Abstract
The blastogenic response of peripheral blood leukocytes to lipopolysaccharide (LPS) was followed over a short course of experimental gingivitis, developed in human volunteers who strictly avoided oral hygeine procedures for periods up to 9 days. Eleven young males initially received thorough dental prophylaxes and supervised oral hygeine until they acquired optimal gingival health. At this point, leukocytes (5 X 10(5)) incubated with 1.5 to 25 mug of LPS in serum-free media showed no response as measured by tritiated thymidine uptake. Coincubation of cells with LPS and phytohemagglutinin (PHA), however, caused synergistic enhancement of blastogenesis in every LPS-PHA dose combination tried. With progressive accumulation of dental plaque and the concomitant development of gingival inflammation, this synergistic response was lost and replaced, proportionately, by a direct response to LPS. The leukocyte response to PHA was marginally enhanced with gingivitis.
Full text
PDF
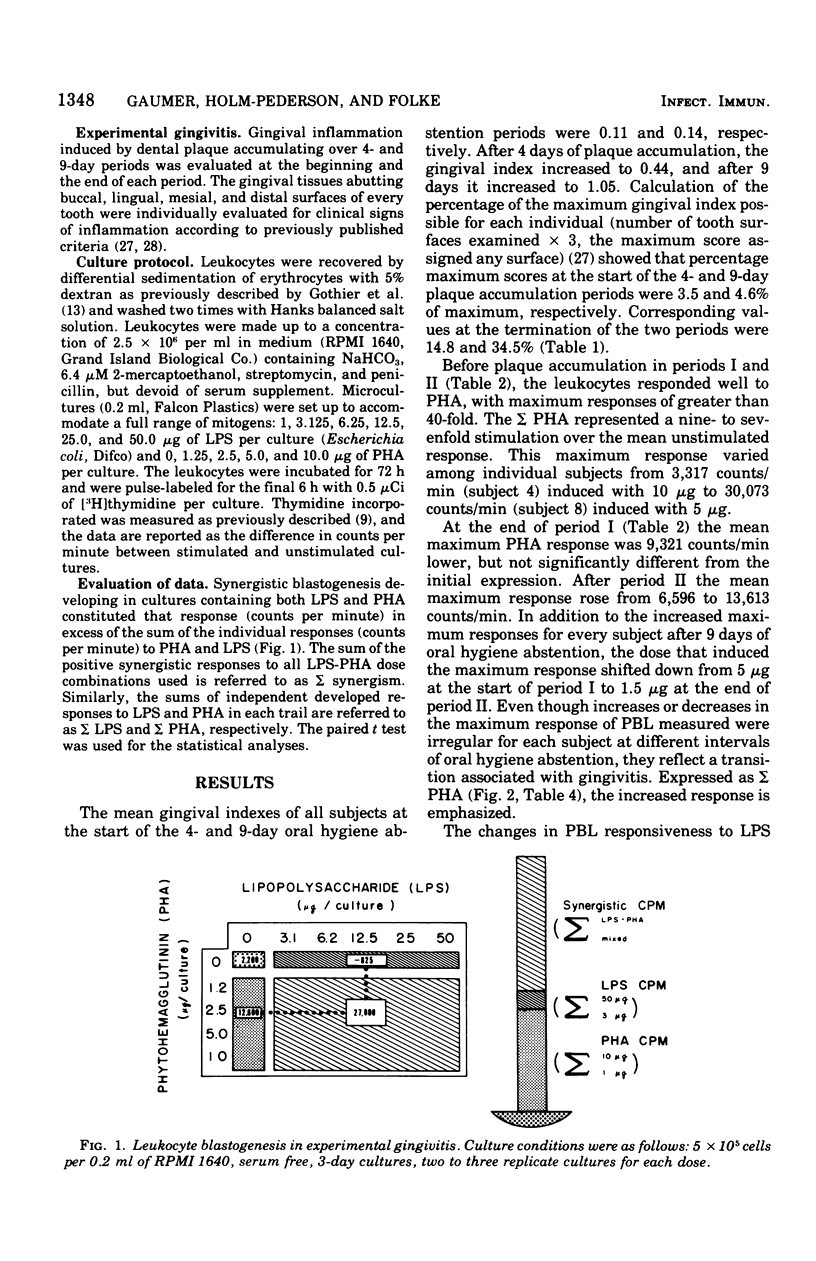
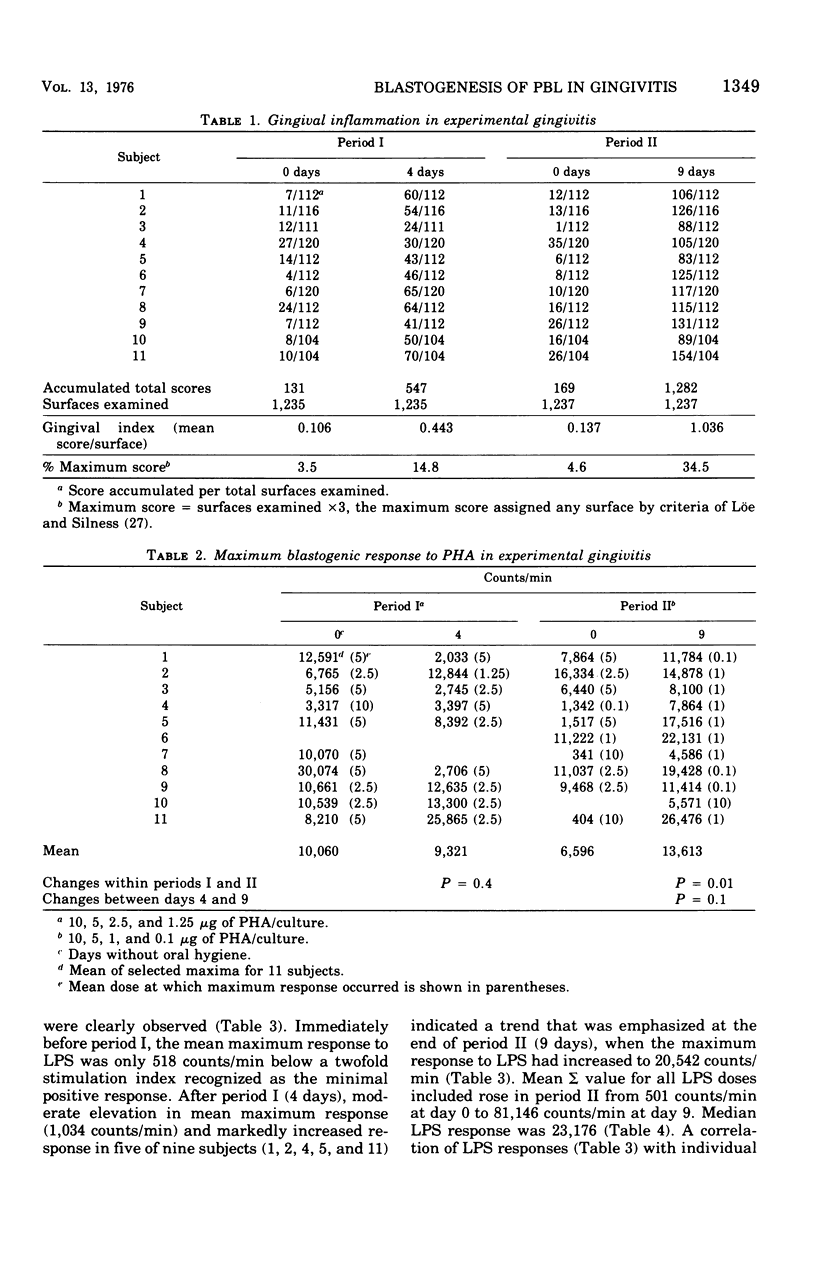
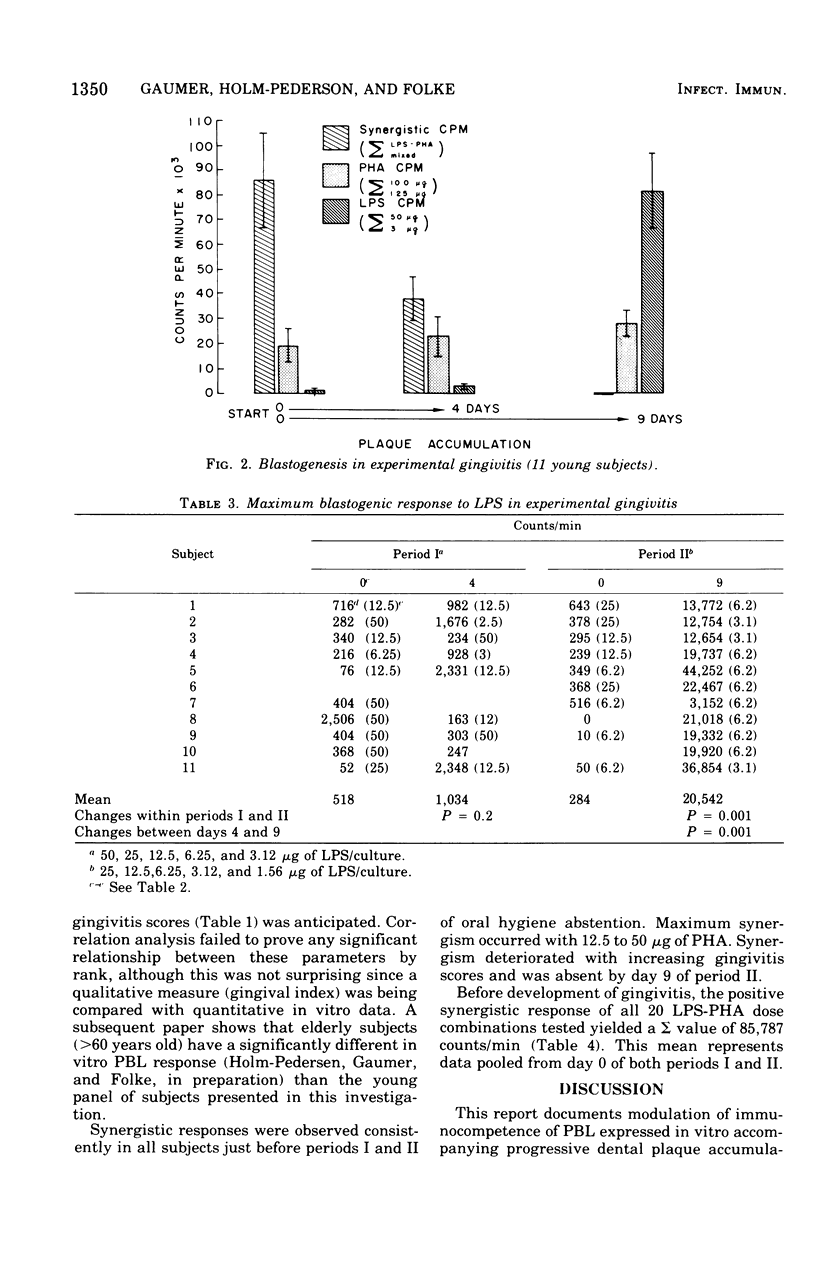



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Möller G., Sjöberg O. B lymphocytes can be stimulated by concanavalin A in the presence of humoral factors released by T cells. Eur J Immunol. 1972 Feb;2(1):99–101. doi: 10.1002/eji.1830020119. [DOI] [PubMed] [Google Scholar]
- Armerding D., Katz D. H. Activation of T and B lymphocytes in vitro. I. Regulatory influence of bacterial lipopolysaccharide (LPS) on specific T-cell helper function. J Exp Med. 1974 Jan 1;139(1):24–43. doi: 10.1084/jem.139.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkenbilt D. A., Bahn A. N. Development of antibodies to cariogenic streptococci in children. J Am Dent Assoc. 1971 Aug;83(2):332–337. doi: 10.14219/jada.archive.1971.0294. [DOI] [PubMed] [Google Scholar]
