Abstract
Drug delivery strategies using cell penetrating peptides (CPPs) have been widely explored to improve the intracellular delivery of a large number of cargo molecules. Electrostatic complexation of pDNA using CPPs has been less explored due to the relatively large complexes formed and the low levels of gene expression achieved when using these low molecular weight polycations as DNA condensing agents. Here, condensing nascent CPP polyplexes using CaCl2 produced small and stable nanoparticles leading to gene expression levels higher than observed for control PEI gene vectors. This simple formulation approach showed negligible cytotoxicity in A549 lung epithelial cells and maintained particle size and transfection efficiency even in the presence of serum.
Keywords: Gene Delivery, Plasmid DNA, Cell-penetrating peptides, A549 cells
Introduction
Nucleic acid therapeutics continue to offer promise for the treatment of both acquired and inherited diseases. One major obstacle impeding the successful application of nucleic acid drugs is the difficulty to develop a simple, safe, and efficacious delivery system 1–3. Gene delivery vectors must compact genetic material into nanoparticles that are colloidally stable, protect nucleic acids from enzymatic degradation, effectively transit nucleic acids to target cells, and achieve a significant transfection yield. Viral vectors remain the most effective method of gene delivery even though problems such as immunogenicity remain a concern.
Polymer-mediated gene delivery has emerged as a viable alternative to viral transfection due to potential attributes such as low immunogenicity, low toxicity, ease of synthesis and low cost 4–6. Many studies have shown that DNA complexes electrostatically with polycations to form "polyplexes" that are endocytosed by many cell types and deliver DNA with varying degrees of efficiency and toxicity 7–16. Frequently, the most effective polyplexes are also the most toxic, thus hampering clinical translation 1–6. As a primary example, polyethylenimine (PEI) exhibits efficient gene delivery but is also very cytotoxic 17,18. Cell-penetrating peptides (CPPs) offer a potential alternative to PEI. These short polycations achieve intracellular access by crossing the plasma membrane directly or by endocytosis 19–25 while typically exhibiting low cytotoxicity 26,27. Covalently conjugating CPPs to gene vectors (e.g. liposomes, polymer nanoparticle, etc.) has shown some promise, but polyplexes of CPPs and DNA have proven to be relatively inefficient and require improvement 28,29.
To provide a simple method for improving the gene delivery of CPP polyplexes, calcium was used to condense large and inefficient CPP polyplexes. The resulting nanoparticles transfected human lung carcinoma cell line A549 more efficiently than PEI and exhibited very low cytotoxicity. Four representative CPPs were studied; Arginine 7 (Arg7), Arginine 9 (Arg9), Antennapedia Heptapeptide (Ahp) and Antennapedia Leader Peptide (Alp) (Table 1). Plasmid DNA encoding firefly luciferase (pGL3, 4.8 kbp) was used as a reporter.
Table 1.
Structure of CPPs, Arginine 7 (Arg7), Arginine 9 (Arg9), Antennapedia Heptapeptide (Ahp) and Antennapedia Leader Peptide (Alp).
| CPP | Sequence | Molecular weight (Da) |
|---|---|---|
| Arg7 | RRRRRRR | 1,111.3 |
| Arg9 | RRRRRRRRR | 1,423.3 |
| Ahp | RRMKWKK | 1,032.6 |
| Alp | KKWKMRRNQFWVKVQRG | 2,276.2 |
Experimental Section
Materials
Plasmid DNA encoding firefly luciferase (pGL3, 4.8 kbp) was obtained from Promega (Madison, WI). Cell penetrating peptides (CPPs) were purchased from Pi Proteomics (Huntsville, AL). Branched polyethylenimine (PEI, 25 kDa) was obtained from Aldrich (Milwaukee, WI). Calcium chloride (CaCl2. 2H2O) was purchased from Fisher Scientific (Pittsburgh, PA). A549 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD). The cell culture medium (Ham’s F-12 Nutrient Mixture, Kaighn’s modified with L-glutamine) was purchased through Fisher Scientic. Fetal bovine serum (FBS) was purchased from Hyclone. Penicillin-streptomycin was purchased from MB Biomedical, LLC. Trypsin-EDTA was purchased through Gibco. MTS reagent [tetrazolium compound; 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] was purchased from Promega.
