Abstract
Peripheral blood leukocytes incubated with a semisynthetic phage antibody library and fluorochrome-labeled CD3 and CD20 antibodies were used to isolate human single-chain Fv antibodies specific for subsets of blood leukocytes by flow cytometry. Isolated phage antibodies showed exclusive binding to the subpopulation used for selection or displayed additional binding to a restricted population of other cells in the mixture. At least two phage antibodies appeared to display hitherto-unknown staining patterns of B-lineage cells. This approach provides a subtractive procedure to rapidly obtain human antibodies against known and novel surface antigens in their native configuration, expressed on phenotypically defined subpopulations of cells. This approach does not depend on immunization procedures or the necessity to repeatedly construct phage antibody libraries.
Full text
PDF

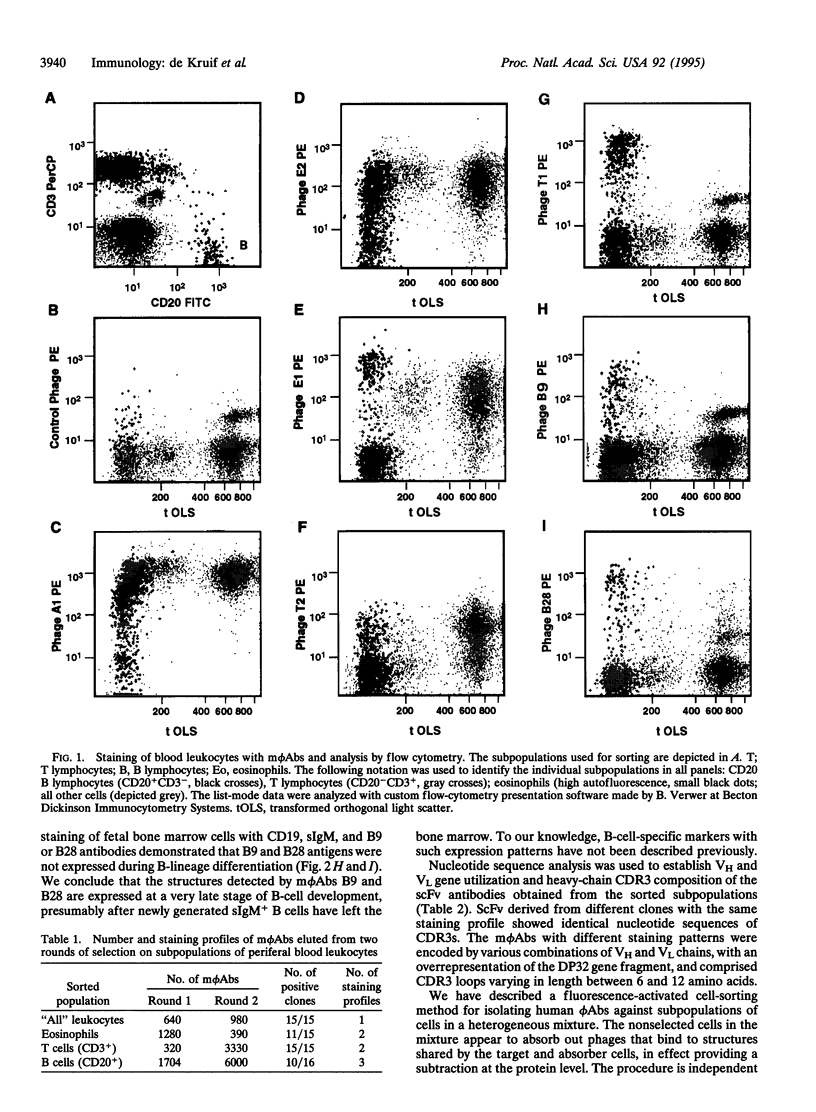
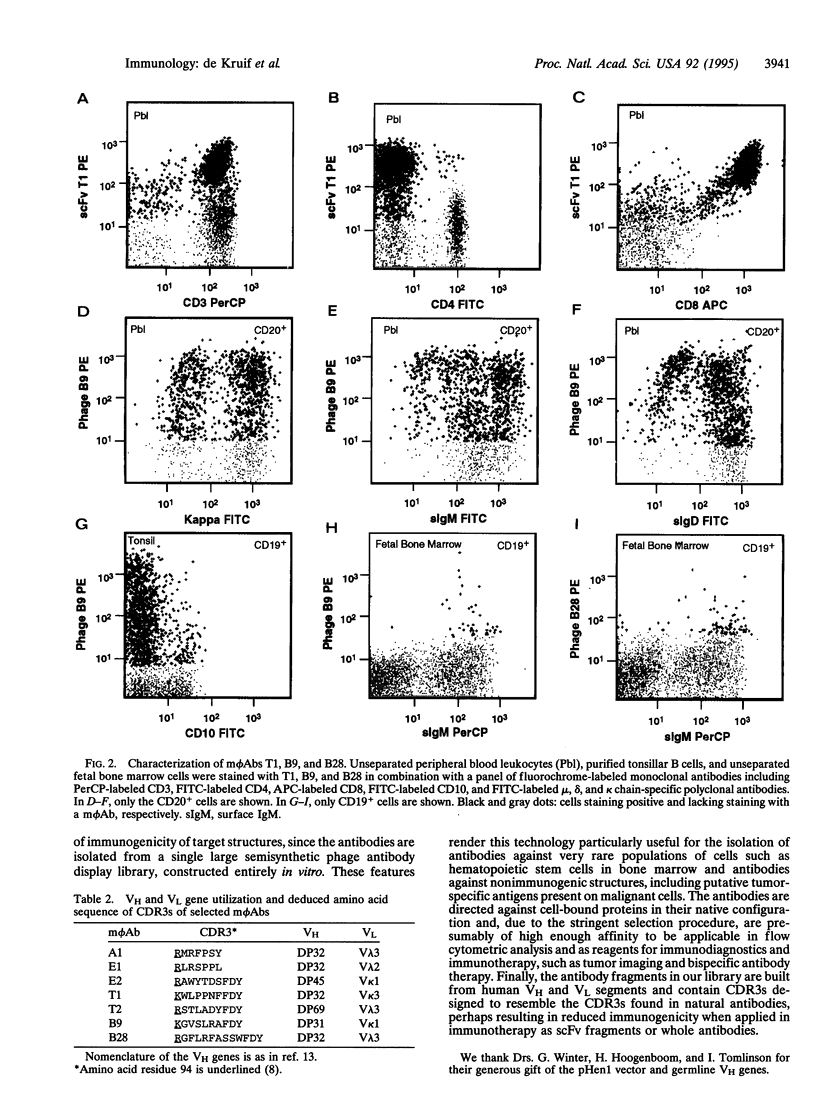

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kabat E. A., Wu T. T. Identical V region amino acid sequences and segments of sequences in antibodies of different specificities. Relative contributions of VH and VL genes, minigenes, and complementarity-determining regions to binding of antibody-combining sites. J Immunol. 1991 Sep 1;147(5):1709–1719. [PubMed] [Google Scholar]
- Marks J. D., Hoogenboom H. R., Bonnert T. P., McCafferty J., Griffiths A. D., Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991 Dec 5;222(3):581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Ouwehand W. H., Bye J. M., Finnern R., Gorick B. D., Voak D., Thorpe S. J., Hughes-Jones N. C., Winter G. Human antibody fragments specific for human blood group antigens from a phage display library. Biotechnology (N Y) 1993 Oct;11(10):1145–1149. doi: 10.1038/nbt1093-1145. [DOI] [PubMed] [Google Scholar]