- Blachman U., Graboff S. R., Haag G. E., Gottfeld E., Pickett M. J. Experimental Cholera in Chinchillas: the Immune Response in Serum and Intestinal Secretions to Vibrio cholerae and Cholera Toxin. Infect Immun. 1974 Nov;10(5):1098–1104. doi: 10.1128/iai.10.5.1098-1104.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratthall D., Gibbons R. J. Changing agglutination activities of salivary immunoglobulin A preparations against oral streptococci. Infect Immun. 1975 Mar;11(3):603–606. doi: 10.1128/iai.11.3.603-606.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Claman H. N. Thymus-marrow immunocompetence. V. Hydrocortisone-resistant cells and processes in the hemolytic antibody response of mice. J Exp Med. 1971 May 1;133(5):1026–1034. doi: 10.1084/jem.133.5.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas S. D., Kamin R. M., Fudenberg H. H. Human lymphocyte response to phytomitogens in vitro: normal, agammaglobulinemic and paraproteinemic individuals. J Immunol. 1969 Dec;103(6):1185–1195. [PubMed] [Google Scholar]
- Forbes J. T., Nakao Y., Smith R. T. T mitogens trigger LPS responsiveness in mouse thymus cells. J Immunol. 1975 Mar;114(3):1004–1007. [PubMed] [Google Scholar]
- GIBBONS R. J., SOCRANSKY S. S., SAWYER S., KAPSIMALIS B., MACDONALD J. B. The microbiota of the gingival crevice area of man. II. The predominant cultivable organisms. Arch Oral Biol. 1963 May-Jun;8:281–289. doi: 10.1016/0003-9969(63)90020-7. [DOI] [PubMed] [Google Scholar]
- Gaumer H. R., Schwab J. H. Differential susceptibility of mouse lymphocytes to an immunosuppressant from group A streptococci. Cell Immunol. 1972 Aug;4(4):394–406. doi: 10.1016/0008-8749(72)90041-x. [DOI] [PubMed] [Google Scholar]
