Abstract
Background
Poor physical performance (PP) is known to be associated with disability, lower quality of life and higher mortality rates. Knee and hip osteoarthritis (OA) might be expected to contribute to poor PP, through joint pain and restricted range of movement. Both clinical and self-reported OA are often used for large scale community and epidemiological studies.
Objective
To examine the relationships between hip and knee OA and PP in a large dataset comprising cohorts from six European countries.
Methods
2942 men and women aged 65-85 years from the Germany, Italy, Netherlands, Spain, Sweden, and the United Kingdom were recruited. Assessment included an interview and clinical assessment for OA. PP was determined from walking speed, chair rises and balance (range 0-12); low PP was defined as a score of ≤9.
Results
The mean(SD) age was 74.2(5.1) years. Rates of self-reported OA were much higher than clinical OA. Advanced age, female gender, lower educational attainment, abstinence from alcohol, and higher BMI were independently associated with low PP. Clinical knee OA, hip OA, or both were associated with a higher risk of low PP; OR(95%CI) 2.93(2.36,3.64), 3.79(2.49,5.76), and 7.22(3.63,14.38) respectively, with relationships robust to adjustment for the confounders above as well as pain.
Conclusion
Lower limb OA at the hip and knee is associated with low PP, and for a clinical diagnosis relationships are robust to adjustment for pain. Those at highest risk have clinical OA at both sites.
Keywords: osteoarthritis, physical performance, European, prevalence
Introduction
Osteoarthritis (OA) is the most common chronic joint disorder in older people(1) and is associated with significant disability. Worldwide it was estimated to account for the loss of over 17 million DALYs in 2010(2). Poor physical performance has been shown to be strongly associated with disability, short term mortality, nursing home admission, poor health and quality of life(3-5). A greater understanding of the relationship between such a score and OA is timely, and might benefit agencies trying to evaluate the likely impact of a diagnosis of OA on ability to self care.
Although relationships between OA and physical performance have been examined previously, these studies have usually focussed on those individuals with severe disease rather than community-based populations(6;7) and have often concentrated on OA in one joint alone when the condition is frequently polyarticular(7-9). Typically investigators have considered subjects with radiographic OA(6-9). However, there is a growing awareness that this often correlates poorly with symptoms(10), in contrast to the American College of Rheumatology (ACR) criteria, which are now thought to reflect accepted clinical practice(11). As we aim to determine the effects of OA on physical performance, it is clearly important that we use a definition that is analogous to clinical practice and that the research is undertaken in cohorts that are representative of the general older population.
There is also increasing utilisation of self-reported diagnoses in large scale community and epidemiological studies, including for OA, as a valid predictor of certain clinical outcomes(12-14). Furthermore, self-report has been used not only to define OA but additionally measures of disease status, physical function, pain, and disability(15-17). Diagnosing OA using a self-reported definition is both less costly and less invasive than making either a clinical or radiographic diagnosis. However, there is a need to determine whether when using this definition, similar relationships are consistently observed to those identified using a more rigorous, clinical definition.
Therefore, in this study, we performed an analysis using a large cohort with representation from several European countries to assess: to what extent joint pain, self-reported OA and clinical OA correlate with physical performance in population-based cohorts; whether location or number of sites affected by OA is important; and what factors mediate any such associations found.
Methods
Study design and sample characteristics
The European Project on Osteoarthritis (EPOSA) involves six cohort studies each performed in a different country: Germany, United Kingdom (UK), Italy, The Netherlands, Spain and Sweden. Random samples from these population-based cohorts are included. In each cohort, around 750 potential participants were contacted with the aim of recruiting 500 participants. In Italy, a new sample was drawn, with recruitment procedures and age/sex-distributions similar to those in the other studies. Further details are available from the EPOSA design paper(18). The overall age range was 65-85 years (with oversampling of the oldest respondents 80-85 years) in all cohorts except for the UK, which has an age range of 71-79 years. The design and procedures of all six cohort studies were approved by the Medical Ethics Committee of the respective institutions.
Procedure
Data collection started between November 2010 and March 2011 in all countries, and ended between September and November 2011. The EPOSA interview incorporated the Western Ontario and McMaster Universities Arthritis Index (WOMAC) questionnaire providing information on degree of joint pain during various activities, joint stiffness, and physical functioning(19). The interview also obtained information on general health (as assessed by number of chronic diseases), medications, lifestyle (such as smoking and alcohol consumption), level of physical activity (using the validated LASA physical activity questionnaire (LAPAQ)(20), quality of life, health care utilisation, and social care utilisation. Self-reported OA was assessed by asking participants, “Do you have OA?”. If they answered “yes”, then the location of OA was asked. The different sites were fingers, hand/wrist, elbows, shoulders, toes, feet, knee, hip, neck, and back.
Measurements of height and weight; a clinical examination for OA; and an assessment of physical performance were also carried out. Algorithms for clinical OA were developed based on the clinical classification criteria of the ACR(21). The clinical diagnoses of hip and knee OA used both history and physical examination findings(18).
Specifically, a clinical diagnosis of hip OA required pain in the hip as evaluated by the WOMAC pain subscale score, plus all of: pain associated with hip internal rotation in at least one side; morning stiffness, evaluated by the WOMAC stiffness subscale; and age over 50 years. The knee OA clinical diagnosis required pain in the knee as evaluated by the WOMAC pain subscale score, plus any 3 of: age over 50 years; morning stiffness, evaluated by the WOMAC stiffness subscale; crepitus on active motion in at least one side; bony tenderness in at least one side; bony enlargement in at least one side; and no palpable warmth of synovium in both knees. Joint pain was assessed using the relevant subscale of the WOMAC questionnaire.
