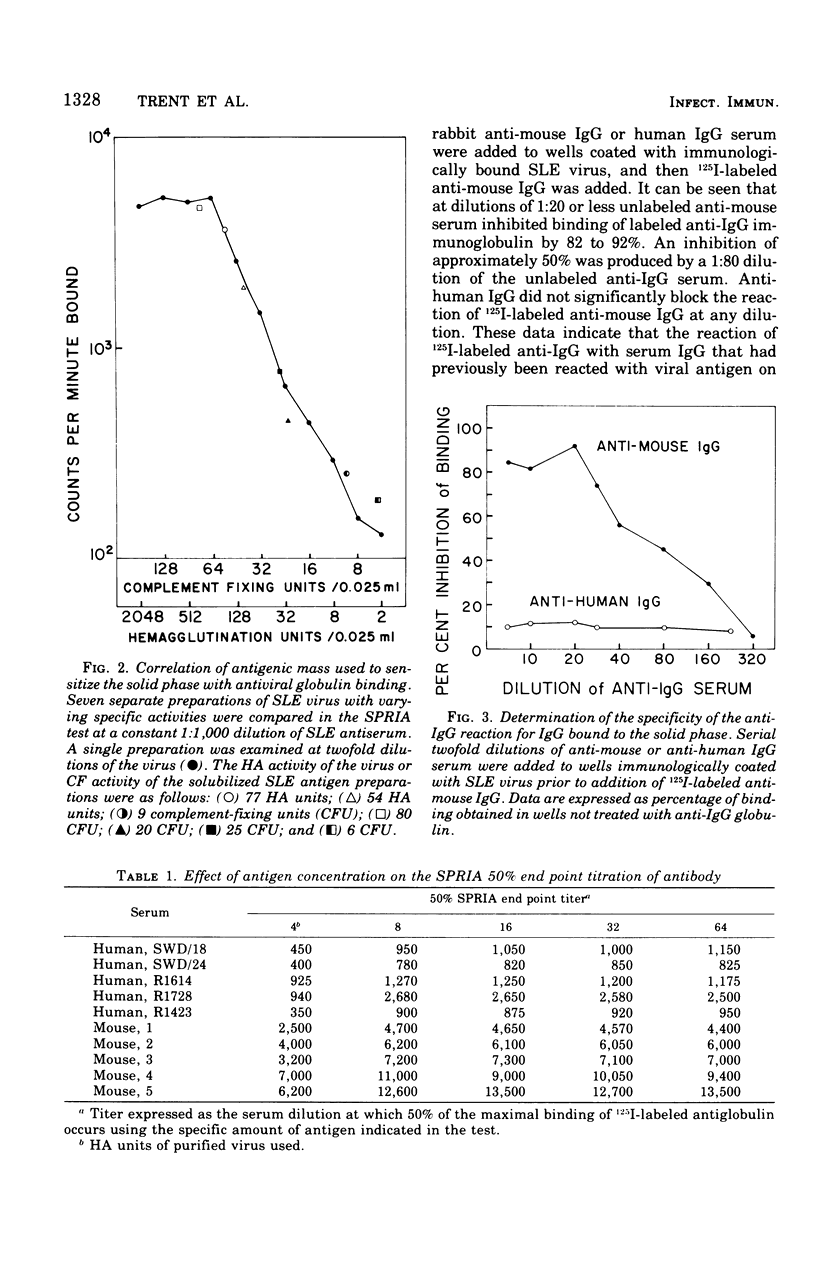
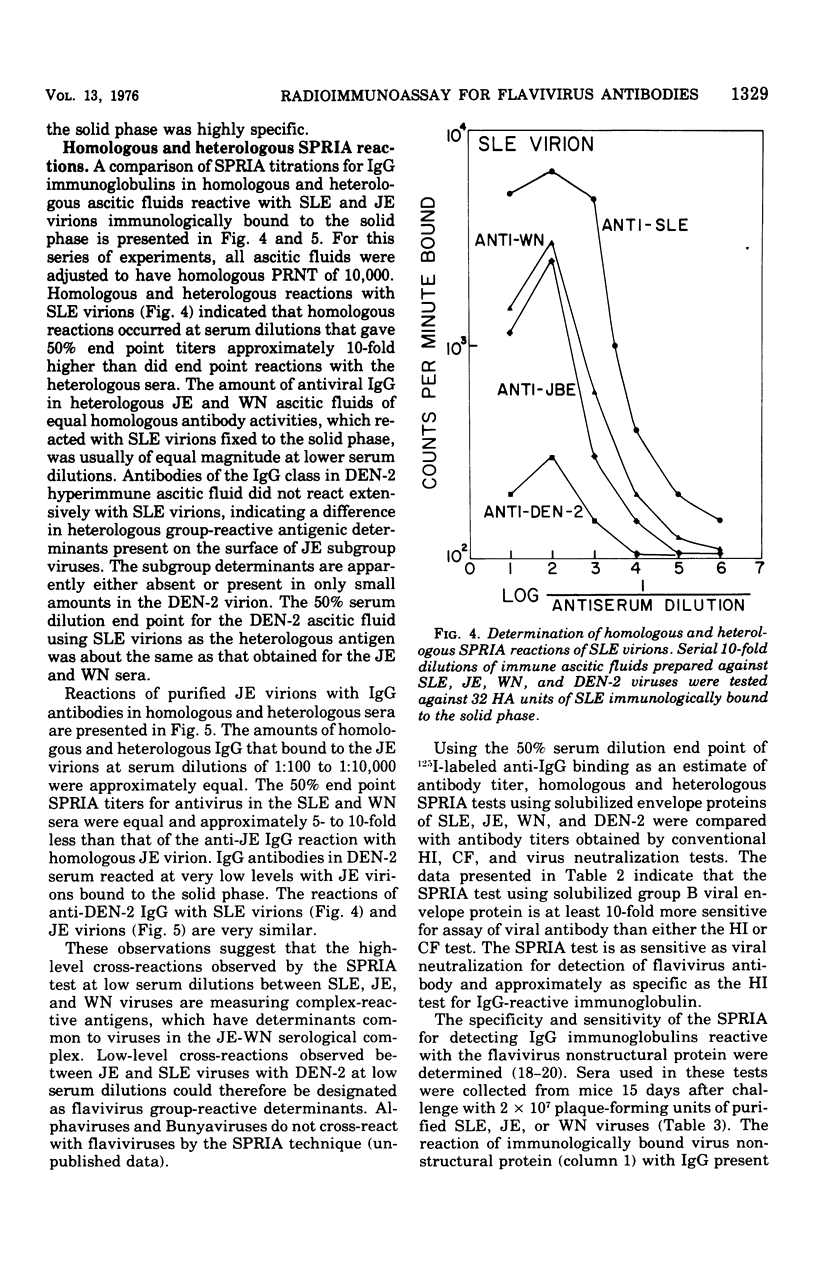
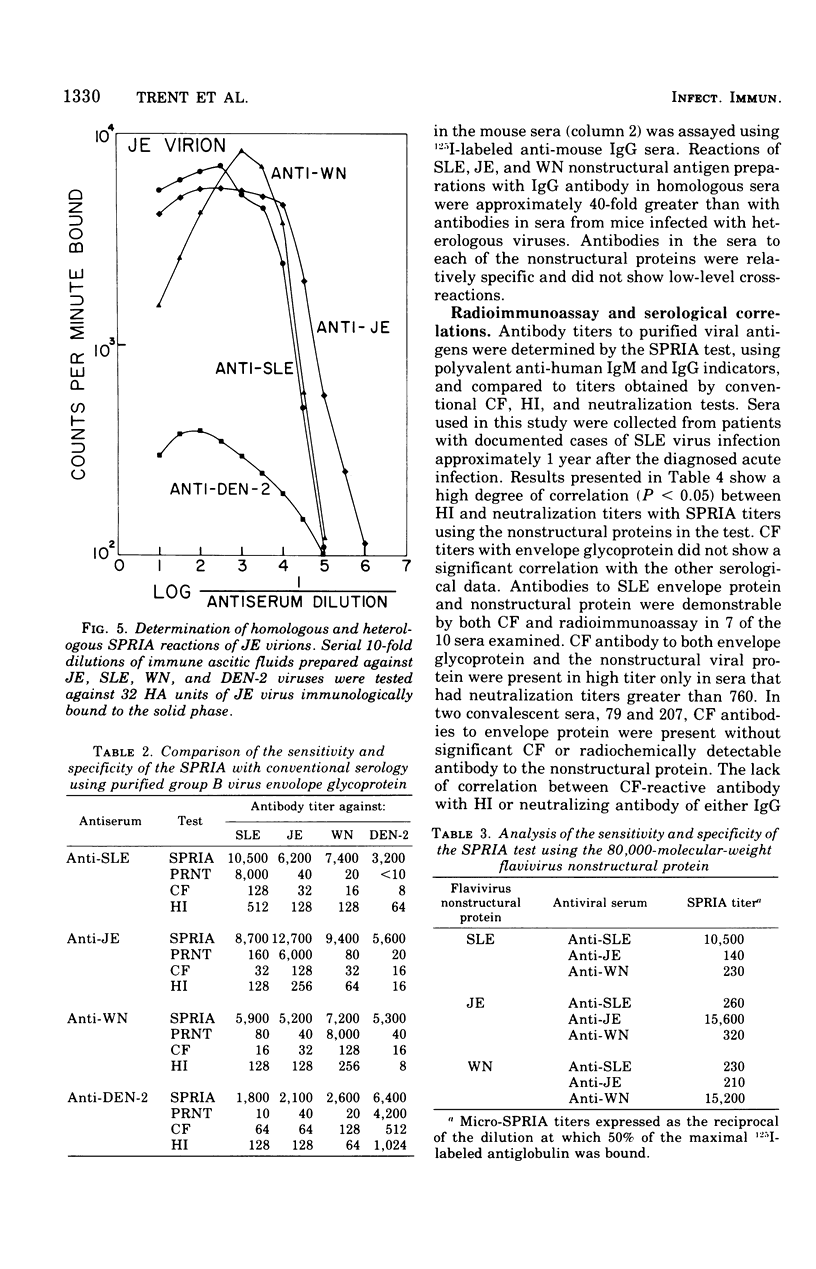
Abstract
A micro-solid-phase radioimmunoassay (SPRIA) is described for quantitation of antibodies to purified flaviviruses as well as to the purified envelope glycoprotein and 80,000-molecular-weight viral nonstructural protein. Sera from mice experimentally infected with Saint Louis encephalitis (SLE) virus or from humans after a primary SLE virus infection reacted more specifically with the major viral envelope protein in the SPRIA test than with antigens conventionally used in the complement fixation (CF) and hemagglutination inhibition tests. A high degree of correlation (P is less than 0.05) was observed between SPRIA anti-immunoglobulin G binding values with the 80,000-molecular-weight nonstructural protein of SLE virus and antibody titers obtained by plaque reduction neutralization and CF with the nonstructural protein. In five of seven human sera in which CF antibody titers to the nonstructural protein were 4 or less, SPRIA testing revealed significant titers of IgG immunoglobulin reactive with this viral protein. The SPRIA test for antibodies reactive with group B togavirus nonstructural protein is as specific and sensitive as the plaque reduction neutralization test for titrating viral antibody in human and animal sera. Antibodies reactive with viral envelope proteins are broadly cross-reactive by the Spria technique, demonstrating both group- and complex-reactive antigenic determinants. The SPRIA test, using wells precoated with antigen, can be completed in 1 day, providing a rapid, highly sensitive test which can be adapted to use in testing a large number of sera.