- Genco R. J., Mashimo P. A., Krygier G., Ellison S. A. Antibody-mediated effects on the periodontium. J Periodontol. 1974 May;45(5):330–337. doi: 10.1902/jop.1974.45.5.330. [DOI] [PubMed] [Google Scholar]
- Gilmour M. N., Nisengard R. J. Interactions between serum titres to filamentous bacteria and their relationship to human periodontal disease. Arch Oral Biol. 1974 Nov;19(11):959–968. doi: 10.1016/0003-9969(74)90081-8. [DOI] [PubMed] [Google Scholar]
- Gothier D. E., Gaumer H. R., Pihlstrom B. L., Folke L. E. Elevation of a serum component in periodontal disease capable of modulating chemotactic infiltration. J Periodontal Res. 1975 May;10(2):65–72. doi: 10.1111/j.1600-0765.1975.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Möller G. Differentiation of B cells: sequential appearance of responsiveness to polyclonal activators. Scand J Immunol. 1974;3(4):413–421. doi: 10.1111/j.1365-3083.1974.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Reactions in the periodontium to continuous antigenic stimulation in sensitized gnotobiotic rats. Infect Immun. 1974 Sep;10(3):565–577. doi: 10.1128/iai.10.3.565-577.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. E., Leikin S., Oppenheim J. J. Human lymphoproliferative reaction to saliva and dental plaque-deposits: an in vitro correlation with periodontal disease. J Periodontol. 1972 Sep;43(9):522–527. doi: 10.1902/jop.1972.43.9.522. [DOI] [PubMed] [Google Scholar]
- Häyry P., Defendi V. I. Cultivation conditions and mixed lymphocyte interaction of mouse peripheral lymphocytes. Clin Exp Immunol. 1970 Mar;6(3):345–361. [PMC free article] [PubMed] [Google Scholar]
