Abstract
The use of various cell-penetrating peptides (CPPs) to deliver genetic material for gene therapy applications has been a topic of interest for more than 20 years. The delivery of genetic material by using CPPs can be divided into two categories: covalently bound and electrostatically bound. Complexity of the synthesis procedure can be a significant barrier to translation when using a strategy requiring covalent binding of CPPs. In contrast, electrostatically complexing CPPs with genetic material or with a viral vector is relatively simple and has been demonstrated to improve gene delivery in both in vitro and in vivo studies. This review highlights gene therapy applications of complexes formed noncovalently between CPPs and genetic material or viruses.
The past decade has witnessed a dramatic acceleration in the production of new therapeutic macromolecules, such as proteins, peptides, and nucleic acids, with the interest of overcoming limitations of small-molecule drugs such as specificity, poor potency and rapid elimination. Gene therapy, which involves the delivery of large macromolecules of nucleic acids, has emerged as a popular topic among researchers because of its potential to treat severe and challenging-to-cure diseases, such as inherited genetic diseases, viral infections and various cancers [1]. Genetic treatment of these diseases has garnered significant attention over the past 20 years [2] and is nearly a reality, as supported by the hundreds of clinical trials [3].
Gene therapy utilizes the delivery of genetic material into target cells to replace a gene that is missing, mutated or poorly expressed. Gene therapy also used to silence gene expression and production of proteins using siRNA [4]. Clinical applications, however, have been hindered by formulation issues, poor in vivo stability, problems with delivery to target cells, and inefficient cellular uptake [5]. One of the main hindrances for administering genetic material is the inability of DNA or RNA to reach cellular and intracellular target sites such as nuclei and mitochondria [6,7]. The cellular plasma membrane is an impermeable barrier for most hydrophilic macromolecules [8]. Transporting nucleic acids across the plasma membrane, however, is necessary since these therapeutic agents must be internalized for biological activity [9].
Notable effort is being made to develop viral and nonviral vectors that can cross the plasma membrane and deliver therapeutic agents into target cells [10]. Viral vectors (e.g., adenoviruses, adeno-associated viruses and retroviruses) can be highly efficient and are currently used in the majority of ongoing clinical trials. Viral vectors have the natural ability to attach and enter target cells while protecting genetic material and providing long-term gene expression. In some cases, however, dependency of the virus on certain cellular receptors compromises the efficiency of the viral vector. In addition, viral vectors introduce safety concerns such as the risk of oncogenesis and immunogenicity [11]. Consequently, non-viral vectors are being pursued as flexible, easy, and potentially safer alternatives [12,13], yet, they continue to lack the necessary efficiency required for clinical application [14].
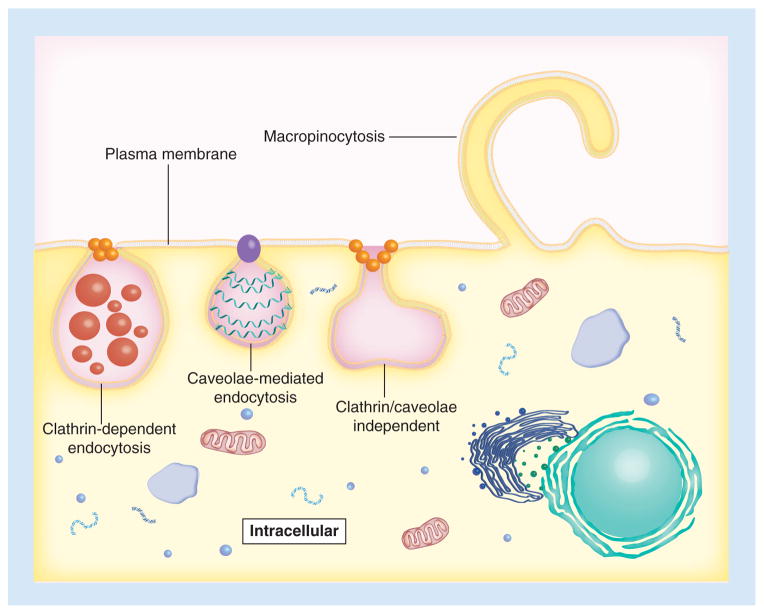
Since their discovery two decades ago, cell-penetrating peptides (CPPs) have been used for a diverse number of applications in numerous types of cells [1]. CPPs are generally defined as cationic or amphipathic peptides consisting of 30 or fewer amino acids with a structure that can mediate movement across a plasma membrane into the cell cytoplasm [15–17]. CPPs translocate into cells without cytotoxic effects, are efficiently internalized across cell membranes and aid in carrying cargo into live cells [18]. Although the internalization mechanisms are debatable, the application of CPPs is growing rapidly [19]. Some studies suggest that CPPs directly penetrate (e.g., carpet, inverted micelle, toroidal) through the lipid bilayer of the cell membrane, and others suggest that CPPs employ diverse endocytic pathways (e.g., macropinocytosis, clathrin-dependent, caveolae-mediated and clathrin-/caveolae-independent) (Figure 1) [16,20,21]. Additionally, some CPPs are capable of using both direct penetration and endocytosis for cellular internalization. One study indicated that the internalization mechanism of arginine-containing CPPs is dominated by direct membrane penetration, while the internalization mechanism of arginine-containing CPPs mediating delivery of DNA is dominated by endocytosis [22]. Another study found that direct membrane penetration occurs at low extracellular CPPs concentrations, while endocytosis is activated at higher peptide concentrations [23]. This review focuses mainly on current classifications, formulation approaches, and applications relating to strategies that use noncovalent association of CPPs and gene vectors (viral and nonviral) to improve delivery of genetic material.
Figure 1. Three proposed endocytic pathways for cell-penetrating peptide entry.
Cell-penetrating peptides may enter target cells through macropinocytosis, clathrin-dependent endocytosis, caveolae-mediated endocytosis, or clathrin-/caveolae-independent endocytosis.
Overview of current classifications
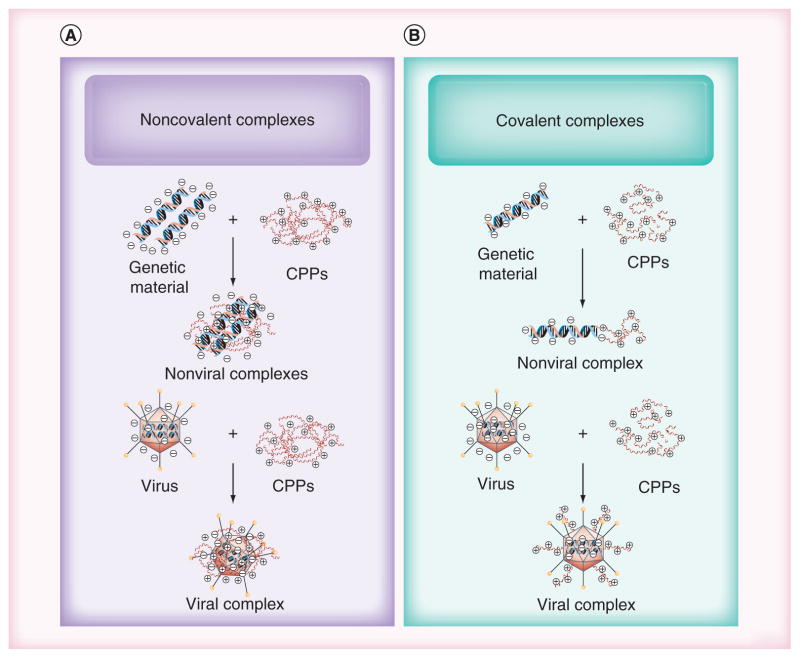
CPPs are used to enhance cellular internalization of different biomolecules or vectors (e.g., pDNA, siRNA, oligonucleotides, liposomes, peptides, proteins and viruses) [4]. The existence of diverse internalization mechanisms is of great importance when considering the use of CPPs as drug transporters. The exact mechanism, however, is not well understood. Some researchers indicated that uptake pathways of CPPs do not involve cellular receptors [24]. Other studies have reported that the electrostatic interaction of cationic CPP with negatively charged heparan sulfate proteoglycans that are found abundantly on the cell membrane triggers endocytotic internalization [25]. The exact mechanism of internalization is governed by cell type, cargo type and CPP properties, such as sequence, molecule length, and secondary structure [26]. The nature of the CPPs (e.g., size, surface charge or hydrophilicity) [27] and whether the CPPs are covalently or noncovalently attached (Figure 2) influence the internalization mechanism [28]. The origin of a CPP also provides some insight into the mechanism of internalization.
Figure 2. Approaches to forming viral and nonviral vector complexes.
Formation of (A) covalently and (B) noncovalently formed CPP–genetic material and CPP–virus complexes. CPPs: Cell-penetrating peptides.
Over the past two decades, more than 100 diverse peptide sequences (5–40 peptide residues), isolated from various sources, have been identified as being capable of mediating the transport of diverse biological molecules, such as pDNA, siRNA, and large molecules, such as viruses [29]. The TAT of HIV, which was discovered in 1988, was found to efficiently enter mammalian cells [30]. In 1991 Joliot et al. discovered that Drosophila Antennapedia homeodomain could translocate into neuronal cells [31]. A short while later in 1996, Derossi and colleagues demonstrated that the peptide Antennapedia, commonly called penetratin (RQIKIYFQNRRMKWKK), could be covalently bound to cargo and translocate into cells [32]. The minimum peptide sequence of TAT (YGRK-KRRQRRR) thought to be necessary for cellular uptake was identified by Vives et al. in 1997 [33].
CPPs can be classified in different ways, and one manner of classification is on the basis of their origin. This classification sort CPPs into three major classes:
Protein derived peptides (e.g., TAT and penetratin);
Chimeric peptides (e.g., transportan);
Synthetic peptides (e.g., polylysine [PLL]) [34].
CPPs can also be divided into two groups based on the internalization mechanism: energy-dependent (endocytosis) and energy-independent (direct translocation) transport through the cell membrane [35]. Another way of classifying CPPs is based on their physicochemical properties. Here, CPPs are divided into three classes:
Cationic CPPs, which are short peptide residue sequences that mostly consist of arginine, lysine, and/or histidine (e.g., TAT and PLL);
Amphipathic CPPs, which have a lipophilic (non-polar) domain and a hydrophilic (polar) domain (e.g., MPG);
Hydrophobic CPPs, which consist of hydrophobic residues, such as valine, leucine and tryptophan (e.g., Bip and FGF12) [36].
