Abstract
Background
Prior research suggests that 60–74% of males and 16–45% of females with fragile X syndrome (FXS) meet criteria for autism spectrum disorder (ASD) in research settings. However, relatively little is known about the rates of clinical diagnoses in FXS and whether such diagnoses are consistent with those performed in a research setting using gold standard diagnostic tools.
Method
This study explored whether boys and girls with FXS met criteria for ASD in a research setting using the Autism Diagnostic Observation Schedule (ADOS) and the Autism Diagnostic Interview-Revised (ADI-R), and then compared these data with the frequency of parent-reported clinical diagnoses. We also examined child and family characteristics as potential diagnostic predictors across settings. Participants included 35 females and 51 males with FXS (mean age: 10 years), who were from Eastern and Midwestern regions of the United States.
Results
About half of the children met criteria for ASD on either the ADOS or ADI-R, with ASD occurring three times more frequently in males than females (~75% versus ~25%). In contrast, ~25% of participants of both genders had received a clinical diagnosis of ASD. While cognitive and language skills predicted diagnostic outcome on the ADOS and ADI-R, these skills did not predict clinical diagnoses. Executive functions predicted clinical diagnoses, but not diagnoses per the ADOS or ADI-R.
Conclusions
ASD in FXS may be under-diagnosed in clinical/educational settings, which raises questions regarding access to ASD-related services.
Keywords: autism, fragile X syndrome, autism spectrum disorder, ADOS, ADI-R
Introduction
Fragile X syndrome (FXS) is the most common inherited cause of intellectual disability, and the most common known monogenic disability associated with autism spectrum disorder (ASD; Cohen et al. 2005). FXS occurs in 1 in 2,500 individuals (Hagerman 2008, Fernandez-Carvajal et al. 2009) and is caused by a mutation in the Fragile X Mental Retardation 1 (FMR1) gene. This mutation prevents the production of Fragile X Mental Retardation Protein (FMRP), a protein that is essential for brain development, giving rise to the neurocognitive phenotype that defines FXS (Loesch et al. 2004, Tassone et al. 1999). Notably, there is significant overlap in the behavioral phenotypes of FXS and ASD, including shared social and communication deficits (Hagerman and Hagerman 2002). While reported rates vary according to diagnostic method and participant characteristics (e.g., age, gender), it is estimated that 25–50% of males with FXS meet diagnostic criteria for autistic disorder in a research setting, which is defined by social and communication impairments, and the presence of restricted/repetitive behaviors (American Psychological Association 1994). Rates are much higher when considering the number of males who meet diagnostic criteria for ASD (i.e., an umbrella term, including autistic disorder as well as milder variants such as pervasive developmental disorder-not otherwise specified, or PDD-NOS). Sixty to seventy-four percent of males with FXS meet criteria for ASD in a research setting (Harris et al. 2008, Clifford et al. 2007, Rogers et al. 2001). Reported rates in females with FXS are lower than those in males, with 13–20% meeting criteria for autistic disorder, and 45% for ASD (Clifford et al. 2007, Hall et al. 2008). See Table 1 for detailed review of reported rates of ASD in FXS.
Table 1.