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASALS J. Viruses: the versatile parasites; the arthropod-borne group of animal viruses. Trans N Y Acad Sci. 1957 Jan;19(3):219–235. doi: 10.1111/j.2164-0947.1957.tb00526.x. [DOI] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- DORRANCE W. R., FRANKEL J. W., GORDON I., PATTERSON P. R., SCHLESINGER R. W., WINTER J. W. Clinical and serologic response of man to immunization with attenuated dengue and yellow fever viruses. J Immunol. 1956 Nov;77(5):352–364. [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple J. M., Teramoto A. Y., Cardiff R. D., Russell P. K. Radioimmune precipitation of group A arboviruses. J Immunol. 1972 Sep;109(3):426–433. [PubMed] [Google Scholar]
- Dalrymple J. M., Vogel S. N., Teramoto A. Y., Russell P. K. Antigenic components of group A arbovirus virions. J Virol. 1973 Nov;12(5):1034–1042. doi: 10.1128/jvi.12.5.1034-1042.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugharty H., Warfield D. T., Davis M. L. Solid-phase radioimmunoassay of total and influenza-specific immunoglobulin G. Appl Microbiol. 1972 Feb;23(2):360–367. doi: 10.1128/am.23.2.360-367.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugharty H., Warfield D. T., Hemingway W. D., Casey H. L. Mumps class-specific immunoglobulins in radioimmunoassay and conventional serology. Infect Immun. 1973 Mar;7(3):380–385. doi: 10.1128/iai.7.3.380-385.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman R., Nisalak A., Pariyanonda A., Udomsakdi S., Johnsen D. O. Immunoglobulin response and viremia in dengue-vaccinated gibbons repeatedly challenged with Japanese encephalitis virus. Am J Epidemiol. 1973 Mar;97(3):208–218. doi: 10.1093/oxfordjournals.aje.a121501. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L., HORBETT A. P. Human gamma globulin fractionation on anion exchange cellulose columns. J Biol Chem. 1959 Oct;234:2645–2651. [PubMed] [Google Scholar]