Formulation strategies
Two main strategies have explored the use of CPPs to deliver cargo (Figure 2). The first strategy uses chemical linkers to covalently attach CPPs to their cargo, and the second strategy relies on electrostatic, self-assembly to form noncovalent complexes between CPPs and their cargo. Various CPPs including peptides derived from TAT [37], penetratin, [38], transportan [39] and polyarginine peptides [40] have been covalently attached to viral and nonviral gene and [41–43] shown to increase transfection and biological effects [44].
Approaches for forming conjugates include mainly thioester or disulfide bonds [45]. Muratovska and Eccles investigated the transfection efficiency of different CPPs (e.g., penetratin) conjugated to siRNA via disulfide bonds and reported that these CPP–siRNAs efficiently reduced transient and stable expression of reporter transgenes in different cell types equivalent or better than cationic liposomes. Disulfide bonds were said to increase CPP affinity for genetic material (e.g., DNA or RNA) by the ‘chelate effect’, which increases the complex stability and reduces cyto-toxicity by rapid degradation in the cytoplasmic space [46,47]. Other studies have observed that cysteine residues added onto CPPs can have a significant impact on delivery. One study, for example, demonstrated that the introduction of a single cysteine residue on the C-terminal of various CPPs (e.g., TAT and transportan) resulted in formation of peptide dimers that greatly enhanced the transfection efficiency of DNA in HEK293T cells [48].
Cargos without thiol groups or disulfide bonds can be altered by chemical synthesis and/or recombinant techniques. One example is a study where two types of CPP–oligonucleotide conjugates were prepared in order to improve the transfection efficiency. Unexpectedly, the CPP–oligonucleotide conjugates were more effective in the presence of serum than when used with serum-free medium, which is in notable contrast to most other approaches to gene delivery [49]. The development of CPP-conjugated cargo necessitates careful assessment to determine if there is a therapeutic benefit gained by conjugation [24].
The second strategy of using CPPs foregoes the formation of chemical bonds and simply complexes CPPs or CPP-containing molecules with cargo via electrostatic interactions. MPG [50,51], Pep-1 [52] and TAT [53] were among the first CPPs used following this method for complex formation and gene delivery [33]. This strategy has recently been extended to other CPPs, such as polyarginine [54]. CPP complexation with genetic material originally devised to facilitate exploration of numerous peptides that may condense nucleic acids or favor endosomal escape [24].
Motivation for using noncovalently associated CPPs
Both viral and nonviral vectors suffer from drawbacks that hinder the advancement of gene therapy beyond the clinical trial stages, and there is much emphasis on design of improved vectors [55]. CPPs provide an additional strategy for improving existing vectors. Such improvements include enhanced transfection efficiency, increased cargo capacity, low cytotoxicity and reduced immunogenicity [2]. While there is the potential for significant enhancement of existing vectors, there are also some drawbacks associated with CPPs and the methods by which they are associated with the genetic cargo. For example, one shortcoming of CPPs is their general lack of specificity [56,57]. A second drawback is associated with the covalent attachment of CPPs. While covalently bound CPPs form well-defined chemical entities that are more likely to be approved for clinical use than less defined nanoparticles formed through electrostatics, the chemical linking of CPPs to the cargo has been reported to lead to loss of biological activity in some cases. For example, one study found that chemical conjugation of CPPs to methotrexate improved the intracellular concentration of the drug but reduced its potency by 20-fold compared with the unconjugated drug [58].
Covalent conjugation of cationic CPPs has been observed to enhance cellular uptake by engaging the cell surface [59], but many have asserted that the covalent bond between CPP and vector must be reversible inside the cellular environment for desired cytoplasmic and nuclear localization [60]. Since conjugation of cargo with CPPs may affect nuclear localization and lead to loss of biological activity, an attractive approach is to develop linker strategies that will enable genetic material to be cleaved from CPPs as soon as the vector reaches the cytoplasm [24]. The linker chemistry, however, becomes more difficult and complicated when dealing with CPPs and nucleic acids or viruses, since several functional components are represented. In addition, the CPP-conjugated vector should be easy to produce and cost-effective to manufacture in order to develop this strategy at an industrial scale for clinical applications [55]. Furthermore, as mentioned earlier, the risk of diminishing the biological activity of the cargo may limit the benefit of this strategy. Although chemical conjugation of CPPs appears promising, several concerns and questions are still under investigation [61].
Strategies that do not rely on chemical conjugation of CPPs also offer promise for developing gene vectors [1]. This method is advantageous because it usually employs simple mixing of the two components, thus eliminating the need to imbue reactive sites or optimize synthetic schemes. The dense positive charge of CPPs assists with electrostatic packaging of the negatively charge genetic material and often yields a positively charged particle [62–64]. This strategy is often favored over chemical reactions when exploring gene therapy in preclinical studies [1]. In addition, the use of cationic or amphipathic CPPs to form complexes with nucleic acids was found to improve the transfection efficiency and nuclear uptake of siRNA within non-dividing cells [63,64]. The following sections review some current applications of noncovalently associated CPPs for improving viral and nonviral gene delivery. Table 1 contains a list of classical CPPs that have been investigated for their ability to penetrate the cell membrane.
Table 1.
Current applications of noncovalent strategies of classical cell-penetrating peptides
| Function | Peptide | Sequence | Ref. |
|---|---|---|---|
| Nonviral | |||
|
| |||
| Genetic material condensation | Polylysine | K(n) | [72,95] |
| Lysine-rich CPPs | Krich | [95,138,139] | |
| Polyarginine | R(n) | [47,48,85,140] | |
| Arginine-rich CPPs | Rrich | [40,134] | |
| TAT | RKKRRQRRR | [61,89] | |
|
| |||
| Endosomolytic CPPs | Histidine-rich CPPs | Hrich | [93,141,142] |
| MPG | GALFLGFLGAAGSTMGAWSQPKKKRKV | [85] | |
| Penetratin | RQIKIWFQNRRMKWKK | [85] | |
|
| |||
| Fusogenic CPPs | Transportan | GWTLNSAGYLLGKINLKALAALAKKIL | [95] |
| Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ | [95] | |
| GALA | WEAALAEALAEALAEHLAEALAEALEALAA | [97] | |
| KALA | WEAKLAKALAKALAKHLAKALAKALKACEA | [36] | |
|
| |||
| Nuclear localization CPPs | SV40 T antigen | CGPGDDEAAADAQHAAPPKKKRKVGY | [99,100] |
| M9 | NQSSNFGPMKGGNFGGRSSGPYGGGGQYFAKPRNQGGY | [102] | |
| TAT | RKKRRQRRR | [143,144] | |
|
| |||
| Noncovalently associated CPPs for cellular targeting | RVG | YTIWMPENPRPGTPCDIFTNSRGKRASNG | [85] |
|
| |||
| Viral | |||
|
| |||
| Noncovalent CPP/virus | Penetratin | RQIKIWFQNRRMKWKK | [43,125,126] |
| TAT | RKKRRQRRR | [43,125,126] | |
| Polyarginine | R(n) | [137] | |
| HP4 | RRRRPRRRTTRRRR | [126] | |
| Hph-1 | YARVRRRGPRR | [126] | |
CPPs: Cell-penetrating peptides.
Current applications using the noncovalent strategy
CPP-enhanced nonviral gene delivery
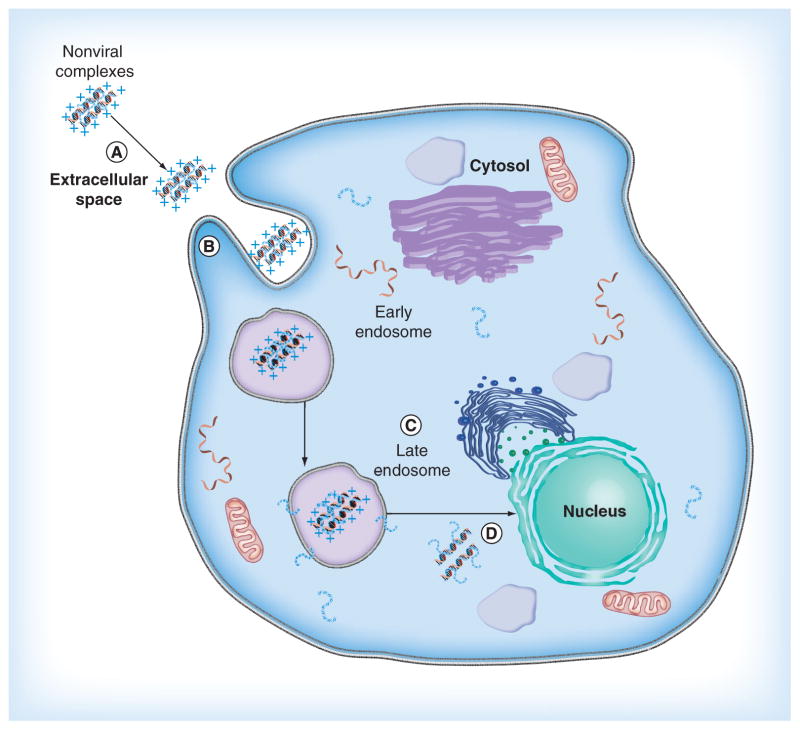
The inefficiency of nonviral vectors is due mainly to difficulty overcoming barriers between the administration site and the nucleus of the target cells. Examples of barriers include the physico-chemical stability of the genetic material and its delivery vehicle in the extracellular space; efficient cellular uptake, which may depend on the size of the complex; escape from the endosomal network before degradation within lysosomes; and genetic material unpackaging and entry into the nucleus (Figure 3). Overcoming these barriers is one of the greatest challenge for efficacious nonviral gene delivery [3]. Because of the ability of CPPs to transport cargo across the cell membrane, these peptides are an attractive option for helping nonviral particle overcome some of the barriers to gene delivery. A common approach for complexing CPPs to the nonviral vector is simple electrostatic formation of a complex between cationic CPPs and anionic nucleic acids. One example of how useful CPPs can be was the incorporation of CPPs in a PLL–siRNA polyplex [44]. The investigators in this study used a PLL, modified to bind to CPPs, to first form an electrostatic complex with siRNA. The resulting polyplex was subsequently bound by CPPs to produce a CPP–PLL–siRNA polyplex. The investigators reported high transfection efficiency, but also emphasized the importance of the siRNA being released from the polyplex in order to function. While activity is not adversely affected as it may be when CPPs are chemically conjugated directly to the genetic material [65], unpackaging remains a concern with vectors formed through noncovalently bound CPPs. Additional studies have established various applications of electrostatic complexes by using diverse CPPs and conditions. The following section discusses CPPs that are able to perform several functions to carry out various gene delivery applications.
Figure 3. Barriers to nonviral gene delivery.