Preparation of CPP/pGL3 complexes
CPP/pGL3 complexes were prepared by adding 10 µL (0.1 µg/µL) of pGL3 to 15 µL CPP solution while pipetting. To this solution, 15 µL of known molarity (e.g. 113 mM) CaCl2 was added and mixed by vigorous pipetting. Before performing experiments, the complexes were allowed to equilibrate for 20 min at 4° C.
Preparation of PEI/pGL3 complexes
PEI/pGL3 complexes were prepared by adding 10 µl (0.1 µg/µL) of pGL3 solution to 15 µL (N/P ratio 10) PEI solution while pipetting followed by 20 min incubation at 4° C. Complexes were freshly prepared before each individual experiment.
Characterization of CPPs and PEI complexes
The size and zeta potential of the different complexes were measured by using ZetaPALS dynamic light scattering (DLS) (Brookhaven, Holtsville, NY).
Cell culture
Culturing of human epithelial lung cell line A549 was performed according to the protocol provided by the American Type Culture Collection. A549 cells were grown in F-12K supplemented with 10% v/v FBS and 1% v/v Penicillin/streptomycin at 37° C in a humidified air atmosphere containing 5% CO2.
In vitro cell transfection studies
A549 cells were trypsinized, counted and diluted to a concentration of approximately 80,000 cells/ mL. Then 0.1 mL of that dilution was added to each well of a 96-well plate and the cells were incubated in a humidified atmosphere at 5% CO2 and 37°C for 24 h. Immediately before transfection, the cells were washed once with PBS and 100 µl sample (20% of complex to 80% of serum free cell culture medium) was added to each well. Cells were incubated with the complexes for 5 h. The transfection agent was then removed by aspiration and 100 µL of fresh serum medium was added followed by further incubation. The Luciferase Assay System from Promega was used to determine gene expression following the manufacturer’s recommended protocol. The light units were normalized against protein concentration in the cells extracts, which were measured using the BCA™ Protein Assay (Thermo Scientific). The transfection results were expressed as Relative Light Units (RLU) per mg of cellular protein.
Assessment of cytotoxicity (MTS Assay)
Cytotoxicity of polymers was determined by the CellTiter 96® Aqueous Cell Proliferation Assay (Promega). A549 cells were grown as described in the transfection experiments. Cells were treated with the samples for ~24 h. The media were then removed and replaced with a mixture of 100 µL fresh culture media and 20 µL MTS reagent solution. The cells were incubated for 3 h at 37°C in the 5% CO2 incubator. The absorbance of each well was then measured at 490 nm using a microtiter plate reader (SpectraMax, M25, Molecular Devices Corp., CA) to determine cell viability.
Statistical analysis
Statistical evaluation of comparing the significance of the difference in expression between the means of two groups was performed using the t-test, a value of p <0.05 was accepted as significant.
Results and Discussion
Peptides offer a highly attractive feature of incorporating various biological activities required for biomedical applications30–34. Cell-penetrating peptides, a group of short peptides with the potent ability to translocate across the plasma membrane of the cells, have been reported to mediate plasmid DNA delivery into cells35–37; however, improving the transfection efficiency of their DNA complexes remains a major challenge. The relatively low transfection level of certain complexes may be due to inadequate escape from endosomes or the inefficient release of DNA from the complexes38. Previous studies demonstrated that the HIV-1 TAT peptide could only provide a high level of gene expression when chloroquine (an endosomolytic agent) was added, which is not feasible for gene delivery in vivo28,39–41. One approach for overcoming this limitation was to link the CPPs to produce high molecular weight polypeptides42–44 or to directly conjugate the CPPs using histidine or cysteine residues38,45. Others have tried a low molecular weight PEI with covalently linked TAT to overcome the poor transfection efficiency of the CPP alone39. Targeting studies have also been explored. For example, the YIGSR pentapeptide, known to target cell surface laminin receptors, or the LK15 peptide was conjugated to TAT. In each case, the transfection efficiency improved, but gene expression levels were still low compared to PEI46–48.