Reported Rates of ASD among Individuals with FXS
| Authors (Year) | Sample characteristics | Diagnostic procedures | Detected rates of Autism |
|---|---|---|---|
| Bailey, Raspa, Olmsted, & Holiday (2008) | 976 males; 259 females (national survey) 6–30+ years (Mage not reported) |
Caregivers asked: “Has this child ever been diagnosed or treated by a medical professional for any of the following conditions… autism?” |
Males: 46% Females: 16% |
| Clifford, Dissanayake, Bui, Huggins, Taylor, & Loesch (2007) | 33 males; 31 females (ascertained via clinical referrals) 5–60 years (M23.2) |
Participants who scored above SCQ thresholds were administered the ADI-R & ADOS |
Males: 36% on ADI-R; 67% on ADOS for autism/spectrum; 27% on ADI-R+ ADOS autism/spectrum Females: 3% on ADI-R; 13% on ADOS for autism/spectrum; 13% on ADI-R+ ADOS autism/spectrum |
| Garcia-Nonell, Ratera, Harris, Hessl, Ono, et al. (2008) | 90 males 3–25 years (M ~8) |
ADOS, DSM-IV | 63% on ADOS for autism/spectrum + DSM-IV for autism or PDD-NOS |
| Hall, Lightbody, & Reiss (2008) | 31 males; 29 females 5–20 years (M~13) |
ADOS |
Males: 74% for autism/spectrum Females: 45% for autism/spectrum |
| Harris, Hessl, Bacalman, Goodlin-Jones, Ferranti, et al. (2008) | 63 males 2–19 years (M7.9) |
ADI-R, DSM-IV & ADOS | 49% on ADI-R; 49% on ADOS for autism/spectrum; 35% on DSM-IV for autism; 59% on DSM-IV for PDD-NOS; 27% on ADI-R+DSM-IV+ADOS autism/spectrum |
| Hernandez, Feinberg, Vaurio, Passanante, Thompson, et al. (2009) | 56 males Age range not reported (M 4.7 years |
ADI-R, DSM-IV | 43% on ADI-R+DSM-IV |
| Rogers, Wehner, & Hagerman (2001) | 23 males; 1 female (ascertained via clinical referrals) 2–4 years (M 2.9) |
ADOS, DSM-IV, & ADI-R (used reduced cut-off scores for the ADI-R repetitive behavior domain) | 46% on ADI-R; 33% on ADI-R+ DSM-IV+ ADOS autism/spectrum |
Note: ADOS rates are reported for children who met criteria for either “autism” or “autism spectrum”. ADI-R rates report the percentage of children who met criteria of “autism” (the ADI-R does not provide diagnostic cut-offs for “autism spectrum”). ADOS=Autism Diagnostic Observation Scale; ADI-R=Autism Diagnostic Interview-Revised; SCQ=Social Communication Questionnaire; DSM-IV=American Psychiatric Association Diagnostic and Statistical Manual-4th Edition; PDD-NOS=Pervasive developmental disorder-not otherwise specified.
While several research groups have examined the rate of ASD comorbidity in FXS within a research context, much less is known about the rate at which individuals with FXS are diagnosed with ASD in real-life clinical or educational settings. Our knowledge of this topic is limited to one report by Bailey and colleagues (2008), who found that ~45% of males and ~15% of females were reported to have been diagnosed with and/or treated for autism in a large, nationally-representative survey of families of individuals with FXS. While these figures are generally consistent with the rates of research-derived diagnoses of autistic disorder, they are considerably lower than rates reported for ASD. Thus, questions remain regarding clinical ASD diagnostic practices and how these diagnoses might agree with estimates using gold standard diagnostic tools. Better understanding of ASD diagnostic practices in FXS has important implications for clinical practice, given that diagnostic classification may dictate the type/intensity of services that a child will qualify for in certain medical and educational settings.
Research strongly suggests that ASD comorbidity has a significant impact on functional outcomes for individuals with FXS. Children with FXS and co-occurring ASD present with a behavioral phenotype that is distinct from that of FXS without ASD, and which is characterized by more significant cognitive impairment (Bailey et al. 2000, Bailey et al. 2001, Clifford et al. 2007, Loesch et al. 2007, Dissanayake et al. 2009, Rogers et al. 2001), greater social impairment (Hernandez et al. 2009, Kau et al. 2004, Budimirovic et al. 2006, Kaufmann et al. 2004, Hessl et al. 2006), poorer adaptive functions (Rogers et al. 2001, Kau et al. 2004), increased likelihood for developing secondary medical conditions (Garcia-Nonell et al. 2008), and, according to some reports, decreased receptive language skills (Lewis et al. 2006, Rogers et al. 2001). Furthermore, it appears that ASD symptoms in FXS remain relatively stable over time (Hernandez et al. 2009, Sabaratnam et al. 2003), suggesting that children are not likely to “grow out of” autistic behaviors without specialized intervention. Given evidence that individuals with FXS and comorbid ASD are at risk for suboptimal outcomes, ASD comorbidity should be a key factor in determining the type and intensity of interventions for this population. Further, because individuals with FXS and ASD may present with unique phenotypic profiles, it is essential to consider the presence of ASD features in studies of behavior, brain, and genetic effects in FXS.