(A) The physiochemical stability of nonviral complexes in the extracellular space. (B) Cellular uptake. (C) Escape from the endosome. (D) Genetic material unpackaging and entry into the nucleus.
Genetic material condensation with CPPs
Cationic CPPs interact with the negatively charged phosphate backbone of genetic material (e.g., pDNA and siRNA) through electrostatic interaction [62,66]. This process can result in genetic material condensation and protection from nuclease enzyme digestion [67]. If carried out properly, this approach leads to small nano-particles with a net positive charge that are capable of interaction with moieties such as heparan sulfate proteoglycans on cell surfaces [68,69].
PLL, a well-known nonviral DNA condensing agent used to mediate gene delivery, was one of first cationic peptides studied. There is some toxicity associated with PLL, however, and as with many polycations, the cytotoxicity increases as the molecular weight of the PLL increases. One group investigating the structure property relationships of PLL describe the in vitro cytotoxicity of PLL-based cations and demonstrated that both molecular weight and architecture were important factors [70]. Another group studied a library of 13 PLL–graft–imidazoleacetic acid conjugates to examine the collective effects of polymer molecular weight, DNA:polymer ratio, and side chain change on DNA/polycation interaction, transfection efficiency, and cytotoxicity. They found that the in vitro cytotoxicity of the polymers increased, while total protein expression decreased, with increasing molecular weight [71].
While higher-molecular-weight PLL possesses greater toxicity, it is able to bind DNA tighter and form more stable complexes than low-molecular-weight PLL due to the greater abundance and density of positive charge [72]. Numerous scientists have turned to the development of homogenous PLL-containing peptides due to the lack of chemical control and poly-dispersity of PLL [73,74]. In order to determine the optimal lysine chain length for DNA condensation and transfection efficiency, a group of researchers systematically studied different from lengths of the cationic peptide Cys-Trp-Lysn three to 36 lysine residues. Shorter peptides of eight or fewer lysine residues formed large particles due to weakly bound DNA, while peptides containing 13 or more lysine residues were able to strongly bind DNA and form small particles (from 50 to 200 nm) [75]. Another study reported that peptides containing 18 lysine residues could condense and protect DNA from degradation, while a short peptide containing less than eight residues could not prevent DNA degradation [76].
The chemical nature of cationic residues has been found to influence cellular uptake [77–79]. Arginine tends to mediate cell uptake better than ornithine or lysine and at least six–eight cationic residues were reported to be the minimum number required for DNA condensation [79]. Studies demonstrated that when lysine residues were replaced with arginine residues in a penetratin variant, the modified penetratin had better cellular uptake that penetratin itself [80,81]. Arginine-rich CPPs have also been reported to be more efficient than lysine-rich CPPs [81,82]. In fact, polyarginine peptides have been demonstrated to play an important role in cytosol-to-nucleus transport and nuclear localization of plasmid DNA (Figure 4) [83,84]. The polyarginine peptides used by Kim et al. demonstrated significant co-localization of CPP and DNA near the nucleus, with some material located within the nucleus, but the majority of the complexes appears to have remained outside the nucleus in a perinuclear compartment. Location of the complexes near the nucleus will undoubtedly improve gene delivery to the nucleus, but the limited nuclear entry indicates the additional need for some type of active transport into the nucleus, such as an addition of a nuclear localization signal (NLS).
Figure 4. Arginine peptide-improved transportation of DNA around and into the nucleus in live cells.
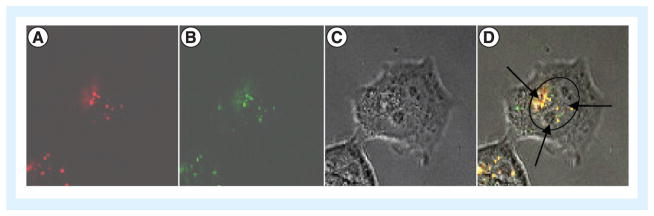
The cells were treated with FITC-labeled arginine peptide/rhodamine-labeled DNA complexes and imaged using confocal microscopy. (A) Rhodamine-labeled DNA (red); (B) FITC-labeled arginine peptide (green); (C) differential interference contrast; and (D) a merge of A, B, and C. Panel D illustrates the nucleus circled and the presence of both peptide-bound DNA and free DNA.
Reproduced from [83], © (2013), with permission from Elsevier.
Polyarginine peptides have a strong tendency to electrostatically interact with both siRNA [85,86] and with DNA [66]. One study indicated that as with PLL and other polycations, the length of polyarginine may affect transfection efficiency and cytotoxicity. A minimum chain length of six–ten amino acids is needed for delivery of genetic material, which is enough to condense DNA into stable complexes. With polyarginine 9, only four–five arginine residues were thought to be involved in forming the complexes with genetic material, while the extra arginine residues were available for interaction with the cell membrane. Furthermore, they have considered the possibility that genetic material may not be released from the complexes when the cationic CPPs bind too strongly with the negatively charged genetic material [47].
The size of polyarginines has been explored by several groups. One group studied the transfection efficiency of different polyarginine peptides (arginine 9, 12 and 16), which was found to increase when the molecular weight increased [66]. Another group examined the transfection efficiency of different polyarginine molecular weights complexed with pDNA. They found that the high-molecular-weight polyarginines (41 and 83 kDa) demonstrated 100-times higher transfection efficiency (RLU/ug protein) than the lower-molecular-weight polyarginine approximately (10 kDa) [87].
In addition to molecular weight and residue charge, peptide hydrophobicity has also been studied. One study examined the effects of introducing a hydrophobic group onto CPPs such as TAT and polyarginine. They found that N-terminal stearylation of these CPPs increased the transfection efficiency by approximately 100-fold to reach the same order of magnitude as that of Lipofectamine 2000™ (Invitrogen, Paisley, UK). They suggested that the hydrophobic moieties contributed to absorption of the complexes on the surface of cell membrane thereby destabilizing the membrane. This explanation provides a possible explanation for the high transfection efficiency of theses complexes [88]. Another study tested the hypothesis that a hydrophobically modified CPPs, cholesteryl polyarginine 9, may stabilize and improve tumor regression efficacy of the VEGF targeting siRNA. They indicated that this nonconjugate complexation of the siRNA with cholesteryl polyarginine 9 efficiently transfected siRNA into cells in vitro. Polyarginine peptide offers efficient siRNA packaging and cell membrane interaction. The cholesterol moiety was able to engage the hydrophobic residues of the extracellular cell surface, which enhanced the transfection efficiency of the complexes [86].
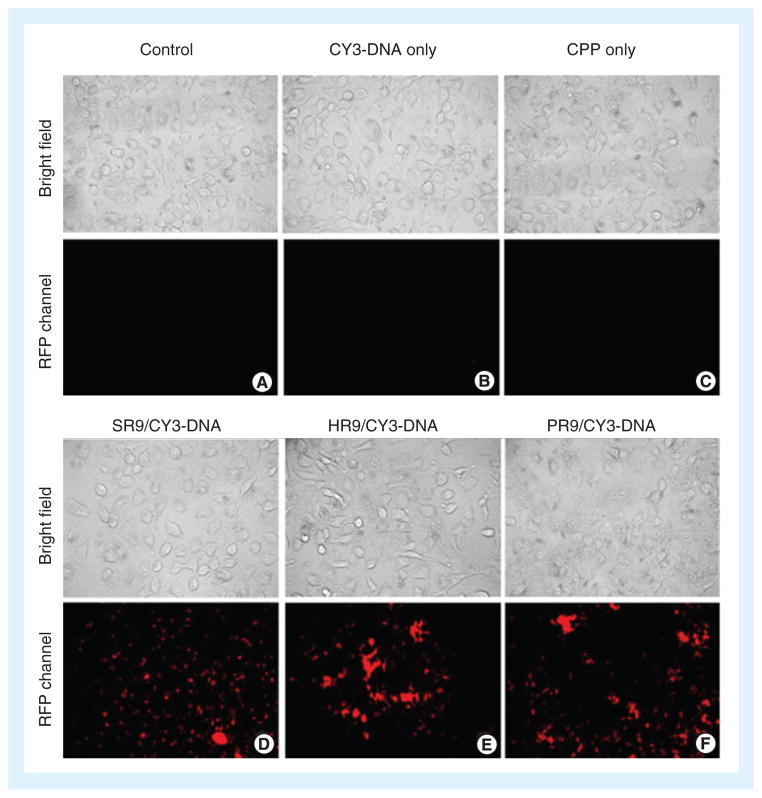
Furthermore, calcium chloride was used as a condensing agent for large CPP complexes with DNA or RNA. The transfection of different CPPs (e.g., arginine 7 and TAT) complexed with siRNA or pDNA was practically nil; however, the addition of calcium chloride decreased the large size of these complexes through ‘soft’ cross-links. Adding calcium chloride to the CPP complexes led to an increase in transfection efficiency [61,89]. In another report, arginine-rich CPPs (PR9, SR9 and HR9) were used to deliver genetic material into target cells. The researchers proposed that calcium condensed CPPs/DNA complexes into small particles and that including calcium chloride caused a significant increase in cellular internalization and gene expression (Figure 5) [46].
Figure 5. Cell-penetrating peptide-mediated delivery of Cy3-labeled DNA into cells.
Cells were treated with: (A) phosphate-buffered saline as a negative control; (B) Cy3-labeled DNA only; (C) peptide HR9 only; (D) SR9/Cy3-labeled DNA; (E) HR9/Cy3-labeled DNA; or (F) PR9/Cy3-labeled DNA. The fluorescent microscopy indicates the location of Cy3-labeled DNA inside cells. CPP: Cell-penetrating peptide.
Reproduced from [46], © (2013), with permission from Elsevier.
Endosomolytic & fusogenic CPPs
One of major limitations in the delivery of genetic material using CPPs complexes is the entrapment of the complexes within endosomal vesicles. In order to avoid this problem and enhance the transfection efficiency, an endosomolytic agent, such as chloroquine, may be used, but, unfortunately, this is not feasible for in vivo gene delivery [61]. Endosomolytic CPPs can be used to overcome this obstacle as they are able to enhance gene delivery by inducing release from endosomes. Several peptides with high charge density were surmised to act as a ‘proton sponge’ and become endosomolytic at the acidic environment of the endosome. These peptides can either fuse with the endosomal membrane leading to pore formation or act as a ‘proton sponge’ causing lysis [67,90–92]. In order to apply the lysis activity to CPPs, histidine residues were added to CPP sequences. The imidazole group approximately 6.0) can remain of histidine (pKa neutrally charged at physiological pH. Then, it can become protonated in the acidic environment of the endosome, thus, imparting selective membrane disruption [67]. Histidine residues have been inserted into PLL in order to enhance the transfection efficiency of noncovalent complexes [93]. A published study offered a comparative analysis of the ability of various CPPs (e.g., MPG and penetratin) to complex siRNA molecules and induces efficient cellular internalization [94]. Other investigators studied the gene expression of three arginine-rich CPPs (SR9, PR9 and HR9). Researchers indicated that HR9 was superior, possibly because the histidine facilitated DNA endosomal escape [46]. Another group indicated that efficient endosomolytic peptides, such as EB1, can be applied to enhance the ability of CPP/siRNA complexes to effectively deliver siRNA across the endosomal membrane [94].