- Falkler W. A., Jr, Diwan A. R., Halstead S. B. Human antibody to dengue soluble complement-fixing (SCF) antigens. J Immunol. 1973 Dec;111(6):1804–1809. [PubMed] [Google Scholar]
- GOLDBLUM N., STERK V. V., JASINSKAKLINGBERG W. The natural history of West Nile fever. II. Virological findings and the development of homologous and heterologous antibodies in West Nile infection in man. Am J Hyg. 1957 Nov;66(3):363–380. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- PARKS J. J., GANAWAY J. R., PRICE W. H. Studies on immunologic overlap among certain arthropod-borne viruses. III. A laboratory analysis of three strains of West Nile virus which have been studied in human cancer patients. Am J Hyg. 1958 Sep;68(2):106–119. [PubMed] [Google Scholar]
- POND W. L. ARTHROPOD-BORNE VIRUS ANTIBODIES IN SERA FROM RESIDENTS OF SOUTH-EAST ASIA. Trans R Soc Trop Med Hyg. 1963 Sep;57:364–371. doi: 10.1016/0035-9203(63)90100-7. [DOI] [PubMed] [Google Scholar]
- Pond W. L., Ehrenkranz N. J., Danauskas J. X., Carter M. J. Heterotypic serologic responses after yellow fever vaccination; detection of persons with past St. Louis encephalitis or dengue. J Immunol. 1967 Apr;98(4):673–682. [PubMed] [Google Scholar]
- Purcell R. H., Wong D. C., Alter H. J., Holland P. V. Microtiter solid-phase radioimmunoassay for hepatitis B antigen. Appl Microbiol. 1973 Oct;26(4):478–484. doi: 10.1128/am.26.4.478-484.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A. A., Trent D. W. Group B arbovirus structural and nonstructural antigens. 3. Serological specificity of solubilized intracellular viral proteins. Infect Immun. 1973 Dec;8(6):993–999. doi: 10.1128/iai.8.6.993-999.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A. A., Trent D. W. Group B arbovirus structural and nonstructural antigens. I. Serological identification of Saint Louis encephalitis virus soluble antigens. Infect Immun. 1973 Feb;7(2):242–248. doi: 10.1128/iai.7.2.242-248.