A physical performance score was derived from tests of walking speed, repeated chair stands and standing balance based on the methods described by Guralnik et al.(3). To test walking speed, a 3 metre course, with no obstructions for an additional 2 feet at either end was marked out on the floor. Participants were asked to “walk to the other end of the course as quickly as you can, but do not run” and time taken was assessed using a stopwatch. In the UK, the instruction was to “walk to the other end of the course at your usual speed, just as if you were walking down the street to go to a shop.” The use of assistive devices was permitted if required. In Germany, 361 of the participants were measured using a GAITRite® walkway system and were standardised to timings using a stopwatch. The GAITrite® system consisted of a portable walkway embedded with pressure-activated sensors(22). The walkway detected the timing of sensor activation during walking as well as the relative distances between the activated sensors. This information was fed back to the application software for analysis. Gait aspeed was determined by dividing the distance traversed by the time between the first and last step.
Chair stands tested each participant’s ability to rise from a straight-backed chair. Participants folded their arms across their chest and stood up. If they were able to complete this they were asked to stand up and sit down again a total of five times at their normal pace. The UK and the German cohorts were instructed to perform this task as quickly as possible. Participants were timed from their initial sitting position until they were standing on the fifth repetition.
The test of standing balance was a tandem stand. The interviewer demonstrated the task and then supported one arm of the participant whilst they positioned their feet. When the participant was ready, the support was released and timing began. Timing was stopped if the participant moved their feet or grasped the interviewer for support, or when 10 seconds were up. If the participant maintained balance for 10 seconds they were given a score of 4; if they obtained a time of between 4 and <10 seconds they scored 2; and if they were unable to attempt the stand or managed a shorter time they were given a score of 0.
For the walking test and chair stands, those who could not complete the test were given a score of 0. The rest of the participants’ times were divided into country-specific quartiles to take account of the specific methodology used in each country. They were then given scores of 1 to 4 corresponding to these with those producing times in the fastest quartile being given a score of 4. The scores for the walking test, chair rises and balance were then summed. The maximum possible score was 12 and the minimum was 0. In keeping with previous work, those with a score equal to or lower than 9 were designated as low physical performance(23;24).
Statistical methods
Based on current prevalence rates for clinical OA(25-27) and low physical performance(28) in the age range of the participants studied, the sample size used gave over 80% power to demonstrate a 7% difference in rates of low physical performance in those with and without OA.
As age distribution and sex split varied between the cohorts of different countries a weighting variable was created for each individual within each country. The variables were derived from the European standard population in 2010 and calculated per sex and per five-year age category, using the formula: W=Nexp/Nobs (Nobs is the number of persons in a specific age/sex category in the cohort; and Nexp is the number of persons in a specific age/sex category in the European standard population in 2010). These were applied to all data with the exception of age and sex. This technique allowed direct comparisons of the demographic findings across countries.
Differences between countries were assessed using ANOVA for continuous variables and chi-squared test for categorical variables. Logistic regression was used to assess the relationships between demographic variables and low physical performance, and to model the associations between of subjective OA, clinical OA, and WOMAC pain score, with low physical performance, before and after adjustment for potential covariates.
Declaration of sources of funding
The study was funded by a non-commercial private funder. The funder had no role in the design, execution, analysis or interpretation of the data, or writing of the study.
Results
Fifty-six individuals that took part in the EPOSA study (n=2942) did not have physical performance assessed and were therefore excluded from the analyses. This left a total of 2886 men and women. Those excluded were on average older and more likely to be single or widowed; they did not differ significantly in height, weight, body mass index (BMI), sex, education, smoking status or alcohol consumption.
The mean age in the cohort as a whole was 74.2 years and the mean BMI was 27.3; there was some variation in sex distribution by country but overall females were slightly overrepresented compared with males. Table 1 shows the summary characteristics of the cohorts in each country after weighting to the European standard population in 2010. Clear differences were observed between countries with regard to educational attainment and alcohol consumption; only 0.8% of the Italian cohort had a university education, compared with 42.8% of the Swedish cohort. In the Spanish cohort 38.9% reported drinking alcohol, compared with 74.9-90% in other countries.
Table 1. Baseline characteristics of study participants by country after weighting to the European standard population in 2010.