Fusogenic CPPs are short peptides with the potential to promote membrane destabilization, endosomal escape and delivery of genetic material to the cytosol and/or nucleus. Numerous fusogenic CPPs are derived from different proteins that interact with cell membranes such as TAT, transportan, and melittin. Fusogenic CPPs have hydrophilic and hydrophobic domains resulting in helical structures of the CPPs at endosomal pH, which can interact with the endosomal membrane to cause pore formation [95]. A group of researchers used short peptides derived from the influenza virus hemagglutinin HA-2 and found that DNA polyplexes containing the influenza peptide as a fusogenic peptide conjugated to PLL could mediate significant gene expression when compared with complexes without the fusogenic peptide [92]. Melittin has been developed into gene delivery CPPs that can condense DNA [96]. GALA is the synthetic fusogenic CPP that has a repeating unit of glutamic acid-alanine-leucine-alanine that gives melittin an amphipathic character. GALA can bind to bilayer membranes and induce leakage. Although GALA has fusogenic activity, it cannot bind and condense DNA for effective transfection due to the negative charge of this peptide. In order to increase the DNA condensation and transfection efficiency of GALA, some alanine residues have been substituted with lysine residues and glutamic acid was reduced, yielding the cationic KALA peptide. DNA complexes prepared with KALA were able to effectively transfect different cell lines [97].
Nuclear localization CPPs
The passage of pDNA from the cytoplasm into the nucleus is one of the main barriers to non-viral gene delivery. The nuclear membrane of human cells is permeable to particles of up to approximately 9 nm. Transfer of large molecules such as pDNA through nuclear pore complexes is signal-mediated and energy dependent [98]. NLS peptides mediate the transport of pDNA or RNA from the cytoplasm into the nucleus through nuclear pore complexes [99]. The highly positively charged NLS peptides can efficiently interact with the negatively charged pDNA. There is a possibility that the NLS peptides dissociate from the pDNA before reaching the nucleus. In order to enhance the nuclear targeting of complexes, another condensation agent or CPPs may be added [95]. NLS of SV40 large T-antigen is the most common nuclear localization peptide that has been used [100] for efficiently transferring pDNA from cytoplasm to nucleus by forming noncovalent complexes with pDNA [101]. Another NLS peptide noncovalently complexed with pDNA is the M9 peptide, which was also complexed with SV40 large T-antigen to enhance ionic interaction with pDNA and increase gene expression [102]. TAT, another NLS peptide, has been demonstrated to mediate the import of diverse cargos into the cell nucleus, including dye-labeled streptavidin protein and quantum dots for kinetic measurement. One study reported that TAT peptide could import 90 nm beads into the cell nucleus, suggesting that its interaction with the nuclear envelope follows a mechanism different from that of other NLS [103].
Noncovalently associated CPPs for targeted delivery
Targeted gene delivery seeks to differentiate between healthy cells and diseased cells. The specificity for a target site is one of the main challenges that belie the efficiency of non-covalent gene complexes [104]. The first example of this approach was done by a group of scientists who fused a short peptide derived from RVG to RVG-9R, and then complexed RVG-9R with siRNA to specifically target neuronal cells [54]. Additionally, gene vectors that bind integrin with the help of peptide ligands have been used in the improvement of targeted vectors. Integrins are cell receptors found on cells, which have many roles such as cell migration, signal transduction, and cell–cell interactions. Integrin-binding peptides commonly have short sequences of amino acids and can bind a widespread variety of the receptors or be specific for a single peptide [105]. A group used the lectin-like oxidized LDL receptor as a target receptor. They sequenced and identified 60 innovative peptides, which will be beneficial for the selective target of gene transfer vectors to endothelial cells expressing the lectinlike oxidized LDL receptor and in particular dysfunctional endothelial cells associated with atherosclerosis and hypertension [106]. Lastly, a study observed differential transfection of cells overexpressing ICAM-1 when treated with a TAT–PEG–LABL peptide complexed with pDNA and condensed with calcium chloride. The results demonstrated the possibility of targeting gene transfection to inflammation sites [107].
CPP-enhanced viral vectors
Viral vectors are the most widely used gene vectors. In fact, 70% of the gene therapy in clinical trials have used or are using viral vectors as the means of delivering the therapeutic gene [108]. Naturally occuring viruses introduce their own genes into cells for replication and often result in disease. Viral vectors used in gene therapy, however, have been modified to be replication defective and carry therapeutic genetic material instead of their own genes, all while utilizing the normal efficiency of the virus. Viruses, such as adenovirus, retro-virus, herpes simplex virus (HSV) and adeno- associated virus, have demonstrated promise as gene vectors [109,110].
The most serious drawbacks associated with viral vectors, such as pathogenesis and viral replication, have been largely addressed by genetic alterations [111]. Also, most studies have transitioned away from retrovirus and other viral vectors that integrate their genetic cargo in the cell genome to avoid issues with oncogenesis. Viral immunogenicity and tropism, however, remain major issues that hinder the use of viral vectors [112,113]. Since viral vectors depend on their native receptor, existing vectors are unable to transduce a number of important therapeutic targets [114]. The use of noncovalently associated CPPs, however, is improving existing viral vectors by improving uptake and infection of cell types that have low levels of the virus receptor or lack the receptor entirely [115–118].
Benefits of combining CPPs with viral vectors
Surface properties vary for different types of viruses. Retroviruses, such as HIV and murine leukemia virus (MLV), have their protein capsid enclosed in a lipid envelope while adenovirus and adeno-associated virus are non-enveloped viruses with only a protein capsid and envelope protein on their surface. In both cases, the outer layer of the virus has a negative surface charge at physiological pH [119] and is responsible for initiating infection. Proteins responsible for the binding of the virus with the cell membrane are located on the virus capsid or embedded in the lipid envelope of the virus. For example, HSV has a lipid envelope with multiple viral envelope glycoproteins on the surface that mediate a strong tropism for neurons as well as other types of tissue [120]. Similarly, the fiber and knob proteins on adeno-virus contain a domain that binds to a plasma membrane protein called the coxsackie B virus and adenovirus receptor (CAR) [116,121]. Cellular uptake of viruses is determined by the interaction of the viral coat proteins and their specific cellular receptor on the plasma membrane [121]. The efficiency of this initial receptor-dependent step correlates well with the efficiency of viral infection.
Receptor-dependent binding of virus to the plasma membrane restricts the performance of the virus and compromises gene transfer efficiency. For example, adenovirus is dependent on the CAR [116], and the absence of this receptor on diseased cells compromises the efficiency of the virus as a gene delivery vector to this specific cell type. Similarly, retroviral infection is mediated by virus surface glycoprotein and cellular receptors, such as CD4 [115]. Thus, increasing the efficiency of this initial binding and internalization step by using an alternative pathway can substantially enhance gene transfer.
Past studies have attempted to improve tropism issues associated with viral vectors by combining parts of viruses. These efforts involved the generation of chimeric viruses by pseudotyping, where the envelope protein or capsid protein from one virus or serotype was used to augment the native tropism or impart improved properties. For example, vesicular stomatitis virus G proteins were used to pseudotype MLV, altering the MLV tropism and improving its stability [122]. Adenovirus serotype 5 pseudotyped using fiber isolated from a different adenovirus serotype, such as adenovirus serotype 19 and 37, improved targeted delivery of the virus to vascular endothelial and smooth muscle cells [123]. In a similar fashion, CPPs have been used to enhance viral vectors by enabling the virus to infect cells with low levels of the virus receptor or lacking the receptor entirely. For example, the CPPs TAT, MPG and VP22 have been isolated from viruses (HIV, simian virus 40 and HSV-1, respectively) and used with adenovirus and retrovirus to infect cells the virus would not infect normally on its own [43,124–129].
Formation of non-covalent CPP/virus vectors
CPPs have been a promising strategy for overcoming the receptor-dependency of viral vectors and have been used with viruses in three ways: genetic fusion, chemical conjugation, and non-covalent complexing. Genetic fusion involves genetically modifying the virus to insert DNA encoding the CPP into a gene for the relevant viral protein. An example of this approach was modification of adenovirus by inserting a TAT peptide in the HI loop or the C-terminus of the fiber protein [130]. Chemical conjugation to covalently link a CPP to the virus uses specific linker molecules to form the association between CPPs and the virus. For example, TAT, polyarginine and polyproline have been covalently linked to adenovirus using a 6-maleimidohexanoic acid N-hydroxysuccinimide ester that reacts with lysine residues on the surface of adenovirus [124,131]. Genetic fusion limits complex formation flexibility whereas chemical conjugation employs chemical linkers that may have undesired side effects such as toxicity of the linker itself or failure to dissociate from the virus once inside the cell. A simpler approach and the focus of this paper is non-covalent CPP/virus complex formation, which occurs when cationic CPPs are incubated with the negatively charged virus to form an electrostatic CPP/virus complex (Figure 2). A non-covalent complex between adenovirus and polycationic CPPs has been demonstrated to improve adenoviral and retroviral transduction. These approaches have included the use of monomeric CPPs, branched oligomeric CPPs and CPPs conjugated to polymers.
Monomeric & branched oligomeric CPPs
Incubation of viral vectors with cationic CPPs such as penetratin, TAT and polyarginine results in noncovalent electrostatic binding between positively charged CPPs and negatively charged virus and has produced a remarkable improvement in viral transduction of cells lacking the native virus receptor. Gratton and colleagues demonstrated that penetratin and TAT improved transduction of viral vectors by more than tenfold compared with native adenovirus alone [43]. Further, the study observed that adenovirus pre-incubated with these CPPs improved gene expression on a wide range of cells in vitro, such as human umbilical vascular endothelial, monkey COS 7 cells and ovine aortic endothelial cells. In addition, the study demonstrated an improvement in transduction of mouse muscle, skin and arteries in vivo. Angiogenesis resulting from adenoviral delivery of a gene encoding VEGF was evaluated in mice dosed with penetratin/adenovirus or adenovirus alone. The desired outcome of vascular leakage and formation of new blood vessels was improved when Ad-VEGF was complexed with penetratin (Figure 6). The results of a similar study by Lehmusvaara et al. reported that using the same method of pre-incubation of penetratin and TAT with adenovirus or lentivirus improved in vitro transduction of human tumor cells such as ovarian carcinoma, prostate carcinoma and osteosarcoma [125]. Although the levels of gene expression observed by Lehmusvaara and colleagues were less than those observed by Gratton, the study demonstrated that the CPPs were able to enhance transduction efficiency on a wide range of cancer cells. The variation in the transduction efficiency results can probably be attributed to the quality of the adenovirus, peptides, cells, and minor differences in the complex formation and/or transduction protocols.