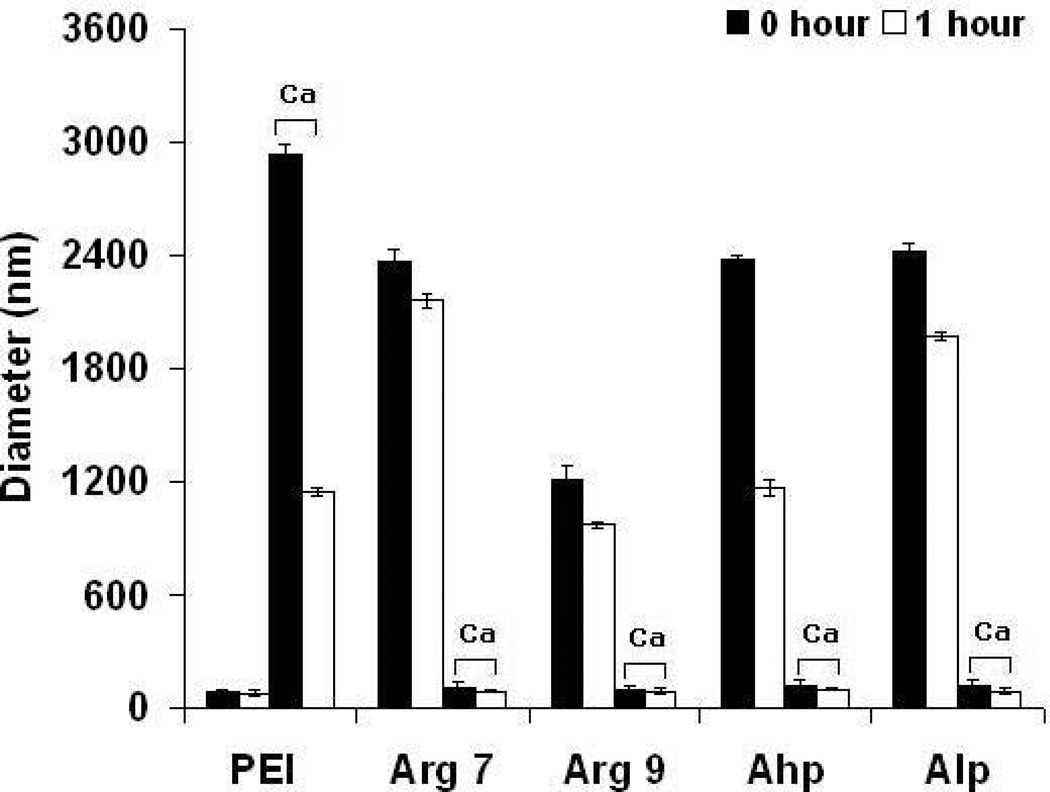
In studies reported here, CPP/pGL3 complexes were synthesized by rapidly adding pGL3 to CPP. These complexes were thoroughly mixed by pipetting and then CaCl2 was added to decrease the large size of these complexes. Calcium was previously reported to interact with both amines (e.g. on CPPs) and pDNA within polyplexes to form compact complexes through "soft" crosslinks. Here, the reduction in the size of CPP/pGL3 complexes likely led to some of the noted increase in transfection. A CaCl2 concentration of 113 mM consistently produced small (100–140 nm) and stable CPP polyplexes with a single particle population (polydispersity < 0.23). In general, the zeta potential of CPP polyplexes increased significantly from 8 to 26 mV with increasing concentration of CaCl2 (Figure 1). The CPP polyplexes synthesized with CaCl2 remained stable in the absence and presence of 10% FBS at 37° C for 1 hr. Conversely, CPP polyplexes without added calcium remained large (Figure 2).
Figure 1.
The effect of CaCl2 (113 mM) on the charge of PEI and CPPs complexes. Results are presented as mean ± SD (n = 3). PEI, polyethylenimine; CPPs, cell-penetrating peptides; Arg7, arginine 7; Arg9, arginine 9; Ahp, antennapedia heptapeptide; Alp, antennapedia leader peptide.
Figure 2.
The diameter of CPPs–Ca/pGL3 and PEI complexes (without and with 113 mM CaCl2) in (a) the presence and (b) absence of 10% fetal bovine serum. Results are presented as mean ± SD (n = 3). PEI, polyethylenimine; CPPs, cell-penetrating peptides; Arg7, arginine 7; Arg9, arginine 9; Ahp, antennapedia heptapeptide; Alp, antennapedia leader peptide.