| All countries (n=2942) | Germany (n=407) | Italy (n=468) | Netherlands (n=574) | Spain (n=539) | Sweden (n=510) | United Kingdom (n=444) | p value3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | Mean | SD | N1 | Mean | SD | N1 | Mean | SD | N1 | Mean | SD | N1 | Mean | SD | N1 | Mean | SD | N1 | Mean | SD | ||
| Age (yrs)2 | 2942 | 74.2 | 5.1 | 407 | 74.4 | 5.0 | 468 | 73.3 | 5.2 | 574 | 75.2 | 5.7 | 539 | 75.0 | 5.5 | 510 | 72.1 | 5.0 | 444 | 75.2 | 2.6 | <0.001 |
| Height (cm) | 2906 | 164.8 | 9.7 | 407 | 167.9 | 9.0 | 467 | 162 | 8.2 | 564 | 167.3 | 9 | 539 | 159.1 | 9.5 | 490 | 168.1 | 9.2 | 439 | 165 | 9.3 | <0.001 |
| Weight (kg)4 | 2880 | 73.9 | 1.2 | 398 | 75.8 | 1.2 | 467 | 72.2 | 1.2 | 566 | 76.2 | 1.2 | 539 | 71.6 | 1.2 | 467 | 1.34 | 1.21 | 443 | 75.2 | 1.2 | <0.001 |
| BMI (kg/m2)4 | 2869 | 27.3 | 1.2 | 398 | 27.1 | 1.2 | 467 | 27.6 | 1.2 | 559 | 27.3 | 1.2 | 539 | 28.4 | 1.2 | 467 | 25.89 | 1.16 | 439 | 27.7 | 1.2 | <0.001 |
| N1 | % | N1 | % | N1 | % | N1 | % | N1 | % | N1 | % | N1 | % | |||||||||
| Gender2 | 2942 | 407 | 468 | 574 | 539 | 510 | 444 | |||||||||||||||
| Male | 48.1 | 59.7 | 46.8 | 44.8 | 50.3 | 40.0 | 50.0 | |||||||||||||||
| Female | 51.9 | 40.3 | 53.2 | 55.2 | 49.7 | 60.0 | 50.0 | <0.001 | ||||||||||||||
| Marital status | 2942 | 407 | 468 | 574 | 539 | 510 | 444 | |||||||||||||||
| Single5 | 5.7 | 4.8 | 4.7 | 6.1 | 6.1 | 7.2 | 4.1 | 0.318 | ||||||||||||||
| Cohabiting6 | 65.0 | 67.4 | 73.0 | 58.8 | 69.3 | 56.6 | 69.1 | <0.001 | ||||||||||||||
| Widowed | 22.5 | 17.9 | 21.2 | 29.4 | 22.1 | 19.8 | 22.3 | <0.001 | ||||||||||||||
| Divorced7 | 6.8 | 9.9 | 1.1 | 5.8 | 2.6 | 16.4 | 4.5 | <0.001 | ||||||||||||||
| Education8 | 2891 | 404 | 468 | 574 | 539 | 462 | 444 | |||||||||||||||
| <Elementary | 11.5 | 2.3 | 7.7 | 6.1 | 35.1 | 11.3 | 0 | <0.001 | ||||||||||||||
| Elementary | 34.4 | 47.7 | 69.9 | 19.3 | 37.3 | 14.8 | 20.9 | <0.001 | ||||||||||||||
| Secondary9 | 33.2 | 29.3 | 21.6 | 56.3 | 15.2 | 31.1 | 45.1 | <0.001 | ||||||||||||||
| University10 | 20.9 | 20.8 | 0.8 | 18.2 | 12.4 | 42.8 | 34 | <0.001 | ||||||||||||||
| Consumes alcohol (yes) | 2924 | 407 | 468 | 571 | 539 | 495 | 444 | |||||||||||||||
| 74.9 | 90.0 | 78.1 | 80.7 | 38.9 | 88.8 | 79.0 | <0.001 | |||||||||||||||
| Smoker status | 2931 | 407 | 468 | 571 | 539 | 502 | 444 | |||||||||||||||
| Never smoked | 49.5 | 50.5 | 58.5 | 36.9 | 58.5 | 43.2 | 52.8 | <0.001 | ||||||||||||||
| Current smoker | 8.5 | 7.0 | 8.2 | 10.5 | 9.2 | 10.1 | 3.7 | 0.004 | ||||||||||||||
| Ex-smoker | 42.0 | 42.5 | 33.2 | 52.5 | 32.4 | 46.6 | 43.5 | <0.001 | ||||||||||||||
Variable-specific N values;
age and gender are not weighting to the European standard population in 2010;
p-value for differences between countries;
Geometric mean and SD;
Never married;
Married/registered partnership/cohabiting;
Divorced/living apart;
Highest level of education completed;
Secondary or vocational education;
Univeristy or college.
Table 2 provides a description of physical performance (including walking test, chair rises and standing balance individually) and prevalence of self-reported and clinical OA by country. Over 50% of participants at each site had a physical performance score of ≤9 indicative of significant impairment; however rates varied by country. There were also significant differences between walking test, chair rises and prevalence of poor balance depending on country. Sweden had the highest proportion of individuals not reporting any chronic diseases (46.8%) and Spain had the lowest (19.7). Rates of self reported OA were considerably higher than clinical OA in each joint and in all countries (table 2). The highest rates of clinical OA were seen in Italy and the lowest in Germany. Specifically, 8.1% of Italian subjects had a clinical diagnosis of OA hip and knee, compared with just 0.2% in Germany. The highest rates of self-reported knee OA were found in Spain and Italy.