Figure 6. Penetratin-improved in vivo gene delivery.
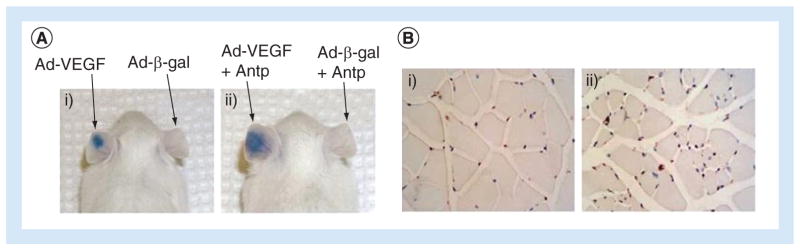
(A) Ad-VEGF (left ear) and Ad-β-gal (right ear) were injected intradermally into the ears of a mouse in the absence (i) and presence (ii) of penetratin for 4 days and vascular leakage was observed. The results show that delivery of the VEGF gene and resulting angiogenesis are greater in mice dosed with CPP/adenovirus than in mice dosed with adenovirus alone. (B) Ischematic lower limbs of mice injected with Ad-VEGF (i) or Ad-VEGF with penetratin (ii) showed intramuscular injection of Ad-VEGF in the presence of penetratin increased angiogenesis.
Adapted with permission from [43].
Similar to penetratin and TAT, other arginine-rich CPPs or amphipathic CPPs can augment transduction of viruses. Studies have illustrated that arginine-rich CPPs derived from herring protamine (HP) enhanced adenovirus transduction efficiency of mesenchymal stem cells (MSCs), dendritic cells and cancer cells [126]. The study evaluated the efficiency of TAT and various HP-derived peptides and found that one peptide in particular, HP4, works up to tenfold better than TAT or the other HP-derived peptides. In addition to TAT and penetratin, our group examined polyarginine and pep-1 for their ability to improve adenoviral transduction of CAR-negative cells [132]. Similar to penetratin/adenovirus and TAT/adenovirus, the resulting polyarginine/adenovirus complexes showed 100-fold higher transduction compared with native adenovirus while pep-1/adenovirus complexes showed 66-fold higher transduction. The difference in transduction efficiency between CPP/adenovirus was due to the properties of the CPPs such as the number and arrangement of amino acid residues and whether they were cationic or hydrophobic [32,133,134].
Branched oligomeric CPPs, such as tetrameric decaarginine and tetrameric TAT peptides, have been reported to improve internalization and intracellular delivery of plasmid DNA [135]. Recently, similar branched oligomeric CPPs were shown to efficiently enhance adenoviral transduction more that monomeric CPPs [129]. Park et al. synthesized branched oligomers of 2, 4 and 8 moieties with TAT, HP4, penetratin and Hph-1 peptide by conjugating the C-termini of the peptides to lysine linkers. The tetrameric oligomers, when combined with adenovirus, performed the best, improving ex vivo transduction of human bone marrow-derived MSCs and umbilical cord blood-derived MSCs, both of which do not express the native adenovirus CAR receptor. The resulting improved transduction of MSCs was attributed to more efficient binding to both adenovirus and the cell membrane compared with monomeric CPPs alone.
CPP–polymer grafts
Another approach to noncovalently associating CPPs with viruses has been the use of cationic polymers. One example includes arginine peptides grafted onto a poly(disulfide amine) polymer backbone to produce an arginine-grafted bioreducible polymer (ABP). This polymer was initially used with nonviral vectors, and several reports showed grafting of arginine peptides greatly improved the transfection efficiency of polymeric vectors with minimal toxicity [136]. Recently, it has been demonstrated that electrostatically coating adenovirus with ABP significantly increased transduction efficiency and reduced immunogenicity of the viral vector [137]. The transduction efficiency of adenovirus coated with ABP was significantly greater than adenovirus alone and adenovirus coated with PEI for delivering genes to CAR-deficient C2C12 murine cells, MCF7 breast carcinoma cells and high CAR-expressing A549 lung carcinoma cells. In addition, coating of the virus with ABP showed a reduction in the innate immune response associated with the native adenovirus.
Future perspective
Gene therapy has incredible potential to revolutionize the way we treat human diseases and other medical conditions. Improved gene delivery, however, remains a major obstacle that must be overcome before gene therapy can advance beyond the clinical setting and into the doctor’s office. Major strides are being made currently in terms of developing improved vectors to transport genes from the laboratory bench top to the diseased tissue within a patient and ultimately to the cell nucleus. CPPs are being used with viral vectors to improve transduction efficiencies and extend target cells beyond the native tropism of the viral vector. Further, CPPs are opening possibilities for addressing other drawbacks that are currently associated with viral vectors.
While improving viral gene delivery vectors will probably have the most immediate impact, the long-term goal for many of us in the field is to develop completely synthetic vectors that perfectly mimic the efficiency of a virus with none of the drawbacks. As this article has described, CPPs are having immediate impacts on moving us one step closer to this goal. Nonviral vectors complexed with CPPs are being delivered directly to the cell cytosol across the cell membrane, escaping endosomal vesicles with greater efficiencies, and being transported to the cell nucleus in ways that are more traditionally only associated with viruses.
CPPs have been studied for more than 20 years, but their impact on the field of gene delivery has been limited predominantly to the last several years. While they have improved significantly existing vectors, further design and development of simpler and more effective CPPs will undoubtedly continue to greatly benefit the development of both viral and nonviral gene delivery vectors as the field continues to advance.
Executive summary.
Background
Difficulty delivering genetic material to cellular and intracellular target sites is one of the main hindrances to clinical applications of gene therapy.
Cell-penetrating peptides (CPPs) are internalized by cells in a highly efficient manner and have been used to transport nucleic acids and viral vectors into cells.
Overview of current classification
CPPs are classified based on their internalization mechanism, origin and physicochemical properties.
Formulation strategies
The two main methods for attaching CPPs to nucleic acids or viruses are covalent linkages or noncovalent, electrostatic association.
Motivation for using noncovalently associated CPPs
The noncovalent approach to complexing CPPs to nucleic acids or viruses has been shown to improve gene delivery in both in vitro and in vivo studies.
The noncovalent strategy is often favored over covalent strategies in gene therapy preclinical studies.
Current application using the noncovalent strategy
CPPs are an attractive option for improving the efficiency with which viral and nonviral vectors overcome the barriers to gene delivery.
CPPs condense and protect nucleic acids.
Endosomolytic and fusogenic CPPs enhance gene delivery by facilitating release from endosomes.
Nuclear localization signal peptides improve the transport of nucleic acids through the cytoplasm and into the nucleus.
CPPs enhance viral vectors by enabling the virus to transform cells independent of the native virus receptor, thereby infecting target cells with low levels of the receptor or cells lacking the receptor entirely.
Monomeric CPPs, branched oligomeric CPPs and CPPs grafted onto bioreducible polymers have been shown to greatly improve the gene delivery efficiency of viral vectors.
Future perspective
Further development of more effective CPPs will certainly continue to greatly benefit the development of both viral and nonviral gene delivery vectors as the field continues to advance.
Acknowledgments
The authors would like to acknowledge support for this work from the Coulter Foundation and the Higuchi Biosciences Center. They also acknowledge Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia, for their support.
Key Terms
- Gene therapy
Treats diseases through targeted delivery of a therapeutic gene into a patient’s own cells. To be successful, the therapy must navigate and overcome numerous barriers including extracellular barriers, cellular association, internalization, endolysosomal escape, intracellular trafficking, unpackaging and nuclear import
- Cell-penetrating peptides
Typically have less than 30 amino acid residues, and as their name implies, they have the ability to cross the cell membrane and carry cargo into cells
- Gene vectors
Protect nucleic acids as they are transported ultimately to the cell cytosol or cell nucleus. Vectors are typically classified as either viral or nonviral. Approximately 70% of the vectors used in gene therapy clinical trials are viral vectors. The remaining 30% are nonviral vectors, including naked plasmid DNA, lipofection and various other methods
- Endosomolytic cell-penetrating peptides
Actively promote endosome escape and help avoid entrapment of vectors within the endolysosomal network, by forming pores or lysing endosomal vesicles and releasing the contents into the cell cytosol
- Fusogenic cell-penetrating peptides
Destabilize lipid bilayers by directly interacting with the cell membrane or vesicle membrane to form pores that allow vectors to either enter a cell or escape the endolysosomal network.