- Ivanyi L., Lehner T. Lymphocyte transformation by sonicates of dental plaque in human periodontal disease. Arch Oral Biol. 1971 Sep;16(9):1117–1121. doi: 10.1016/0003-9969(71)90216-0. [DOI] [PubMed] [Google Scholar]
- Ivanyi L., Lehner T. Stimulation of lymphocyte transformation by bacterial antigens in patients with periodontal disease. Arch Oral Biol. 1970 Nov;15(11):1089–1096. doi: 10.1016/0003-9969(70)90121-4. [DOI] [PubMed] [Google Scholar]
- Ivanyi L., Lehner T. The significance of serum factors in stimulation of lymphocytes from patients with periodontal disease by Veillonella alcalescens. Int Arch Allergy Appl Immunol. 1971;41(4):620–627. doi: 10.1159/000230554. [DOI] [PubMed] [Google Scholar]
- Kauffman C. A., Phair J. P., Linnemann C. C., Jr, Schiff G. M. Cell-mediated immunity in humans during viral infection. I. Effect of rubella on dermal hypersensitivity, phytohemagglutinin response, and T lymphocyte numbers. Infect Immun. 1974 Jul;10(1):212–215. doi: 10.1128/iai.10.1.212-215.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger R. D., Wright W. H., Creamer H. R. The significance of lymphocyte transformation responses to various microbial stimulants. J Periodontol. 1974 Nov;45(11):780–785. doi: 10.1902/jop.1974.45.11.780. [DOI] [PubMed] [Google Scholar]
- LOE H., SILNESS J. PERIODONTAL DISEASE IN PREGNANCY. I. PREVALENCE AND SEVERITY. Acta Odontol Scand. 1963 Dec;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E., Pardon P. Effects of bacterial lipopolysaccharide on the induction and expression of cell-mediated immunity. I. Depression of the afferent arc. J Immunol. 1975 Jan;114(1 Pt 2):442–446. [PubMed] [Google Scholar]