Table 2. Descriptives of physical performance, clinical OA and subjective OA by country after weighting to the European standard population 2010.
| All countries (n=2942) | Germany (n=407) | Italy (n=468) | Netherlands (n=574) | Spain (n=539) | Sweden (n=510) | United Kingdom (n=444) | p value2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | Mean | SD | N1 | Mean | SD | N1 | Mean | SD | N1 | Mean | SD | N1 | Mean | SD | N1 | Mean | SD | N1 | Mean | SD | ||
| Physical performance | ||||||||||||||||||||||
| Walk time (sec)3 | 2850 | 3.04 | 1.5 | 401 | 2.41 | 1.2 | 466 | 2.93 | 1.3 | 552 | 3.29 | 1.6 | 535 | 3.16 | 1.5 | 483 | 2.69 | 1.4 | 413 | 4.11 | 1.3 | <0.001 |
| Chair rises (sec)3 | 2699 | 13.0 | 1.4 | 390 | 10.1 | 1.3 | 435 | 12.2 | 1.3 | 518 | 11.8 | 1.4 | 502 | 13.6 | 1.4 | 470 | 15.1 | 1.3 | 384 | 16.6 | 1.4 | <0.001 |
| N1 | % | N1 | % | N1 | % | N1 | % | N1 | % | N1 | % | N1 | % | |||||||||
| Poor balance4 | 2739 | 10.3 | 380 | 5.0 | 454 | 0.2 | 513 | 16.1 | 505 | 12.1 | 476 | 8.3 | 411 | 21.0 | <0.001 | |||||||
| Low physical performance5 | 2886 | 59.6 | 405 | 52.6 | 467 | 59.3 | 559 | 58.5 | 531 | 60.9 | 480 | 58.7 | 444 | 68.9 | <0.001 | |||||||
| Number of chronic diseases6 | 2942 | 407 | 468 | 574 | 539 | 510 | 444 | |||||||||||||||
| 0 | 30.4 | 23.5 | 18.9 | 31.7 | 19.7 | 46.8 | 41.7 | <0.001 | ||||||||||||||
| 1 | 36.2 | 36.6 | 41.4 | 34.6 | 35.6 | 33.1 | 37.3 | 0.109 | ||||||||||||||
| 2 | 21.4 | 26.6 | 29.4 | 20.5 | 24.9 | 13.2 | 13.8 | <0.001 | ||||||||||||||
| 3+ | 11.6 | 13.3 | 10.3 | 12.8 | 19.8 | 5.3 | 7.1 | <0.001 | ||||||||||||||
| Self-reported OA | ||||||||||||||||||||||
| Knee | 2929 | 34.2 | 396 | 28.4 | 467 | 44.3 | 573 | 26.2 | 539 | 45.0 | 510 | 34.9 | 444 | 22.0 | <0.001 | |||||||
| Hip | 2930 | 21.9 | 396 | 7.0 | 468 | 35.5 | 573 | 19.1 | 539 | 24.1 | 510 | 27.3 | 444 | 11.6 | <0.001 | |||||||
| Knee and hip | 2931 | 13.8 | 396 | 4.5 | 467 | 21.6 | 573 | 10.7 | 539 | 19.6 | 510 | 16.9 | 444 | 4.9 | <0.001 | |||||||
| Clinical OA | ||||||||||||||||||||||
| Knee | 2904 | 20.2 | 405 | 11.0 | 467 | 28.8 | 558 | 18.2 | 535 | 24.1 | 506 | 20.0 | 433 | 15.9 | <0.001 | |||||||
| Hip | 2915 | 6.1 | 405 | 0.8 | 467 | 13.8 | 565 | 6.7 | 535 | 4.4 | 507 | 5.0 | 436 | 4.7 | <0.001 | |||||||
| Knee and hip | 2927 | 3.6 | 406 | 0.2 | 467 | 8.1 | 567 | 3.9 | 536 | 3.0 | 509 | 3.4 | 442 | 2.5 | <0.001 | |||||||
| WOMAC Pain 7 | ||||||||||||||||||||||
| Knee | 2924 | 39.8 | 406 | 21.8 | 467 | 46.3 | 566 | 41.2 | 537 | 48.5 | 509 | 40.1 | 439 | 34.7 | <0.001 | |||||||
| Hip | 2928 | 29.0 | 406 | 14.6 | 467 | 41.2 | 568 | 31.4 | 536 | 30.2 | 510 | 31.7 | 441 | 18.0 | <0.001 | |||||||
| WOMAC Stiffness 7 | ||||||||||||||||||||||
| Knee | 2925 | 32.5 | 406 | 12.2 | 467 | 40.7 | 567 | 45.7 | 537 | 23.3 | 509 | 36.9 | 439 | 29.2 | <0.001 | |||||||
| Hip | 2931 | 22.8 | 406 | 5.3 | 467 | 26.1 | 570 | 33.0 | 537 | 16.8 | 510 | 27.2 | 441 | 22.9 | <0.001 | |||||||
Variable-specific N values
p-value for differences between countries
Geometric mean and SD
Time <10 sec
Score ≤9
Chronic diseases include chronic lung disease, cardiovascular disease, peripheral arterial disease, stroke, diabetes, cancer and osteoporosis
Score >0
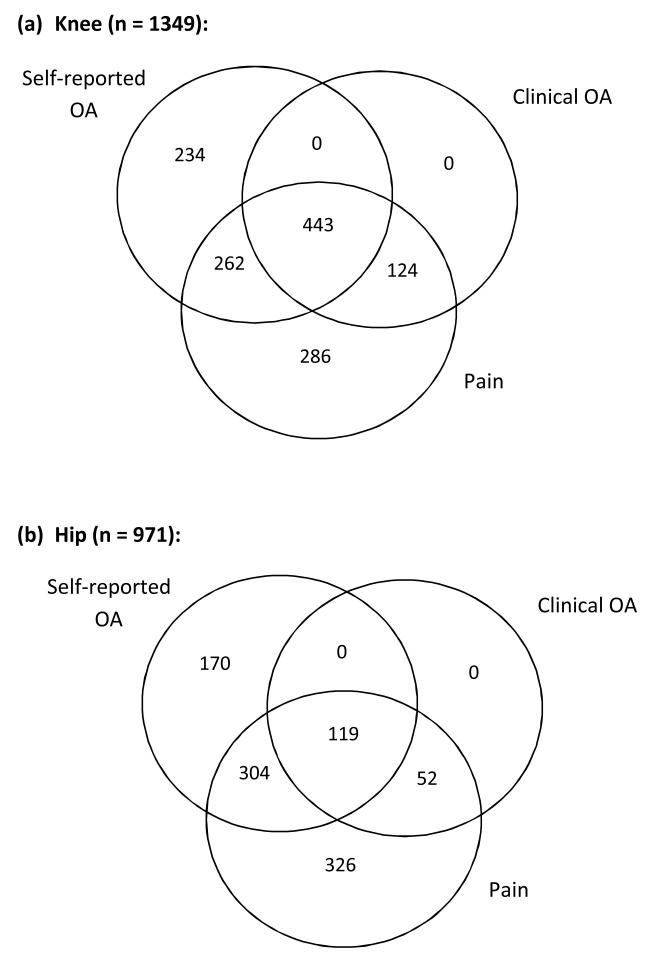
Figure 1 demonstrates the overlap between the different definitions of OA and joint pain. Based on the ACR criteria, a clinical diagnosis of hip or knee OA cannot be made in the absence of pain and this is reflected in the figure. OA was not infrequently self-reported in the absence of joint pain (n=234 and 170 for knee and hip respectively). Pain without self-reported OA was present in the knee in 410 and hip in 378 individuals. Of the 939 participants with self-reported knee OA, 443 (47.2%) also had clinical knee OA; whereas clinical hip OA was diagnosed in only 20.1% of individuals with self-reported hip OA.