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure
The authors would like to acknowledge additional laboratory funding from the NIH (R03 AR054035, P20 RR016443 and T32 GM08359-11) and the Department of Defense. In addition, the authors acknowledge the support of the NSF (CHE 0719464) and the support of the NSF (CHE 0968972 and 0966614). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009;157(2):195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoyer J, Neundorf I. Peptide vectors for the nonviral delivery of nucleic acids. Acc Chem Res. 2012;45(7):1048–1056. doi: 10.1021/ar2002304. [DOI] [PubMed] [Google Scholar]
- 3.Wiethoff CM, Middaugh CR. Barriers to nonviral gene delivery. J Pharm Sci. 2003;92(2):203–217. doi: 10.1002/jps.10286. [DOI] [PubMed] [Google Scholar]
- 4.Munyendo WLL, Lv H, Benza-Ingoula H, Baraza LD, Zhou J. Cell penetrating peptides in the delivery of biopharmaceuticals. Biomolecules. 2012;2(2):187–202. doi: 10.3390/biom2020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilina P, Hyvonen Z, Saura M, Sandvig K, Yliperttula M, Ruponen M. Genetic blockage of endocytic pathways reveals differences in the intracellular processing of non-viral gene delivery systems. J Control Release. 2012;163(3):385–395. doi: 10.1016/j.jconrel.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Medina-Kauwe L, Xie J, Hamm-Alvarez S. Intracellular trafficking of nonviral vectors. Gene Ther. 2005;12(24):1734–1751. doi: 10.1038/sj.gt.3302592. [DOI] [PubMed] [Google Scholar]
- 7.Torchilin VP. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu Rev Biomed Eng. 2006;8:343–375. doi: 10.1146/annurev.bioeng.8.061505.095735. [DOI] [PubMed] [Google Scholar]
- 8.Mäe M, Myrberg H, El-Andaloussi S, Langel Ü. Design of a tumor homing cell-penetrating peptide for drug delivery. Int J Pept Res Ther. 2009;15(1):11–15. [Google Scholar]
- 9.Wender PA, Galliher WC, Goun EA, Jones LR, Pillow TH. The design of guanidinium-rich transporters and their internalization mechanisms. Adv Drug Deliv Rev. 2008;60(4):452–472. doi: 10.1016/j.addr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshayes S, Morris M, Divita G, Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci. 2005;62(16):1839–1849. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7(1):33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 12.De Jong G, Telenius A, Vanderbyl S, Meitz A, Drayer J. Efficient in vitro transfer of a 60-Mb mammalian artificial chromosome into murine and hamster cells using cationic lipids and dendrimers. Chromosome Res. 2001;9(6):475–485. doi: 10.1023/a:1011680529073. [DOI] [PubMed] [Google Scholar]
- 13.Kreiss P, Mailhe P, Scherman D, et al. Plasmid DNA size does not affect the physicochemical properties of lipoplexes but modulates gene transfer efficiency. Nucleic Acids Res. 1999;27(19):3792–3798. doi: 10.1093/nar/27.19.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Said Hassane F, Saleh A, Abes R, Gait M, Lebleu B. Cell penetrating peptides: overview and applications to the delivery of oligonucleotides. Cell Mol Life Sci. 2010;67(5):715–726. doi: 10.1007/s00018-009-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundberg M, Wikström S, Johansson M. Cell surface adherence and endocytosis of protein transduction domains. Mol Ther. 2003;8(1):143–150. doi: 10.1016/s1525-0016(03)00135-7. [DOI] [PubMed] [Google Scholar]
- 16.Nakase I, Niwa M, Takeuchi T, et al. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol Ther. 2004;10(6):1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Foerg C, Ziegler U, Fernandez-Carneado J, et al. Decoding the entry of two novel cell-penetrating peptides in HeLa cells: lipid raft-mediated endocytosis and endosomal escape. Biochemistry. 2005;44(1):72–81. doi: 10.1021/bi048330+. [DOI] [PubMed] [Google Scholar]
- 18.Järver P, Langel U. Cell-penetrating peptides – a brief introduction. Biochim Biophys Acta. 2006;1758(3):260–263. doi: 10.1016/j.bbamem.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Lorents A, Kodavali PK, Oskolkov N, Langel Ü, Hällbrink M, Pooga M. Cell-penetrating peptides split into two groups based on modulation of intracellular calcium concentration. J Biol Chem. 2012;287(20):16880–16889. doi: 10.1074/jbc.M111.318063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richard JP, Melikov K, Brooks H, Prevot P, Lebleu B, Chernomordik LV. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J Biol Chem. 2005;280(15):15300–15306. doi: 10.1074/jbc.M401604200. [DOI] [PubMed] [Google Scholar]
- 21.Fittipaldi A, Ferrari A, Zoppé M, et al. Cell membrane lipid rafts mediate caveolar endocytosis of HIV-1 Tat fusion proteins. J Biol Chem. 2003;278(36):34141–34149. doi: 10.1074/jbc.M303045200. [DOI] [PubMed] [Google Scholar]
- 22.Guterstam P, Madani F, Hirose H, et al. Elucidating cell-penetrating peptide mechanisms of action for membrane interaction, cellular uptake, and translocation utilizing the hydrophobic counter-anion pyrenebutyrate. Biochim Biophys Acta. 2009;1788(12):2509–2517. doi: 10.1016/j.bbamem.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Jiao C-Y, Delaroche D, Burlina F, Alves ID, Chassaing G, Sagan S. Translocation and endocytosis for cell-penetrating peptide internalization. J Biol Chem. 2009;284(49):33957–33965. doi: 10.1074/jbc.M109.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolhassani A. Potential efficacy of cell-penetrating peptides for nucleic acid and drug delivery in cancer. Biochim Biophys Acta. 2011;1816(2):232–246. doi: 10.1016/j.bbcan.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs SM, Raines RT. Pathway for polyarginine entry into mammalian cell. Biochemistry. 2004;43(9):2438–2444. doi: 10.1021/bi035933x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel LN, Zaro JL, Shen WC. Cell penetrating peptides: intracellular pathways and pharmaceutical perspectives. Pharm Res. 2007;24(11):1977–1992. doi: 10.1007/s11095-007-9303-7. [DOI] [PubMed] [Google Scholar]
- 27.Nakase I, Akita H, Kogure K, et al. Efficient intracellular delivery of nucleic acid pharmaceuticals using cell-penetrating peptides. Acc Chem Res. 2012;45(7):1132–1139. doi: 10.1021/ar200256e. [DOI] [PubMed] [Google Scholar]
- 28.Silhol M, Tyagi M, Giacca M, Lebleu B, Vives E. Different mechanisms for cellular internalization of the HIV-1 Tat-derived cell penetrating peptide and recombinant proteins fused to Tat. Eur J Biochem. 2002;269(2):494–501. doi: 10.1046/j.0014-2956.2001.02671.x. [DOI] [PubMed] [Google Scholar]
- 29.Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends Mol Med. 2012;18(7):385–393. doi: 10.1016/j.molmed.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 31.Joliot A, Pernelle C, Deagostini-Bazin H, Prochiantz A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc Natl Acad Sci USA. 1991;88(5):1864–1868. doi: 10.1073/pnas.88.5.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the antennapedia homeodomain is receptor-independent. J Biol Chem. 1996;271(30):18188–18193. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- 33.Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272(25):16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 34.Lindgren M, Langel Ü. Classes and prediction of cell-penetrating peptides. In: Langel Ü, editor. Cell-Penetrating Peptides Methods and Protocols. Vol. 683. Springer; NY, USA: 2011. pp. 3–19. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Zhong CY, Wu JF, Huang YB, Liu CB. Enhancement of TAT cell membrane penetration efficiency by dimethyl sulphoxide. J Control Release. 2010;143(1):64–70. doi: 10.1016/j.jconrel.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today. 2012;(15–16):850–860. doi: 10.1016/j.drudis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Fawell S, Seery J, Daikh Y, et al. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA. 1994;91(2):664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269(14):10444–10450. [PubMed] [Google Scholar]
- 39.Pooga M, Soomets U, Hällbrink M, et al. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission invivo. Nat Biotechnol. 1998;16(9):857–861. doi: 10.1038/nbt0998-857. [DOI] [PubMed] [Google Scholar]
- 40.Wender PA, Rothbard JB, Jessop TC, Kreider EL, Wylie BL. Oligocarbamate molecular transporters: design, synthesis, and biological evaluation of a new class of transporters for drug delivery. J Am Chem Soc. 2002;124(45):13382–13383. doi: 10.1021/ja0275109. [DOI] [PubMed] [Google Scholar]
- 41.Pujals S, Fernández-Carneado J, López-Iglesias C, Kogan MJ, Giralt E. Mechanistic aspects of CPP-mediated intracellular drug delivery: relevance of CPP self-assembly. Biochim Biophys Acta. 2006;1758(3):264–279. doi: 10.1016/j.bbamem.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Schwarze SR, Hruska KA, Dowdy SF. Protein transduction: unrestricted delivery into all cells? Trends Cell Biol. 2000;10(7):290–295. doi: 10.1016/s0962-8924(00)01771-2. [DOI] [PubMed] [Google Scholar]
- 43▪▪.Gratton JP, Yu J, Griffith JW, et al. Cell-permeable peptides improve cellular uptake and therapeutic gene delivery of replication-deficient viruses in cells and invivo. Nat Med. 2003;9(3):357–362. doi: 10.1038/nm835. First group to report the use of cell-penetrating peptides as agents to transfer viruses (e.g., adenovirus and lentivirus) into a wide range of cells. [DOI] [PubMed] [Google Scholar]
- 44▪▪.Mo RH, Zaro JL, Shen WC. Comparison of cationic and amphipathic cell penetrating peptides for siRNA delivery and efficacy. Mol Pharm. 2011;9(2):299–309. doi: 10.1021/mp200481g. The authors designed a novel of polyplex for siRNA delivery demonstrated the importance of amphipathic cell-penetrating peptides for delivery of siRNA polyplexes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muratovska A, Eccles MR. Conjugate for efficient delivery of short interfering RNA (siRNA) into mammalian cells. FEBS Lett. 2004;558(1):63–68. doi: 10.1016/S0014-5793(03)01505-9. [DOI] [PubMed] [Google Scholar]
- 46▪.Liu BR, Lin MD, Chiang HJ, Lee HJ. Arginine-rich cell-penetrating peptides deliver gene into living human cells. Gene. 2012;505(1):37–45. doi: 10.1016/j.gene.2012.05.053. Demonstrates that arginine-rich cell-penetrating peptides are capable of delivering genes into living human cells. Also shows that co-treatment with calcium resulted in a significant increase in cell-penetrating peptide-mediated gene expression. [DOI] [PubMed] [Google Scholar]
