In their correspondence, Das and colleagues 1 raise a number of issues about our work, which we address below.
Firstly, we have not claimed that the RNA scissile phosphate is situated in the RNase H active site of HIV-1 RT ready for cleavage. Instead we reported 2 that our structures are “compatible with RNA degradation” (not “catalytically relevant” as incorrectly quoted by Das et al. 1), whereas all previous RT-nucleic acid (NA) complex structures are incompatible with RNA degradation. The incompatibility with RNA cleavage in the previous RT-NA complexes lies in the orientation of the NA substrate, not in the distance between NA and the RNase H active site, as we clearly demonstrated in Figure S5 2. In the previous RT-NA complexes, one DNA strand can be connected with the RNA/DNA hybrid positioned for RNA cleavage (Fig. 1a) according to the human and bacterial RNase H1-RNA/DNA hybrid structure 3,4, but the second strand (RNA equivalent) cannot be connected because of a 14 Å gap. This gap cannot be closed by any amount of bending or unwinding of the duplex 3. In the three RT-RNA/DNA-NNRTI complex structures we reported 2, the RNA/DNA hybrids are oriented such that there is no long a gap (Fig. 1b), and a slight adjustment of the RNA strand would permit hydrolysis as illustrated in Figures 5 2. Indeed, the nearest RNA phosphate in our structures is 8.8 Å from the active site carboxylate as depicted in Figure 5 2. We should clarify that the distance between the scissile phosphate and the active site in the human RNase H1-RNA/DNA complex is 4.6Å (PDB: 2Q39) 3. Thus the scissile phosphate in the 4B3O structure needs to move 4.2Å to be positioned for cleavage. It is not the distance but rather the NA orientation that makes our RNA/DNA hybrid compatible with RNA degradation, which is also evident in Fig. 1a of the correspondence by Das and colleague 1.
Figure 1.
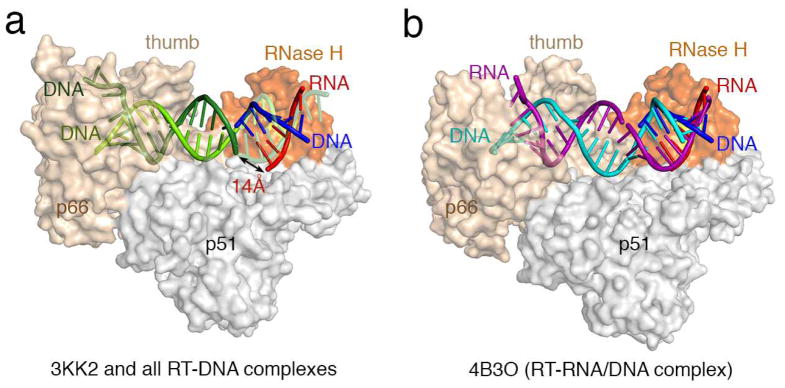
Differences between previous RT-NA complexes and our RT-RNA/DNA structures. (a) All RT-NA structrues previously reported are similar 2 and represented here by the RT-DNA-dATP ternary complex (PDB:3KK2 6) in the polymerization mode. Human RNase H1-RNA/DNA structure (PDB: 2QK9 3) is superimposed onto the RNase H domain of p66 (colored in orange) with the active site highlighted in yellow. The RNA/DNA hybrid from the human RNase H1 complex is shown in red and blue as labeled. The part of the DNA (colored light and dark green) in 3KK2 that overlaps with the RNA/DNA hybrid borrowed from the human RNase H1 complex is shown in semi-transparecy for clarity. When one DNA observed in 3KK2 (light green) and the DNA modeled into the RNase H active site (blue) are connected, the second DNA strand in 3KK2 (dark green) is 14Å from the RNA strand (red) positioned for cleavage by RNase H. (b) In all three of our RT-RNA/DNA hybrid structures, represented here by 4B3O, the RNA/DNA hybrid (purple and cyan, respectively) are A-form, where basepairs are tilted relative to the helical axis rather than perpendicular as in the B-form, and can be connected with the RNA/DNA hybrid in the RNase H active site with minor adjustment. Therefore we suggest that our RT-RNA/DNA complex structures represent the mode compatible with RNA degradation.