Figure 1. Venn diagrams showing overlap between definitions of self-reported OA, clinical OA, and pain in the (a) knee and (b) hip.
Country, sex, and age were all associated with a low physical performance score as shown in Table 3. After adjustment for these three factors, high BMI, low educational attainment, abstinence from alcohol, and stiffness in the hip and knee also showed significant positive relationships with low physical performance. Interestingly, there was a graded relationship between low physical performance and both educational attainment and number of chronic diseases. No associations were demonstrated between either marital status or smoker status and physical performance.
Table 3. Various characteristics as explanatory variables for low physical performance.
| Unadjusted | ||||
|---|---|---|---|---|
| N1 | Odds Ratio | 95% CI | p-value | |
| Country | 2886 | |||
| Germany (reference) | 1 | (1.00, 1.00) | . | |
| Italy | 1.34 | (1.02, 1.75) | 0.034 | |
| Netherlands | 1.22 | (0.95, 1.58) | 0.123 | |
| Spain | 1.31 | (1.01, 1.71) | 0.04 | |
| Sweden | 1.33 | (1.02, 1.74) | 0.037 | |
| United Kingdom | 1.9 | (1.44, 2.51) | <0.001 | |
| Age (yrs) | 2886 | 1.12 | (1.10, 1.14) | <0.001 |
| Gender | 2886 | |||
| Male (reference) | 1 | (1.00, 1.00) | . | |
| Female | 2.18 | (1.87, 2.54) | <0.001 | |
| Adjusted for country, age and gender | ||||
|---|---|---|---|---|
| N1 | Odds Ratio | 95% CI | p-value | |
| BMI (z-score) | 2834 | 1.35 | (1.24, 1.47) | <0.001 |
| Marital status | 2886 | |||
| Single (reference)2 | 1 | (1.00, 1.00) | . | |
| Cohabiting3 | 0.73 | (0.50, 1.05) | 0.089 | |
| Widowed | 0.83 | (0.55, 1.24) | 0.355 | |
| Divorced4 | 0.64 | (0.40, 1.01) | 0.058 | |
| Education1 | 2838 | |||
| <elementary (reference) | 1 | (1.00, 1.00) | . | |
| Elementary | 0.7 | (0.51, 0.96) | 0.026 | |
| Secondary6 | 0.52 | (0.37, 0.72) | <0.001 | |
| University7 | 0.37 | (0.26, 0.52) | <0.001 | |
| Consumes alcohol (yes) | 2878 | 0.78 | (0.63, 0.97) | 0.024 |
| Smoker status: | 2883 | |||
| Never smoked (reference) | 1 | (1.00, 1.00) | . | |
| Current smoker | 1.12 | (0.82, 1.52) | 0.472 | |
| Ex-smoker | 1.1 | (0.92, 1.32) | 0.274 | |
| Number of chronic diseases8 | 2876 | |||
| 0 (reference) | (1.00, 1.00) | . | ||
| 1 | 1.55 | (1.29, 1.86) | <0.001 | |
| 2 | 1.72 | (1.39, 2.12) | <0.001 | |
| 3+ | 2.91 | (2.19, 3.86) | <0.001 | |
| WOMAC Knee Stiffness9 | 2874 | 1.93 | (1.61, 2.32) | <0.001 |
| WOMAC Hip Stiffness9 | 2879 | 2.31 | (1.87, 2.85) | <0.001 |
Variable-specific N values
Never married
Married/registered partnership/cohabiting
Divorced/living apart
Highest level of education completed
Secondary or vocational education
University or college
Chronic diseases include chronic lung disease, cardiovascular disease, peripheral arterial disease, stroke, diabetes, cancer and osteoporosis
Score >0
In table 4, univariate analyses show that those with either pain, self-reported OA or clinical OA in any joint assessed were more likely to have low physical performance. At each joint, the magnitude of the odds ratio was greater with a clinical diagnosis than either pain or self-reported OA. Furthermore, the effect magnitude was also greater in those with both joints affected, as opposed to either hip or knee individually, regardless of the definition used. All of these associations were attenuated by adjustment for other risk factors described in table 3; although each remained statistically significant. The associations between physical performance and both clinical and self-reported OA were further adjusted for pain and stiffness in the corresponding joint. This completely attenuated associations with self-reported OA; in contrast, strong positive relationships were maintained between physical performance and clinical OA at all sites assessed.
Table 4. Associations between low physical performance and clinical OA, self-reported OA and pain before and after adjustments.
| Unadjusted | Adjusted1 | Adjusted including pain and stiffess2 | ||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | |
| Clinical OA | ||||||
| Knee | 2.99 (2.40, 3.71) | <0.001 | 2.31 (1.81, 2.94) | <0.001 | 1.67 (1.23, 2.26) | 0.001 |
| Hip | 3.86 (2.54, 5.87) | <0.001 | 3.68 (2.32, 5.82) | <0.001 | 2.02 (1.21, 3.37) | 0.007 |
| Knee and hip | 7.34 (3.69, 14.6) | <0.001 | 6.67 (3.13, 14.21) | <0.001 | 2.99 (1.36, 6.57) | 0.006 |
| Self-reported OA | ||||||
| Knee | 1.83 (1.55, 2.15) | <0.001 | 1.46 (1.21, 1.76) | <0.001 | 1.05 (0.84, 1.30) | 0.687 |
| Hip | 1.83 (1.51, 2.23) | <0.001 | 1.52 (1.22, 1.89) | <0.001 | 1.05 (0.82, 1.35) | 0.709 |
| Knee and hip | 2.17 (1.69, 2.77) | <0.001 | 1.70 (1.29, 2.24) | <0.001 | 1.04 (0.76, 1.42) | 0.781 |
| Joint Pain | ||||||
| Knee | 2.28 (1.95, 2.68) | <0.001 | 1.78 (1.48, 2.13) | <0.001 | - | - |
| Hip | 2.11 (1.77, 2.52) | <0.001 | 1.85 (1.51, 2.25) | <0.001 | - | - |
| Knee and Hip | 2.86 (2.28, 3.58) | <0.001 | 2.38 (1.86, 3.06) | <0.001 | - | - |
Adjusted for country, age, sex, education, alcohol, BMI and number of chronic diseases
Adjusted for country, age, sex, education, alcohol, BMI, number of chronic diseases and knee pain and stiffness (for knee OA) or hip pain and stiffness (for hip OA) or both knee and hip pain and stiffness (for knee or hip OA)
Discussion
This study has shown that a diagnosis of either clinical or self-reported OA is associated with low physical performance; however, with self-reported OA this relationship is attenuated when covariates, including joint pain and stiffness, are included in the model. The combination of knee and hip OA is associated with a greater risk of low physical performance.