- 47.Won YW, Kim HA, Lee M, Kim YH. Reducible poly (oligo-D-arginine) for enhanced gene expression in mouse lung by intratracheal injection. Mol Ther. 2009;18(4):734–742. doi: 10.1038/mt.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Åmand HL, Nordén B, Fant K. Functionalization with C-terminal cysteine enhances transfection efficiency of cell-penetrating peptides through dimer formation. Biochem Biophys Res Commun. 2012;418(3):469–474. doi: 10.1016/j.bbrc.2012.01.041. [DOI] [PubMed] [Google Scholar]
- 49.Astriab-Fisher A, Sergueev DS, Fisher M, Ramsay Shaw B, Juliano RL. Antisense inhibition of P-glycoprotein expression using peptide–oligonucleotide conjugates. Biochem Pharmacol. 2000;60(1):83–90. doi: 10.1016/s0006-2952(00)00310-5. [DOI] [PubMed] [Google Scholar]
- 50.Morris MC, Vidal P, Chaloin L, Heitz F, Divita G. A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acids Res. 1997;25(14):2730–2736. doi: 10.1093/nar/25.14.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris MC, Chaloin L, Méry J, Heitz F, Divita G. A novel potent strategy for gene delivery using a single peptide vector as a carrier. Nucleic Acids Res. 1999;27(17):3510–3517. doi: 10.1093/nar/27.17.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris MC, Depollier J, Méry J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol. 2001;19(12):1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 53.Tung C-H, Mueller S, Weissleder R. Novel branching membrane translocational peptide as gene delivery vector. Bioorg Med Chem. 2002;10(11):3609–3614. doi: 10.1016/s0968-0896(02)00248-1. [DOI] [PubMed] [Google Scholar]
- 54.Kumar P, Wu H, McBride JL, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448(7149):39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 55.Temsamani J, Vidal P. The use of cell-penetrating peptides for drug delivery. Drug Discov Today. 2004;9(23):1012–1019. doi: 10.1016/S1359-6446(04)03279-9. [DOI] [PubMed] [Google Scholar]
- 56.Nakase I, Hirose H, Tanaka G, et al. Cell-surface accumulation of flock house virus-derived peptide leads to efficient internalization via macropinocytosis. Mol Ther. 2009;17(11):1868–1876. doi: 10.1038/mt.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andreu D, Merrifield R, Steiner H, Boman HG. N-terminal analogs of cecropin A: synthesis, antibacterial activity, and conformational properties. Biochemistry. 1985;24(7):1683–1688. doi: 10.1021/bi00328a017. [DOI] [PubMed] [Google Scholar]
- 58.Lindgren M, Rosenthal-Aizman K, Saar K, et al. Overcoming methotrexate resistance in breast cancer tumour cells by the use of a new cell-penetrating peptide. Biochem Pharmacol. 2006;71(4):416–425. doi: 10.1016/j.bcp.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 59.Wadia JS, Dowdy SF. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv Drug Deliv Rev. 2005;57(4):579–596. doi: 10.1016/j.addr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 60▪▪.Meade BR, Dowdy SF. Exogenous siRNA delivery using peptide transduction domains/ cell penetrating peptides. Adv Drug Deliv Rev. 2007;59(2):134–140. doi: 10.1016/j.addr.2007.03.004. Interesting study with a polycation library of 13 peptides intended to examine the collective effects of polymer molecular weight, side chain substitution, and DNA:polymer ratio on cytotoxicity, transfection efficiency, and polycation–DNA interactions. [DOI] [PubMed] [Google Scholar]
- 61.Männistö M, Vanderkerken S, Toncheva V, et al. Structure–activity relationships of poly (L-lysines): effects of pegylation and molecular shape on physicochemical and biological properties in gene delivery. J Control Release. 2002;83(1):169–182. doi: 10.1016/s0168-3659(02)00178-5. [DOI] [PubMed] [Google Scholar]
- 62.McKenzie DL, Collard WT, Rice KG. Comparative gene transfer efficiency of low molecular weight polylysine DNA-condensing peptides. J Pept Res. 1999;54(4):311–318. doi: 10.1034/j.1399-3011.1999.00104.x. [DOI] [PubMed] [Google Scholar]
- 63.Gottschalk S, Sparrow J, Hauer J, et al. A novel DNA-peptide complex for efficient gene transfer and expression in mammalian cells. Gene Ther. 1996;3(5):448. [PubMed] [Google Scholar]
- 64.Wadhwa MS, Collard WT, Adami RC, McKenzie DL, Rice KG. Peptide-mediated gene delivery: influence of peptide structure on gene expression. Bioconj Chem. 1997;8(1):81–88. doi: 10.1021/bc960079q. [DOI] [PubMed] [Google Scholar]
- 65.Adami RC, Collard WT, Gupta SA, Kwok KY, Bonadio J, Rice KG. Stability of peptide-condensed plasmid DNA formulations. J Pharm Sci. 1998;87(6):678–683. doi: 10.1021/js9800477. [DOI] [PubMed] [Google Scholar]
- 66.Rydberg HA, Matson M, Åmand HL, Esbjörner EK, Nordén B. Effects of tryptophan content and backbone spacing on the uptake efficiency of cell-penetrating peptides. Biochemistry. 2012;51(27):5531–5539. doi: 10.1021/bi300454k. [DOI] [PubMed] [Google Scholar]
- 67.Walrant A, Correia I, Jiao CY, et al. Different membrane behaviour and cellular uptake of three basic arginine-rich peptides. Biochim Biophys Acta. 2011;1808(1):382–393. doi: 10.1016/j.bbamem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 68.Plank C, Tang MX, Wolfe AR, Szoka FC. Branched cationic peptides for gene delivery: role of type and number of cationic residues in formation and in vitro activity of DNA polyplexes. Hum Gene Ther. 1999;10(2):319–332. doi: 10.1089/10430349950019101. [DOI] [PubMed] [Google Scholar]
- 69.Åmand HL, Boström CL, Lincoln P, Nordén B, Esbjörner EK. Binding of cell-penetrating penetratin peptides to plasma membrane vesicles correlates directly with cellular uptake. Biochim Biophys Acta. 2011;1808(7):1860–1867. doi: 10.1016/j.bbamem.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 70.Åmand HL, Fant K, Nordén B, Esbjörner EK. Stimulated endocytosis in penetratin uptake: effect of arginine and lysine. Biochem Biophys Res Commun. 2008;371(4):621–625. doi: 10.1016/j.bbrc.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 71.Mitchell D, Steinman L, Kim D, Fathman C, Rothbard J. Polyarginine enters cells more efficiently than other polycationic homopolymers. J Pept Res. 2000;56(5):318–325. doi: 10.1034/j.1399-3011.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 72.Kim HH, Choi HS, Yang JM, Shin S. Characterization of gene delivery in vitro and in vivo by the arginine peptide system. Int J Pharm. 2007;335(1–2):70–78. doi: 10.1016/j.ijpharm.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 73.Lundberg M, Johansson M. Positively charged DNA-binding proteins cause apparent cell membrane translocation. Biochem Biophys Res Commun. 2002;291(2):367–371. doi: 10.1006/bbrc.2002.6450. [DOI] [PubMed] [Google Scholar]
- 74.Meade BR, Dowdy SF. Enhancing the cellular uptake of siRNA duplexes following noncovalent packaging with protein transduction domain peptides. Adv Drug Deliv Rev. 2008;60(4):530–536. doi: 10.1016/j.addr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim WJ, Christensen LV, Jo S, et al. Cholesteryl oligoarginine delivering vascular endothelial growth factor siRNA effectively inhibits tumor growth in colon adenocarcinoma. Molecular Ther. 2006;14(3):343–350. doi: 10.1016/j.ymthe.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 76.Koo H, Kang H, Lee Y. Analysis of the relationship between the molecular weight and transfection efficiency/cytotoxicity of Poly-L-arginine on a mammalian cell line. Bull Korean Chem Soc. 2009;30(4):927. [Google Scholar]
- 77.Futaki S, Ohashi W, Suzuki T, et al. Stearylated arginine-rich peptides: a new class of transfection systems. Bioconj Chem. 2001;12(6):1005–1011. doi: 10.1021/bc015508l. [DOI] [PubMed] [Google Scholar]
- 78.Baoum A, Ovcharenko D, Berkland C. Calcium condensed cell penetrating peptide complexes offer highly efficient, low toxicity gene silencing. Int J Pharm. 2012;427(1):134–142. doi: 10.1016/j.ijpharm.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 79.Midoux P, LeCam E, Coulaud D, Delain E, Pichon C. Histidine containing peptides and polypeptides as nucleic acid vectors. Somat Cell Mol Genet. 2002;27(1–6):27–47. doi: 10.1023/a:1022931923153. [DOI] [PubMed] [Google Scholar]
- 80.Midoux P, Kichler A, Boutin V, Maurizot J-C, Monsigny M. Membrane permeabilization and efficient gene transfer by a peptide containing several histidines. Bioconj Chem. 1998;9(2):260–267. doi: 10.1021/bc9701611. [DOI] [PubMed] [Google Scholar]
- 81.Wagner E, Plank C, Zatloukal K, Cotten M, Birnstiel ML. Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes: toward a synthetic virus-like gene-transfer vehicle. Proc Natl Acad Sci USA. 1992;89(17):7934–7938. doi: 10.1073/pnas.89.17.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Midoux P, Monsigny M. Efficient gene transfer by histidylated polylysine/pDNA complexes. Bioconj Chem. 1999;10(3):406–411. doi: 10.1021/bc9801070. [DOI] [PubMed] [Google Scholar]
- 83.Lundberg P, El-Andaloussi S, Sütlü T, Johansson H, Langel Ü. Delivery of short interfering RNA using endosomolytic cell-penetrating peptides. FASEB J. 2007;21(11):2664–2671. doi: 10.1096/fj.06-6502com. [DOI] [PubMed] [Google Scholar]
- 84.Martin ME, Rice KG. Peptide-guided gene delivery. AAPS J. 2007;9(1):18–29. doi: 10.1208/aapsj0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen CP, Kim J, Steenblock E, Liu D, Rice KG. Gene transfer with poly-melittin peptides. Bioconj Chem. 2006;17(4):1057–1062. doi: 10.1021/bc060028l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wyman TB, Nicol F, Zelphati O, Scaria P, Plank C, Szoka FC. Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry. 1997;36(10):3008–3017. doi: 10.1021/bi9618474. [DOI] [PubMed] [Google Scholar]
- 87.Zanta MA, Belguise-Valladier P, Behr JP. Gene delivery: a single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc Natl Acad Sci USA. 1999;96(1):91–96. doi: 10.1073/pnas.96.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Escriou V, Carrière M, Scherman D, Wils P. NLS bioconjugates for targeting therapeutic genes to the nucleus. Adv Drug Deliv Rev. 2003;55(2):295–306. doi: 10.1016/s0169-409x(02)00184-9. [DOI] [PubMed] [Google Scholar]
- 89.Lanford RE, Butel JS. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell. 1984;37(3):801. doi: 10.1016/0092-8674(84)90415-x. [DOI] [PubMed] [Google Scholar]