- Lang N. P., Smith F. N. Lymphocyte response to T-cell mitogen during experimental gingivitis in humans. Infect Immun. 1976 Jan;13(1):108–113. doi: 10.1128/iai.13.1.108-113.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Challacombe S. J., Ivanyi L., Wilton J. M. The relationship between serum and salivary antibodies and cell-mediated immunity in oral disease in man. Adv Exp Med Biol. 1974;45(0):485–495. doi: 10.1007/978-1-4613-4550-3_59. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Gibbons R. J. Amino acid fermentation by Fusobacterium nucleatum. Arch Oral Biol. 1968 Feb;13(2):191–202. doi: 10.1016/0003-9969(68)90051-4. [DOI] [PubMed] [Google Scholar]
- Ozato K., Adler W. H., Ebert J. D. Synergism of bacterial lipopolysaccharides and concanavalin A in the activation of thymic lymphocytes. Cell Immunol. 1975 Jun;17(2):532–541. doi: 10.1016/s0008-8749(75)80057-8. [DOI] [PubMed] [Google Scholar]
- Rudbach J. A., Luoma M. K. Endotoxin-altering activity of plasma does not affect antigenicity of native protoplasmic polysaccharide. Infect Immun. 1974 Nov;10(5):1183–1184. doi: 10.1128/iai.10.5.1183-1184.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke J. R., Najarian J. S. Synergistic effects on DNA synthesis of phytohemagglutinin or concanavalin A and lipopolysaccharide in human peripheral blood lymphocytes. J Immunol. 1975 Feb;114(2 Pt 2):742–746. [PubMed] [Google Scholar]
- Schroeder H. E., Münzel-Pedrazzoli S., Page R. Correlated morphometric and biochemical analysis of gingival tissue in early chronic gingivitis in man. Arch Oral Biol. 1973 Jul;18(7):899–923. doi: 10.1016/0003-9969(73)90060-5. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Buimovici-Klein E. Lymphocyte responses to rubella antigen and phytohemagglutinin after administration of the RA 27/3 strain of live attenuated rubella vaccine. Infect Immun. 1975 Apr;11(4):748–753. doi: 10.1128/iai.11.4.748-753.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