We found that rates of self-reported and clinical OA vary between European countries. As expected, there was significant overlap between those individuals with joint pain, self-reported OA, and clinical OA. There were consistently higher rates of self-reported than clinical OA. This may be due to the less stringent and robust nature of the definition. Consequently, if an individual was diagnosed with clinical OA they were highly likely to self-report OA although this does not hold true for the reverse. Overall rates of clinical OA in the knee (11.1-28.8%) and hip (0.8-13.8%) are of similar magnitude to those found in previous population studies(25-27). Interestingly, around a quarter of individuals that described self-reported knee or hip OA, did not have pain in the corresponding joint. This might suggest they are reporting due to other factors such as joint deformity, stiffness, creaking, or due to a previous medical diagnosis, for example on x-ray. Conversely, many individuals with pain did not attribute this to OA (410(37%) for knee and 378(47%) for hip). This is commensurate with a study of 788 individuals over 50 years of age with knee pain that showed that only 33% had radiographic evidence of OA and 30% fulfilled the ACR criteria(29).
All cohorts were non-selectively recruited from population samples, and have been shown to be representative of the populations from which they were drawn. Low physical performance was found to be most common in the UK cohort (68.9%). We might hypothesise that varying levels of physical performance by country might be related to different physical activity patterns. However, in a study of self-reported physical activity in European adults, of the countries that made up our cohorts the UK was second only to Sweden(30). Unlike the walk test and chair rise scores, the balance score was not calculated as country specific quartiles in those able to complete the task; this test may therefore contribute more markedly to inter-country differences. In keeping with this hypothesis, rates of poor balance were highest in the UK.
Low physical performance was found to be related to female sex, advancing age, greater BMI, abstinence from alcohol, lower educational attainment, number of chronic diseases, hip and knee stiffness, and country. In keeping with our results, men have been shown to have higher levels of physical performance than women(31;32) and negative associations of both age and BMI with assessments of physical performance have been well documented(31-33). Graded relationships have also been identified between higher education at 26 years and better physical performance in terms of sitting balance and chair rises(34). Interestingly the same study showed associations between social class and balance but not chair rises. Physical performance and alcohol consumption were assessed by the Osteoporotic Fracture in Men Research Group(35). They found higher gait speed in those with alcohol consumption up to high-moderate use; above this level, and in those with a history of problem drinking and/or sustained excessive drinking, the association was reversed. Clearly the use of a binary variable in our study prevents the differentiation of individuals by the level of their alcohol consumption. The finding of lower physical performance in those that abstain from alcohol likely reflects confounding by factors, such as co-morbidities, and may suggest that problem drinking and/or sustained excessive drinking were not having the overriding effect on our findings.
Pain, self-reported OA and clinical OA at the knee and/or hip increased the risk of low physical performance. This is commensurate with a study of community-dwelling Japanese women that showed relationships between radiographic knee OA and both walking speed and chair stands that remained after adjustment for age and BMI(8). However, an investigation of a US population with radiographic knee OA (Kellgren and Lawrence (K&L) >1) showed no difference in gait speed or balance in relation to knee osteophyte severity or K&L grade(6). They did find an association shown between joint space and gait speed before and after adjustment for age, BMI, comorbidities, gender, knee pain and ankle pain(6).
The maintenance of relationships between physical performance and each of joint pain, self-reported OA, and clinical OA after adjustment for demographic and lifestyle factors suggests that these are not the sole mediates of the associations found. This is an important finding, particularly as we have shown that several of these factors were individually associated with lower physical performance and thus might act as potential confounders. In contrast, the marked attenuation of the relationships between self-reported OA and physical performance after adjustment for pain and stiffness may suggest that an individual reporting OA is largely the result of symptom perception and to a lesser extent to other features of OA. Furthermore, pain and stiffness are likely to be the main mediators of the association between self-reported OA and physical performance.
Clinical OA is more robustly related to physical performance with associations maintained after adjustment for both covariates and pain and stiffness. This enduring effect may be due to reduced joint mobility and associated muscle weakness, both of which have been found in OA and to be related to lower physical performance (7;9). OA may also lead to a reduction in physical activity which in turn results in a further deterioration. It has been shown previously that physical activity levels are lower in those with OA and that physical performance and physical activity are modestly correlated(36). The likelihood of low physical performance was greater when clinical OA was present in both the knee and hip compared to when only one site was affected. This may reflect a cumulative detrimental effect on performance when OA is present at multiple sites.
There are limitations of this study. Specifically, participants were recruited from existing cohorts, where demographic differences existed in a number of domains, including educational level and occupational history. However, these differences between cohorts may allow hypothesis generation regarding the consequences of a clinical report of OA. Another limitation is that the involvement of multiple research centres meant methods of data collection might vary by study site. However, regular planning workshops included standardisation of data acquisition; all questionnaires and examination proformas were similar across centres, and all training for clinical examination was undertaken by one team to minimise such problems.