- 90.Collas P, Husebye H, Aleström P. The nuclear localization sequence of the SV40 T antigen promotes transgene uptake and expression in zebrafish embryo nuclei. Transgenic Res. 1996;5(6):451–458. doi: 10.1007/BF01980210. [DOI] [PubMed] [Google Scholar]
- 91.Subramanian A, Ranganathan P, Diamond SL. Nuclear targeting peptide scaffolds for lipofection of nondividing mammalian cells. Nat Biotechnol. 1999;17(9):873–877. doi: 10.1038/12860. [DOI] [PubMed] [Google Scholar]
- 92.Nitin N, LaConte L, Rhee WJ, Bao G. Tat peptide is capable of importing large nanoparticles across nuclear membrane in digitonin permeabilized cells. Ann Biomed Eng. 2009;37(10):2018–2027. doi: 10.1007/s10439-009-9768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schätzlein AG. Targeting of synthetic gene delivery systems. J Biomed Biotechnol. 2003;2003(2):149–158. doi: 10.1155/S1110724303209116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hart S. Integrin-mediated vectors for gene transfer and therapy. Curr Opin Mol Ther. 1999;1(2):197. [PubMed] [Google Scholar]
- 95.White SJ, Nicklin SA, Sawamura T, Baker AH. Identification of peptides that target the endothelial cell-specific LOX-1 receptor. Hypertension. 2001;37(2):449–455. doi: 10.1161/01.hyp.37.2.449. [DOI] [PubMed] [Google Scholar]
- 96.Khondee S, Baoum A, Siahaan TJ, Berkland C. Calcium condensed LABL–TAT complexes effectively target gene delivery to ICAM-1 expressing cells. Mol Pharm. 2011;8(3):788–798. doi: 10.1021/mp100393j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012 – an update. J Gene Med. 2013;15(2):65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 98.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 99.Robbins PD, Tahara H, Ghivizzani SC. Viral vectors for gene therapy. Trends Biotechnol. 1998;16(1):35–40. doi: 10.1016/S0167-7799(97)01137-2. [DOI] [PubMed] [Google Scholar]
- 100.Warnes A, Fooks AR. Live viral vectors: construction of a replication-deficient recombinant adenovirus. Methods Mol Med. 1996;4:33–45. doi: 10.1385/0-89603-334-1:33. [DOI] [PubMed] [Google Scholar]
- 101.Bergelson JM, Cunningham JA, Droguett G, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275(5304):1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 102.Walther W, Stein U. Viral vectors for gene transfer: a review of their use in the treatment of human diseases. Drugs. 2000;60(2):249–271. doi: 10.2165/00003495-200060020-00002. [DOI] [PubMed] [Google Scholar]
- 103.Mizuguchi H, Hayakawa T. Targeted adenovirus vectors. Hum Gene Ther. 2004;15(11):1034–1044. doi: 10.1089/hum.2004.15.1034. [DOI] [PubMed] [Google Scholar]
- 104.Schnierle BS, Groner B. Retroviral targeted delivery. Gene Ther. 1996;3(12):1069–1073. [PubMed] [Google Scholar]
- 105.Howitt J, Anderson CW, Freimuth P. Adenovirus interaction with its cellular receptor CAR. Curr Top Microbiol Immunol. 2003;272:331–364. doi: 10.1007/978-3-662-05597-7_11. [DOI] [PubMed] [Google Scholar]
- 106.Al-Jamal WT, Kostarelos K. Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc Chem Res. 2011;44(10):1094–1104. doi: 10.1021/ar200105p. [DOI] [PubMed] [Google Scholar]
- 107.Arcasoy SM, Latoche JD, Gondor M, Pitt BR, Pilewski JM. Polycations increase the efficiency of adenovirus-mediated gene transfer to epithelial and endothelial cells in vitro. Gene Ther. 1997;4(1):32–38. doi: 10.1038/sj.gt.3300349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Michen B, Graule T. Isoelectric points of viruses. J Appl Microbiol. 2010;109(2):388–397. doi: 10.1111/j.1365-2672.2010.04663.x. [DOI] [PubMed] [Google Scholar]
- 109.Wolfer DP, Lang R, Cinelli P, Madani R, Sonderegger P. Multiple roles of neurotrypsin in tissue morphogenesis and nervous system development suggested by the mRNA expression pattern. Mol Cell Neurosci. 2001;18(4):407–433. doi: 10.1006/mcne.2001.1029. [DOI] [PubMed] [Google Scholar]
- 110.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh MJ. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Invest. 1997;100(5):1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Emi N, Friedmann T, Yee JK. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J Virol. 1991;65(3):1202–1207. doi: 10.1128/jvi.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Denby L, Work LM, Graham D, et al. Adenoviral serotype 5 vectors pseudotyped with fibers from subgroup D show modified tropism in vitro and invivo. Hum Gene Ther. 2004;15(11):1054–1064. doi: 10.1089/hum.2004.15.1054. [DOI] [PubMed] [Google Scholar]
- 113.Eto Y, Yoshioka Y, Asavatanabodee R, et al. Transduction of adenovirus vectors modified with cell-penetrating peptides. Peptides. 2009;30(8):1548–1552. doi: 10.1016/j.peptides.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 114.Lehmusvaara S, Rautsi O, Hakkarainen T, Wahlfors J. Utility of cell-permeable peptides for enhancement of virus-mediated gene transfer to human tumor cells. Biotechniques. 2006;40(5):573–574. doi: 10.2144/000112152. [DOI] [PubMed] [Google Scholar]
- 115▪.Youn JI, Park SH, Jin HT, et al. Enhanced delivery efficiency of recombinant adenovirus into tumor and mesenchymal stem cells by a novel PTD. Cancer Gene Ther. 2008;15(11):703–712. doi: 10.1038/cgt.2008.45. Demonstrates the utility of cell-penetrating peptides for facilitating the infection of tumor cells and mesenchymal stem cells. [DOI] [PubMed] [Google Scholar]
- 116.Kuhnel F, Schulte B, Wirth T, et al. Protein transduction domains fused to virus receptors improve cellular virus uptake and enhance oncolysis by tumor-specific replicating vectors. J Virol. 2004;78(24):13743–13754. doi: 10.1128/JVI.78.24.13743-13754.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen HZ, Wu CP, Chao YC, Liu CYY. Membrane penetrating peptides greatly enhance baculovirus transduction efficiency into mammalian cells. Biochem Biophys Res Commun. 2011;405(2):297–302. doi: 10.1016/j.bbrc.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118▪▪.Park SH, Doh J, Park SI, et al. Branched oligomerization of cell-permeable peptides markedly enhances the transduction efficiency of adenovirus into mesenchymal stem cells. Gene Ther. 2010;17(8):1052–1061. doi: 10.1038/gt.2010.58. Demonstrates the concept of producing branched cell-permeable peptides to further increase the efficiency of cell-permeable peptides as a translocating agent. [DOI] [PubMed] [Google Scholar]
- 119.Liu SH, Mao QW, Zhang WF, et al. Genetically modified adenoviral, vector with the protein transduction domain of Tat improves gene transfer to CAR-deficient cells. Biosci Rep. 2009;29(2):103–109. doi: 10.1042/BSR20080023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yoshioka Y, Asavatanabodee R, Eto Y, et al. Tat conjugation of adenovirus vector broadens tropism and enhances transduction efficiency. Life Sci. 2008;83(21–22):747–755. doi: 10.1016/j.lfs.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 121.Nigatu AS, Vupputuri S, Flynn N, Neely BJ, Ramsey JD. Evaluation of cell-penetrating peptide/adenovirus particles for transduction of CAR-negative cells. J Pharm Sci. 2013;102(6):1981–1993. doi: 10.1002/jps.23556. [DOI] [PubMed] [Google Scholar]
- 122.Berlose JP, Convert O, Derossi D, Brunissen A, Chassaing G. Conformational and associative behaviours of the third helix of antennapedia homeodomain in membrane-mimetic environments. Eur J Biochem. 1996;242(2):372–386. doi: 10.1111/j.1432-1033.1996.0372r.x. [DOI] [PubMed] [Google Scholar]
- 123.Rothbard JB, Garlingto S, Lin Q, et al. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat Med. 2000;6(11):1253–1257. doi: 10.1038/81359. [DOI] [PubMed] [Google Scholar]
- 124.Rudolph C, Plank C, Lausier J, Schillinger U, Muller RH, Rosenecker J. Oligomers of the arginine-rich motif of the HIV-1 TAT protein are capable of transferring plasmid DNA into cells. J Biol Chem. 2003;278(13):11411–11418. doi: 10.1074/jbc.M211891200. [DOI] [PubMed] [Google Scholar]
- 125.Kim TI, Ou M, Lee M, Kim SW. Arginine-grafted bioreducible poly(disulfide amine) for gene delivery systems. Biomaterials. 2009;30(4):658–664. doi: 10.1016/j.biomaterials.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126▪▪.Kim P-H, Kim T-I, Yockman JW, Kim SW, Yun C-O. The effect of surface modification of adenovirus with an arginine-grafted bioreducible polymer on transduction efficiency and immunogenicity in cancer gene therapy. Biomaterials. 2010;31(7):1865–1874. doi: 10.1016/j.biomaterials.2009.11.043. Demonstrates cell-penetrating peptides can be combined with polymers to reduce the drawbacks often associated with viral vectors. [DOI] [PubMed] [Google Scholar]
- 127.Wadhwa MS, Collard WT, Adami RC, McKenzie DL, Rice KG. Peptide-mediated gene delivery: influence of peptide structure on gene expression. Bioconj Chem. 1997;8(1):81–88. doi: 10.1021/bc960079q. [DOI] [PubMed] [Google Scholar]
- 128.McKenzie DL, Collard WT, Rice KG. Comparative gene transfer efficiency of low molecular weight polylysine DNA-condensing peptides. J Pept Res. 2003;54(4):311–318. doi: 10.1034/j.1399-3011.1999.00104.x. [DOI] [PubMed] [Google Scholar]
- 129.Åmand HL, Rydberg HA, Fornander LH, Lincoln P, Nordén B, Esbjörner EK. Cell surface binding and uptake of arginine-and lysine-rich penetratin peptides in absence and presence of proteoglycans. Biochim Biophys Acta. 2012;1818(11):2669–2678. doi: 10.1016/j.bbamem.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 130.McKenzie DL, Kwok KY, Rice KG. A potent new class of reductively activated peptide gene delivery agents. J Biol Chem. 2000;275(14):9970–9977. doi: 10.1074/jbc.275.14.9970. [DOI] [PubMed] [Google Scholar]
- 131.Read ML, Singh S, Ahmed Z, et al. A versatile reducible polycation-based system for efficient delivery of a broad range of nucleic acids. Nucleic Acids Res. 2005;33(9):e86–e86. doi: 10.1093/nar/gni085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Siomi H, Shida H, Maki M, Hatanaka M. Effects of a highly basic region of human immunodeficiency virus Tat protein on nucleolar localization. J Virol. 1990;64(4):1803–1807. doi: 10.1128/jvi.64.4.1803-1807.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Friedler A, Friedler D, Luedtke NW, Tor Y, Loyter A, Gilon C. Development of a functional backbone cyclic mimetic of the HIV-1 Tat arginine-rich motif. J Biol Chem. 2000;275(31):23783–23789. doi: 10.1074/jbc.M002200200. [DOI] [PubMed] [Google Scholar]