- McCafferty J., Griffiths A. D., Winter G., Chiswell D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990 Dec 6;348(6301):552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- Meerman H. J., Georgiou G. Construction and characterization of a set of E. coli strains deficient in all known loci affecting the proteolytic stability of secreted recombinant proteins. Biotechnology (N Y) 1994 Nov;12(11):1107–1110. doi: 10.1038/nbt1194-1107. [DOI] [PubMed] [Google Scholar]
- Portolano S., McLachlan S. M., Rapoport B. High affinity, thyroid-specific human autoantibodies displayed on the surface of filamentous phage use V genes similar to other autoantibodies. J Immunol. 1993 Sep 1;151(5):2839–2851. [PubMed] [Google Scholar]
- Tomlinson I. M., Walter G., Marks J. D., Llewelyn M. B., Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J Mol Biol. 1992 Oct 5;227(3):776–798. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A. Monoclonal antibodies in diagnosis and therapy. Science. 1991 Jun 21;252(5013):1657–1662. doi: 10.1126/science.2047874. [DOI] [PubMed] [Google Scholar]
- Williamson R. A., Burioni R., Sanna P. P., Partridge L. J., Barbas C. F., 3rd, Burton D. R. Human monoclonal antibodies against a plethora of viral pathogens from single combinatorial libraries. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4141–4145. doi: 10.1073/pnas.90.9.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G., Griffiths A. D., Hawkins R. E., Hoogenboom H. R. Making antibodies by phage display technology. Annu Rev Immunol. 1994;12:433–455. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Johnson G., Kabat E. A. Length distribution of CDRH3 in antibodies. Proteins. 1993 May;16(1):1–7. doi: 10.1002/prot.340160102. [DOI] [PubMed] [Google Scholar]