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A. A., Trent D. W. Group B arbovirus structural and nonstructural antigens. II. Purification of Saint Louis encephalitis virus intracellular antigens. Infect Immun. 1973 Dec;8(6):985–992. doi: 10.1128/iai.8.6.985-992.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J. D., Hayashi K., Notkins A. L. Rapid micro-radioimmunoassay for the measurement of antiviral antibody. J Immunol. 1972 Jul;109(1):171–173. [PubMed] [Google Scholar]
- Scott R. M., McCown J. M., Russell P. K. Human immunoglobulin specificity after group B arbovirus infections. Infect Immun. 1972 Sep;6(3):277–281. doi: 10.1128/iai.6.3.277-281.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. M., Russell P. K. Complement fixation blocking activity of anti-dengue IgM antibody. J Immunol. 1972 Oct;109(4):875–877. [PubMed] [Google Scholar]
- THEILER M., CASALS J. The serological reactions in yellow fever. Am J Trop Med Hyg. 1958 Nov;7(6):585–594. doi: 10.4269/ajtmh.1958.7.585. [DOI] [PubMed] [Google Scholar]
- Tignor G. H., Price W. H. Antibody responses in spider monkeys following single and double infections with group B arboviruses. Am J Epidemiol. 1971 Oct;94(4):386–396. doi: 10.1093/oxfordjournals.aje.a121333. [DOI] [PubMed] [Google Scholar]
- Westaway E. G. Antibody responses in rabbits to the group B arbovirus Kumjin: serologic activity of the fractionated immunoglobulins in homologous and heterologous reactions. J Immunol. 1968 Mar;100(3):569–580. [PubMed] [Google Scholar]
- Westaway E. G., Della-Porta A. J., Reedman B. M. Specificity of IgM and IgG antibodies after challenge with antigenically related togaviruses. J Immunol. 1974 Feb;112(2):656–663. [PubMed] [Google Scholar]
- Wiktor T. J., Koprowski H., Dixon F. Radioimmunoassay procedure for rabies binding antibodies. J Immunol. 1972 Sep;109(3):464–470. [PubMed] [Google Scholar]