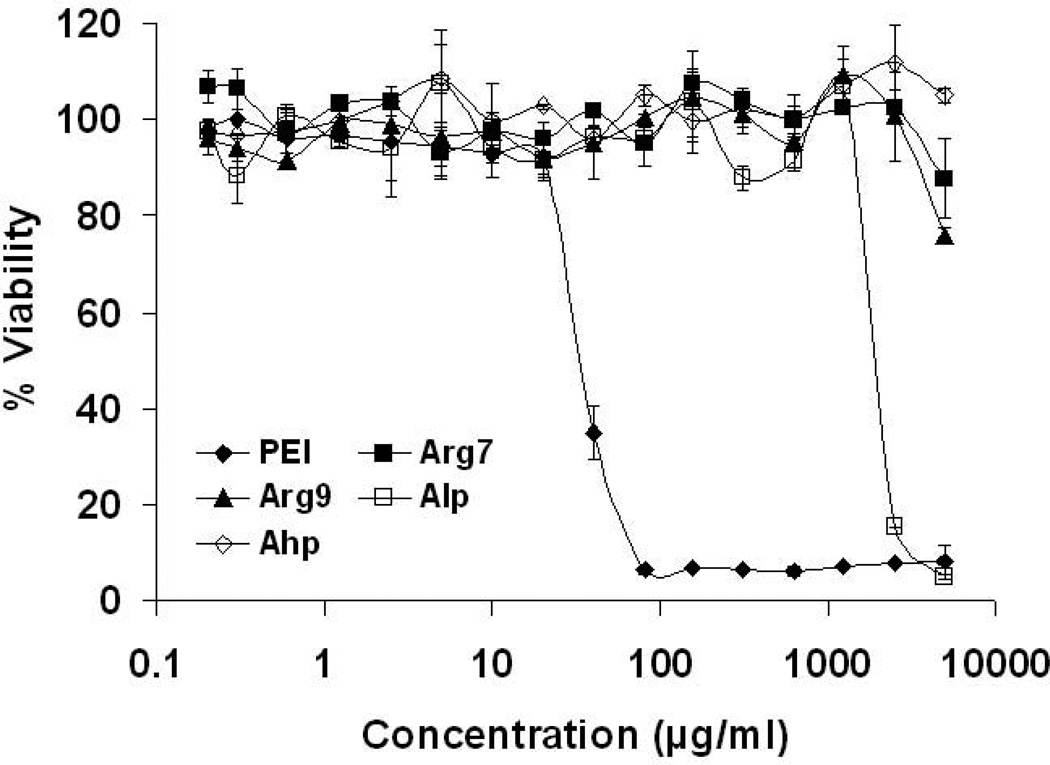
A successful gene delivery vector should be able to deliver gene to the cell without negatively affecting the viability of the host cell. To investigate whether the CPPs affected the viability of A549 human lung carcinoma cells, an MTS cytotoxicity assay of free CPP or branched PEI (25 kDa) was conducted. A549 cells were incubated with up to 5 mg/mL of CPPs or PEI for ~24 hrs. Cytotoxicity profiles of Arg7, Arg9 and Ahp peptides revealed no evidence of cytotoxicity and cells maintained high viability (Figure 3), while Alp peptide showed moderate cytotoxicity (IC50 ~2144 µg/mL). Branched PEI induced substantial cytotoxicity (IC50 ~35 µg/mL).
Figure 3.
Cytotoxicity profiles of PEI andCPPs.. Viability is expressed as a function of polymer concentration. Results are presented as mean ± SD (n = 3). PEI, polyethylenimine; CPPs, cell-penetrating peptides; Arg7, arginine 7; Arg9, arginine 9; Ahp, antennapedia heptapeptide; Alp, antennapedia leader peptide.
Luciferase gene expression was measured 24 h after transfection in order to study the ability of CPP polyplexes to transfect A549 cells. Different N/P ratios of the CPP or branched PEI (N/P 10) polyplexes were studied using different concentrations of CaCl2; 0, 28.3, 56.5, and 113 mM as a condensing agent after complex formation. Most CPP polyplexes showed the highest level of gene expression at 113 mM of added CaCl2 for the various N/P ratios when compared to branched PEI, which had excellent transfection efficiency only in the absence of CaCl2 (Figure 4). Arg7, Arg9, Ahp and Alp revealed the greatest transfection efficiency with 113 mM CaCl2 at N/P ratios of 36, 35, 29 and 15 respectively. It is important to note that gene expression was not detectable for CPPs/pDNA complexes without CaCl2.
Figure 4.
The transfection efficiency of CPP polyplexes using (a) Arg7, (b) Arg9, (c) Ahp, or (d) Alp with different concentrations of added CaCl2. Results are presented as mean ± SD (n = 3), *p < 0.001(Arg7, Ahp, and Alp), and *p < 0.01 (Arg9) as compared with PEI. PEI, polyethylenimine; CPPs, cell-penetrating peptides; Arg7, arginine 7; Arg9, arginine 9; Ahp, antennapedia heptapeptide; Alp, antennapedia leader peptide; RLUs, relative light units.