Secondly, we disagree with the statement that our structure (4B3O) is most closely related to the RT-DNA/DNA-nevirapine structure (PDB 3V8I)5. Both the protein and nucleic acid of the 3V8I structure are more similar to that of RT-DNA complexes than to our RT-RNA/DNA hybrid complexes, as we showed in Figure 3 2. For example, the RNA/DNA hybrid in our structure has the A-form conformation, while the DNA duplex in 3V8I as well as all previously reported RT-NA complexes are largely B-form 2 (Fig. 1).
Thirdly, with regard to the alleged crystal packing effects, we had noticed the lattice contact all along but found it irrelevant to RT-RNA/DNA hybrid complex formation, based on three different crystal forms of RT-RNA/DNA hybrid complexes (Figures 1 and S1 2). The region near the p66 thumb domain, with which Das and colleagues are concerned 1, is not involved in crystal packing in at least one of our three crystal structures 2, and the A-form conformation of our entire RNA/DNA is independent of this lattice contact.
Fourthly, on the issue of the nick in the RNA strand used in one of the three RT-RNA/DNA complex structures presented in our paper, the nick was engineered by design as reported in the main text and Methods section, and depicted in Figures 1 and S1 2. However, the other two RT-RNA/DNA complex structures (PDB: 4B3P and 4B3Q) contain a continuous RNA strand 2. The statement by Das and colleagues that a continuous RNA/DNA duplex would not be able to adopt the conformation or trajectory adopted by the nicked hybrid 1 is simply incorrect. The RNA/DNA hybrids in all three of our structures, including two with a continuous RNA strand are in similar conformations (Figures 1d and S3 2).
Finally, regarding cross-linking between RT and nucleic acid in previous RT complexes, we have not questioned the validity of this strategy in capturing HIV-1 RT in the DNA polymerization mode. Rather, we respectfully pointed out that in the more than 20 RT-DNA crosslinked structures, the DNAs are all in a similar conformation, and one that is incompatible with RNA degradation.
References
- 1.Das K, Sarafianos SG, Arnold E. Understanding Functional States of HIV-1 Reverse Transcriptase. Nat Struct Mol Biol. 2013 doi: 10.1038/nsmb.2725. [DOI] [PubMed] [Google Scholar]
- 2.Lapkouski M, Tian L, Miller JT, Le Grice SF, Yang W. Complexes of HIV-1 RT, NNRTI and RNA/DNA hybrid reveal a structure compatible with RNA degradation. Nat Struct Mol Biol. 2013;20:230–236. doi: 10.1038/nsmb.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nowotny M, et al. Structure of human RNase H1 complexed with an RNA/DNA hybrid: insight into HIV reverse transcription. Mol Cell. 2007;28:264–276. doi: 10.1016/j.molcel.2007.08.015. S1097-2765(07)00558-8 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Nowotny M, Gaidamakov SA, Crouch RJ, Yang W. Crystal structures of RNase H bound to an RNA/DNA hybrid: substrate specificity and metal-dependent catalysis. Cell. 2005;121:1005–1016. doi: 10.1016/j.cell.2005.04.024. S0092-8674(05)00404-6 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Das K, Martinez SE, Bauman JD, Arnold E. HIV-1 reverse transcriptase complex with DNA and nevirapine reveals non-nucleoside inhibition mechanism. Nat Struct Mol Biol. 2012;19:253–259. doi: 10.1038/nsmb.2223. nsmb.2223 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lansdon EB, et al. Visualizing the molecular interactions of a nucleotide analog, GS-9148, with HIV-1 reverse transcriptase-DNA complex. J Mol Biol. 2010;397:967–978. doi: 10.1016/j.jmb.2010.02.019. S0022-2836(10)00177-4 [pii] [DOI] [PubMed] [Google Scholar]