In conclusion, although both self-reported and clinical OA are associated with low physical performance, this relationship is only robust to adjustment for covariates, pain and stiffness in the latter. Consequently, a clinical diagnosis of OA in the hip or knee is an important determinant of physical performance. Those at highest risk have clinical OA at both sites.
Key Points.
Clinical osteoarthritis is associated with low physical performance.
Low physical performance is more likely in those with a combination of both hip and knee OA.
Study participants may report osteoarthritis in the absence of joint pain.
Acknowledgements
The Indicators for Monitoring COPD and Asthma - Activity and Function in the Elderly in Ulm study (IMCA - ActiFE) is supported by the European Union (No.: 2005121) and the Ministry of Science, Baden-Württemberg. The Italian cohort study is part of the National Research Council Project on Aging (PNR). The Longitudinal Aging Study Amsterdam (LASA) is financially supported by the Dutch Ministry of Health, Welfare and Sports. The Peñagrande study was partially supported by the National Fund for Health Research (Fondo de Investigaciones en Salud) of Spain (project numbers FIS PI 05/1898); FIS RETICEF RD06/0013/1013 and FIS PS09/02143). The Swedish Twin Registry is supported in part by the Swedish Ministry of Higher Education. The Hertfordshire Cohort Study was supported by the Medical Research Council of Great Britain; Arthritis Research UK; and the International Osteoporosis Foundation. The work herein was also supported by the NIHR Nutrition BRC, University of Southampton and the NIHR Musculoskeletal BRU, University of Oxford. Finally, we would like to thank all of the men and women who took part in the EPOSA study.
Reference List
- (1).Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987 Aug;30(8):914–8. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- (2).Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 15;380(9859):2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- (3).Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994 Mar;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- (4).Freire AN, Guerra RO, Alvarado B, Guralnik JM, Zunzunegui MV. Validity and reliability of the short physical performance battery in two diverse older adult populations in Quebec and Brazil. J Aging Health. 2012 Aug;24(5):863–78. doi: 10.1177/0898264312438551. [DOI] [PubMed] [Google Scholar]
- (5).Fusco O, Ferrini A, Santoro M, Lo Monaco MR, Gambassi G, Cesari M. Physical function and perceived quality of life in older persons. Aging Clin Exp Res. 2012 Feb;24(1):68–73. doi: 10.1007/BF03325356. [DOI] [PubMed] [Google Scholar]
- (6).McDaniel G, Renner JB, Sloane R, Kraus VB. Association of knee and ankle osteoarthritis with physical performance. Osteoarthritis Cartilage. 2011 Jun;19(6):634–8. doi: 10.1016/j.joca.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Pua YH, Wrigley TV, Collins M, Cowan SM, Bennell KL. Association of physical performance with muscle strength and hip range of motion in hip osteoarthritis. Arthritis Rheum. 2009 Apr 15;61(4):442–50. doi: 10.1002/art.24344. [DOI] [PubMed] [Google Scholar]
- (8).Osaki M, Tomita M, Abe Y, Ye Z, Honda S, Yoshida S, et al. Physical performance and knee osteoarthritis among community-dwelling women in Japan: the Hizen-Oshima Study, cross-sectional study. Rheumatol Int. 2012 Aug;32(8):2245–9. doi: 10.1007/s00296-011-1949-0. [DOI] [PubMed] [Google Scholar]
- (9).Maly MR, Calder KM, Macintyre NJ, Beattie KA. Relationship of intermuscular fat volume in the thigh with knee extensor strength and physical performance in women at risk of or with knee osteoarthritis. Arthritis Care Res. 2013 Jan;65(1):44–52. doi: 10.1002/acr.21868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).McAlindon TE. Regional musculoskeletal pain. The knee. Baillieres Best Pract Res Clin Rheumatol. 1999 Jun;13(2):329–44. doi: 10.1053/berh.1999.0023. [DOI] [PubMed] [Google Scholar]
- (12).van Leeuwen DM, Peeters GM, de Ruiter CJ, Lips P, Twisk JW, Deeg DJ, et al. Effects of self-reported osteoarthritis on physical performance: a longitudinal study with a 10-year follow-up. Aging Clin Exp Res. 2013 Aug 15; doi: 10.1007/s40520-013-0110-1. [DOI] [PubMed] [Google Scholar]
- (13).Prieto-Alhambra D, Nogues X, Javaid MK, Wyman A, Arden NK, Azagra R, et al. An increased rate of falling leads to a rise in fracture risk in postmenopausal women with self-reported osteoarthritis: a prospective multinational cohort study (GLOW) Ann Rheum Dis. 2013 Jun;72(6):911–7. doi: 10.1136/annrheumdis-2012-201451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Eaton CB, Hochberg MC, Assaf A, Cryer BL, Lu B, Sands G, et al. The cross-sectional relationship of hemoglobin levels and functional outcomes in women with self-reported osteoarthritis: results from the Women’s Health Initiative. Semin Arthritis Rheum. 2011 Dec;41(3):406–14. doi: 10.1016/j.semarthrit.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Kirk KM, Bellamy N, O’Gorman LE, Kuhnert PM, Klestov A, Muirden K, et al. The validity and heritability of self-report osteoarthritis in an Australian older twin sample. Twin Res. 2002 Apr;5(2):98–106. doi: 10.1375/1369052022965. [DOI] [PubMed] [Google Scholar]