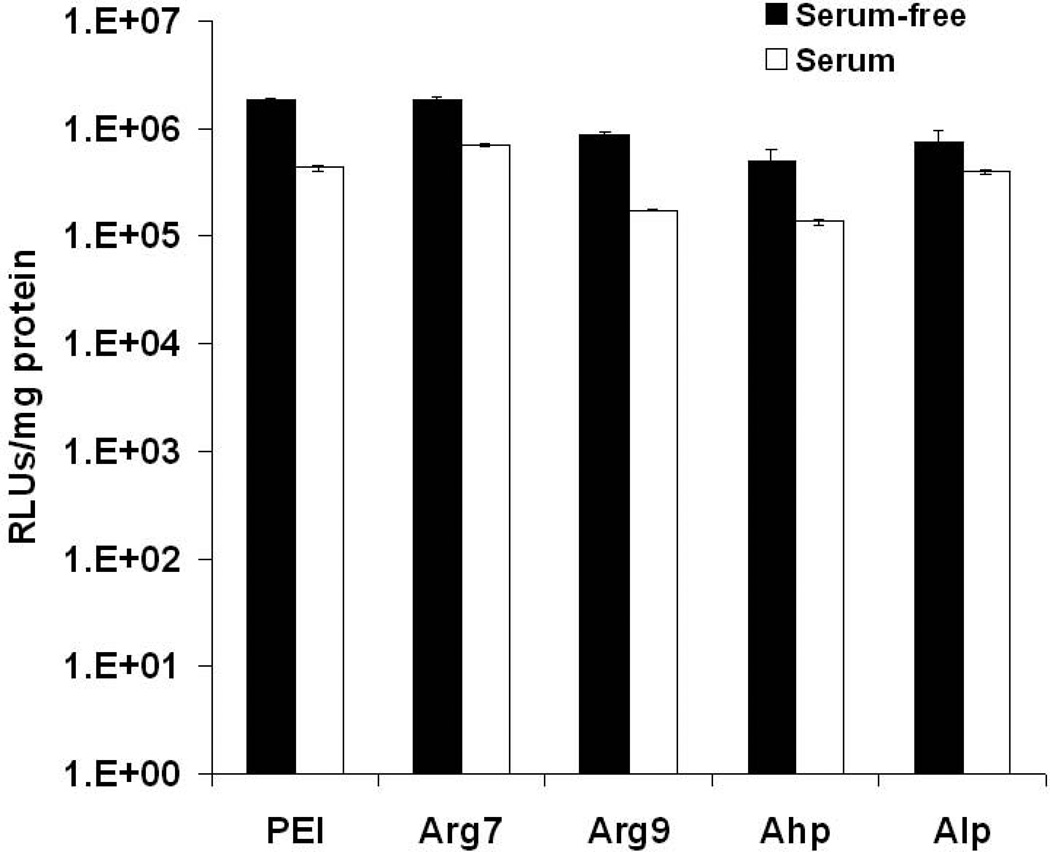
To gain insight into the potential of utilizing calcium condensed CPP polyplexes as delivery vectors in vivo, A549 cells were transfected in the presence of serum (Figure 5). A slight decrease in transfection efficiency was observed and CPP polyplexes exerted a similar reduction in gene expression as that observed for PEI polyplexes. PEI polyplexes have shown effective gene transfection in vivo, but is dose limited due to toxicity.
Figure 5.
The transfection efficiency of CPP polyplexes in the absence and presence of 10% fetal bovine serum. Results are presented asmean ± SD (n = 3). PEI, polyethylenimine; CPPs, cell-penetrating peptides; Arg7, arginine 7; Arg9, arginine 9; Ahp, antennapedia heptapeptide; Alp, antennapedia leader peptide; RLUs, relative light units.
Conclusion
The delivery of therapeutic nucleic acids by CPP polyplexes condensed with calcium may offer a simple and effective gene delivery method with potential for clinical translation. Adding CaCl2 to CPP polyplexes produced small nanoparticles leading to gene expression levels higher than observed for optimized PEI gene vectors in A549 lung epithelial cells. CPP polyplexes were stable, maintaining particle size in the absence and presence of 10% of FBS over a period of 1 h. The CPPs generally showed negligible cytotoxicity up to 5 mg/mL, which may offer an opportunity to increase the dose of nucleic acid therapeutics to achieve a desired therapeutic effect. The simplicity of the formulation in combination with the efficacy and low cytotoxicity of CPP polyplexes makes them highly interesting vectors for future studies in vivo.
Acknowledgments
We acknowledge support for this work from Savara Pharmaceuticals and the Institute for Advancing Medical Innovation. We also acknowledge lab support from the Coulter Foundation, the Higuchi Biosciences Center, the American Heart Association, the NIH (R03 AR054035, P20 RR016443 and T32 GM08359-11) and the NSF (CHE 0719464). We also thank Prof. C. Russ Middaugh for the use of laboratory equipment.
References
- 1.Luo D, Saltzman WM. Synthetic DNA delivery systems. Nature biotechnology. 2000;18(1):33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 2.Ma H, Diamond SL. Nonviral gene therapy and its delivery systems. Current Pharmaceutical Biotechnology. 2001;2(1):1–17. doi: 10.2174/1389201013378770. [DOI] [PubMed] [Google Scholar]
- 3.Mahato RI, Smith LC, Rolland A. Pharmaceutical perspectives of nonviral gene therapy. Advances in genetics. 1999;41:95–156. doi: 10.1016/s0065-2660(08)60152-2. [DOI] [PubMed] [Google Scholar]
- 4.Davis ME. Non-viral gene delivery systems. Current opinion in biotechnology. 2002;13(2):128–131. doi: 10.1016/s0958-1669(02)00294-x. [DOI] [PubMed] [Google Scholar]
- 5.De Smedt SC, Demeester J, Hennink WE. Cationic polymer based gene delivery systems. Pharmaceutical research. 2000;17(2):113–126. doi: 10.1023/a:1007548826495. [DOI] [PubMed] [Google Scholar]
- 6.Godbey WT, Mikos AG. Recent progress in gene delivery using non-viral transfer complexes. Journal of Controlled Release. 2001;72(1–3):115–125. doi: 10.1016/s0168-3659(01)00267-x. [DOI] [PubMed] [Google Scholar]
- 7.Akinc A, Lynn DM, Anderson DG, Langer R. Parallel synthesis and biophysical characterization of a degradable polymer library for gene delivery. Journal of the American Chemical Society. 2003;125(18):5316. doi: 10.1021/ja034429c. [DOI] [PubMed] [Google Scholar]
- 8.Brissault B, Kichler A, Guis C, Leborgne C, Danos O, Cheradame H. Synthesis of linear polyethylenimine derivatives for DNA transfection. Bioconjugate chemistry. 2003;14(3):581–587. doi: 10.1021/bc0200529. [DOI] [PubMed] [Google Scholar]
- 9.Gebhart CL, Kabanov AV. Evaluation of polyplexes as gene transfer agents. Journal of Controlled Release. 2001;73(2–3):401–416. doi: 10.1016/s0168-3659(01)00357-1. [DOI] [PubMed] [Google Scholar]
- 10.Godbey WT, Wu KK, Mikos AG. Poly (ethylenimine) and its role in gene delivery. Journal of Controlled Release. 1999;60(2–3):149–160. doi: 10.1016/s0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 11.Hwang SJ, Bellocq NC, Davis ME. Effects of Structure of [beta]-Cyclodextrin-Containing Polymers on Gene Delivery. Bioconjugate Chem. 2001;12(2):280–290. doi: 10.1021/bc0001084. [DOI] [PubMed] [Google Scholar]
- 12.Jeong JH, Song SH, Lim DW, Lee H, Park TG. DNA transfection using linear poly (ethylenimine) prepared by controlled acid hydrolysis of poly (2-ethyl-2-oxazoline) Journal of Controlled Release. 2001;73(2–3):391–399. doi: 10.1016/s0168-3659(01)00310-8. [DOI] [PubMed] [Google Scholar]
- 13.Putnam D, Gentry CA, Pack DW, Langer R. Polymer-based gene delivery with low cytotoxicity by a unique balance of side-chain termini. Proceedings of the National Academy of Sciences. 2001;98(3):1200. doi: 10.1073/pnas.031577698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reineke TM, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 2. Charge center type. Bioconjugate Chem. 2003;14(1):255–261. doi: 10.1021/bc025593c. [DOI] [PubMed] [Google Scholar]
- 15.Reineke TM, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 1. Carbohydrate size and its distance from charge centers. Bioconjugate Chem. 2003;14(1):247–254. doi: 10.1021/bc025592k. [DOI] [PubMed] [Google Scholar]
- 16.Zelikin AN, Putnam D, Shastri P, Langer R, Izumrudov VA. Aliphatic Ionenes as Gene Delivery Agents: Elucidation of Structure-Function Relationship through Modification of Charge Density and Polymer Length. Bioconjugate Chem. 2002;13(3):548–553. doi: 10.1021/bc015553t. [DOI] [PubMed] [Google Scholar]