- (16).Pua YH, Wrigley TV, Collins M, Cowan SM, Bennell KL. Self-report and physical performance measures of physical function in hip osteoarthritis: relationship to isometric quadriceps torque development. Arthritis Rheum. 2009 Feb 15;61(2):201–8. doi: 10.1002/art.24277. [DOI] [PubMed] [Google Scholar]
- (17).MacDermid JC, Wessel J, Humphrey R, Ross D, Roth JH. Validity of self-report measures of pain and disability for persons who have undergone arthroplasty for osteoarthritis of the carpometacarpal joint of the hand. Osteoarthritis Cartilage. 2007 May;15(5):524–30. doi: 10.1016/j.joca.2006.10.018. [DOI] [PubMed] [Google Scholar]
- (18).van der Pas S, Castell MV, Cooper C, Denkinger M, Dennison EM, Edwards MH, et al. European project on osteoarthritis: design of a six-cohort study on the personal and societal burden of osteoarthritis in an older European population. BMC Musculoskelet Disord. 2013 Apr 18;14(1):138. doi: 10.1186/1471-2474-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Roos EM, Klassbo M, Lohmander LS. WOMAC osteoarthritis index. Reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis. Western Ontario and MacMaster Universities. Scand J Rheumatol. 1999;28(4):210–5. doi: 10.1080/03009749950155562. [DOI] [PubMed] [Google Scholar]
- (20).Stel VS, Smit JH, Pluijm SM, Visser M, Deeg DJ, Lips P. Comparison of the LASA Physical Activity Questionnaire with a 7-day diary and pedometer. J Clin Epidemiol. 2004 Mar;57(3):252–8. doi: 10.1016/j.jclinepi.2003.07.008. [DOI] [PubMed] [Google Scholar]
- (21).Altman RD. Classification of disease: osteoarthritis. Semin Arthritis Rheum. 1991 Jun;20(6 Suppl 2):40–7. doi: 10.1016/0049-0172(91)90026-v. [DOI] [PubMed] [Google Scholar]
- (22).Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003 Feb;17(1):68–74. doi: 10.1016/s0966-6362(02)00053-x. [DOI] [PubMed] [Google Scholar]
- (23).Abbatecola AM, Cherubini A, Guralnik JM, Andres Lacueva C, Ruggiero C, Maggio M, et al. Plasma polyunsaturated fatty acids and age-related physical performance decline. Rejuvenation Res. 2009 Feb;12(1):25–32. doi: 10.1089/rej.2008.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).da Camara SM, Alvarado BE, Guralnik JM, Guerra RO, Maciel AC. Using the Short Physical Performance Battery to screen for frailty in young-old adults with distinct socioeconomic conditions. Geriatr Gerontol Int. 2012 Aug 6; doi: 10.1111/j.1447-0594.2012.00920.x. [DOI] [PubMed] [Google Scholar]
- (25).Haq SA, Davatchi F. Osteoarthritis of the knees in the COPCORD world. Int J Rheum Dis. 2011 May;14(2):122–9. doi: 10.1111/j.1756-185X.2011.01615.x. [DOI] [PubMed] [Google Scholar]
- (26).Dagenais S, Garbedian S, Wai EK. Systematic review of the prevalence of radiographic primary hip osteoarthritis. Clin Orthop Relat Res. 2009 Mar;467(3):623–37. doi: 10.1007/s11999-008-0625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Arden NK, Lane NE, Parimi N, Javaid KM, Lui LY, Hochberg MC, et al. Defining incident radiographic hip osteoarthritis for epidemiologic studies in women. Arthritis Rheum. 2009 Apr;60(4):1052–9. doi: 10.1002/art.24382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000 Apr;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- (29).Peat G, Thomas E, Duncan R, Wood L, Hay E, Croft P. Clinical classification criteria for knee osteoarthritis: performance in the general population and primary care. Ann Rheum Dis. 2006 Oct;65(10):1363–7. doi: 10.1136/ard.2006.051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).European Commission Sport and Physical Activity: Special Eurobarometer 334 / Wave 72.3 – TNS Opinion & Social. 2010.
- (31).Cabrero-Garcia J, Munoz-Mendoza CL, Cabanero-Martinez MJ, Gonzalez-Llopis L, Ramos-Pichardo JD, Reig-Ferrer A. Short Physical Performance Battery reference values for patients 70 years-old and over in primary health care. Aten Primaria. 2012 Sep;44(9):540–8. doi: 10.1016/j.aprim.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Sharkey JR, Branch LG. Gender difference in physical performance, body composition and dietary intake in homebound elders. J Women Aging. 2004;16(3-4):71–90. doi: 10.1300/J074v16n03_06. [DOI] [PubMed] [Google Scholar]
- (33).Bohannon RW, Brennan PJ, Pescatello LS, Marschke L, Hasson S, Murphy M. Adiposity of elderly women and its relationship with self-reported and observed physical performance. J Geriatr Phys Ther. 2005;28(1):10–3. doi: 10.1519/00139143-200504000-00002. [DOI] [PubMed] [Google Scholar]
- (34).Strand BH, Cooper R, Hardy R, Kuh D, Guralnik J. Lifelong socioeconomic position and physical performance in midlife: results from the British 1946 birth cohort. Eur J Epidemiol. 2011 Jun;26(6):475–83. doi: 10.1007/s10654-011-9562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Cawthon PM, Fink HA, Barrett-Connor E, Cauley JA, Dam TT, Lewis CE, et al. Alcohol use, physical performance, and functional limitations in older men. J Am Geriatr Soc. 2007 Feb;55(2):212–20. doi: 10.1111/j.1532-5415.2007.01062.x. [DOI] [PubMed] [Google Scholar]
- (36).Thomas SG, Pagura SM, Kennedy D. Physical activity and its relationship to physical performance in patients with end stage knee osteoarthritis. J Orthop Sports Phys Ther. 2003 Dec;33(12):745–54. doi: 10.2519/jospt.2003.33.12.745. [DOI] [PubMed] [Google Scholar]