- 17.Fischer D, Bieber T, Li Y, Elsässer HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharmaceutical research. 1999;16(8):1273–1279. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- 18.Forrest ML, Koerber JT, Pack DW. A degradable polyethylenimine derivative with low toxicity for highly efficient gene delivery. Bioconjugate Chem. 2003;14(5):934–940. doi: 10.1021/bc034014g. [DOI] [PubMed] [Google Scholar]
- 19.Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 2007;8(7):848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones SW, Christison R, Bundell K, Voyce CJ, Brockbank SMV, Newham P, Lindsay MA. Characterisation of cell-penetrating peptide-mediated peptide delivery. British journal of pharmacology. 2005;145(8):1093. doi: 10.1038/sj.bjp.0706279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meade BR, Dowdy SF. Enhancing the cellular uptake of siRNA duplexes following noncovalent packaging with protein transduction domain peptides. Advanced drug delivery reviews. 2008;60(4–5):530–536. doi: 10.1016/j.addr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richard JP, Melikov K, Brooks H, Prevot P, Lebleu B, Chernomordik LV. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. Journal of Biological Chemistry. 2005;280(15):15300. doi: 10.1074/jbc.M401604200. [DOI] [PubMed] [Google Scholar]
- 23.Thorén PEG, Persson D, Isakson P, Goksör M, Önfelt A, Nordén B. Uptake of analogs of penetratin, Tat (48–60) and oligoarginine in live cells. Biochemical and Biophysical Research Communications. 2003;307(1):100–107. doi: 10.1016/s0006-291x(03)01135-5. [DOI] [PubMed] [Google Scholar]
- 24.Tunnemann G, Martin RM, Haupt S, Patsch C, Edenhofer F, Cardoso MC. Cargo-dependent mode of uptake and bioavailability of TAT-containing proteins and peptides in living cells. The FASEB Journal. 2006;20(11):1775. doi: 10.1096/fj.05-5523com. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Jin Y, Plummer MR, Pooyan S, Gunaseelan S, Sinko PJ. Endocytosis and Membrane Potential Are Required for HeLa Cell Uptake of RI-CKTat9, a Retro-Inverso Tat Cell Penetrating Peptide. Molecular Pharmaceutics. 2009;6(3):836–848. doi: 10.1021/mp800121f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27^ K^ i^ p^ 1 induces cell migration. Nature medicine. 1998;4(12):1998–1912. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- 27.Tréhin R, Krauss U, Beck-Sickinger AG, Merkle HP, Nielsen HM. Cellular uptake but low permeation of human calcitonin-derived cell penetrating peptides and Tat (47–57) through well-differentiated epithelial models. Pharmaceutical research. 2004;21(7):1248–1256. doi: 10.1023/b:pham.0000033013.45204.c3. [DOI] [PubMed] [Google Scholar]
- 28.Rudolph C, Plank C, Lausier J, Schillinger U, Müller RH, Rosenecker J. Oligomers of the arginine-rich motif of the HIV-1 TAT protein are capable of transferring plasmid DNA into cells. Journal of Biological Chemistry. 2003;278(13):11411. doi: 10.1074/jbc.M211891200. [DOI] [PubMed] [Google Scholar]
- 29.Tung CH, Mueller S, Weissleder R. Novel branching membrane translocational peptide as gene delivery vector. Bioorganic & medicinal chemistry. 2002;10(11):3609–3614. doi: 10.1016/s0968-0896(02)00248-1. [DOI] [PubMed] [Google Scholar]
- 30.Ellis-Behnke RG, Liang YX, You SW, Tay DKC, Zhang S, So KF, Schneider GE. Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(13):5054. doi: 10.1073/pnas.0600559103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papapostolou D, Smith AM, Atkins EDT, Oliver SJ, Ryadnov MG, Serpell LC, Woolfson DN. Engineering nanoscale order into a designed protein fiber. Proceedings of the National Academy of Sciences. 2007;104(26):10853. doi: 10.1073/pnas.0700801104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303(5662):1352. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 33.Woolfson DN, Ryadnov MG. Peptide-based fibrous biomaterials: some things old, new and borrowed. Current opinion in chemical biology. 2006;10(6):559–567. doi: 10.1016/j.cbpa.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nature biotechnology. 2003;21(10):1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 35.Heitz F, Morris MC, Divita G. Twenty years of cell penetrating peptides: from molecular mechanisms to therapeutics. British journal of pharmacology. 2009;157(2):195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saleh AFA, Aojula HS, Pluen A. Enhancement of gene transfer using YIGSR analog of Tat derived peptide. Biopolymers. 2008;89(1):62–71. doi: 10.1002/bip.20854. [DOI] [PubMed] [Google Scholar]
- 37.Soundara Manickam D, Bisht HS, Wan L, Mao G, Oupicky D. Influence of TAT-peptide polymerization on properties and transfection activity of TAT/DNA polyplexes. Journal of Controlled Release. 2005;102(1):293–306. doi: 10.1016/j.jconrel.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Midoux P, Monsigny M. Efficient gene transfer by histidylated polylysine/pDNA complexes. Bioconjugate Chem. 1999;10(3):406–411. doi: 10.1021/bc9801070. [DOI] [PubMed] [Google Scholar]
- 39.Alexis F, Lo SL, Wang S. Covalent attachment of low molecular weight Poly (ethylene imine) improves Tat peptide mediated gene delivery. Advanced Materials. 2006;18(16):2174–2178. [Google Scholar]
- 40.Hyndman L, Lemoine JL, Huang L, Porteous DJ, Boyd AC, Nan X. HIV-1 Tat protein transduction domain peptide facilitates gene transfer in combination with cationic liposomes. Journal of Controlled Release. 2004;99(3):435–444. doi: 10.1016/j.jconrel.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 41.Sloots A, Wels WS. Recombinant derivatives of the human high mobility group protein HMGB2 mediate efficient nonviral gene delivery. FEBS Journal. 2005;272(16):4221–4236. doi: 10.1111/j.1742-4658.2005.04834.x. [DOI] [PubMed] [Google Scholar]
- 42.McKenzie DL, Smiley E, Kwok KY, Rice KG. Low molecular weight disulfide cross-linking peptides as nonviral gene delivery carriers. Bioconjugate Chem. 2000;11(6):901–909. doi: 10.1021/bc000056i. [DOI] [PubMed] [Google Scholar]
- 43.Oupick D, Parker AL, Seymour LW. Laterally stabilized complexes of DNA with linear reducible polycations: strategy for triggered intracellular activation of DNA delivery vectors. J Am Chem Soc. 2002;124(1):8–9. doi: 10.1021/ja016440n. [DOI] [PubMed] [Google Scholar]
- 44.Trubetskoy VS, Hanson LJ, Slattum PM, Hagstrom JE, Budker VG, Wolff JA. Self-assembly of DNA—polymer complexes using template polymerization. Nucleic acids research. 1998;26(18):4178. doi: 10.1093/nar/26.18.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo SL, Wang S. An endosomolytic Tat peptide produced by incorporation of histidine and cysteine residues as a nonviral vector for DNA transfection. Biomaterials. 2008;29(15):2408–2414. doi: 10.1016/j.biomaterials.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 46.Dufourcq J, Neri W, Henry-Toulme N. Molecular assembling of DNA with amphipathic peptides. FEBS letters. 1998;421(1):7–11. doi: 10.1016/s0014-5793(97)01522-6. [DOI] [PubMed] [Google Scholar]
- 47.Perry HAD, Alhaj Saleh AF, Aojula H, Pluen A. YOYO as a Dye to Track Penetration of LK15 DNA Complexes in Spheroids: Use and Limits. Journal of Fluorescence. 2008;18(1):155–161. doi: 10.1007/s10895-007-0254-5. [DOI] [PubMed] [Google Scholar]
- 48.Saleh AF, Aojula H, Arthanari Y, Offerman S, Alkotaji M. Pluen A Improved Tat-mediated plasmid DNA transfer by fusion to LK15 peptide. Journal of Controlled Release. 143(2):233–242. doi: 10.1016/j.jconrel.2009.12.025. [DOI] [PubMed] [Google Scholar]