Better understanding of the use of ASD diagnostic tools in FXS is also critical for research. It is unclear how different diagnostic tools might be better able to tease apart these complex phenotypes of FXS, and how the use of different tools might influence study results. For example, in a longitudinal study of 56 boys with FXS, Hernandez et al. (2009) found that the Autism Diagnostic Observation Schedule (ADOS) over-identified children with FXS as having ASD as compared to the Autism Diagnostic Interview-Revised (ADI-R) and the Diagnostic and Statistical Manual of Mental Health Disorders (DSM-IV; American Psychological Association 1994). On the other hand, in a different study of 63 boys, Harris et al. (2008) suggested that the ADI-R over-identified ASD in FXS, presumably because of its focus on behaviors during early childhood, when autistic-like symptoms may be most prominent. Clarifying best practices for diagnosing ASD within the context of FXS will allow for more efficient and accurate phenotypic characterization, which is a foundational step in answering broader questions regarding the role of ASD in biobehavioral pathways of FXS.
Recently, FXS has been proposed as a genetic model for informing the etiological basis of ASD (e.g., Abrahams and Geschwind 2010, Belmonte and Bourgeron 2006, Hagerman et al. 2011). As a single-gene disorder that is associated with significant genetic risk to ASD, FXS provides an opportunity to link a known genetic mutation with core behavioral and biological features associated with ASD. FMR1 interacts with other background genes that are involved in ASD, thereby reducing the genetic threshold needed to produce ASD (Bailey et al. 2001, Rogers et al. 2001). A number of molecular-genetic studies support this hypothesis with direct evidence indicating that the protein that is encoded by FMR1, FMRP, is involved in the regulation of a number of autism susceptibility genes (Iossifov et al. 2012, Darnell et al. 2011, Darnell and Klann 2013). For example, a significant proportion of autism candidate genes are now understood to interact with FMRP (Darnell and Klann 2013), and a number of proteins that are implicated in idiopathic ASD (including SHANK3, CYFIP1, PTEN, and NRXNI) are regulated by FMR1-associated mechanisms such as FMRP and components of the mGluR signaling pathway (Bagni et al. 2012, Iossifov et al. 2012, Darnell et al. 2011, Darnell and Klann 2013, Hagerman et al. 2010, De Rubeis and Bagni 2011). Clarifying the rate at which ASD occurs in FXS (and how diagnostic practices impact this estimate) will contribute to our understanding of the coupling between the FMR1 mutation and the development of ASD.
This study examined rates of ASD among boys and girls with FXS by comparing the children’s history of clinical diagnoses of ASD with diagnostic assessments conducted using gold standard instruments in a controlled research setting. The concordance of ASD status across diagnostic methods was examined to inform diagnostic patterns that have implications for clinical and research practices in FXS. Finally, potential predictors of ASD diagnoses were examined, in order to shed light on factors that might influence the identification of ASD in FXS.
Methods
Participants
Eighty-six children with FXS participated in the study (51 boys and 35 girls). The average age of all participants was 10.77 years (SD = 3.64), with a mean age of 11.75 (SD = 3.11) for boys and 9.38 (SD = 3.91) for girls. The children who participated in this study were recruited from a larger, longitudinal study of language in FXS (Losh et al., 2012). Children were selected for participation from the larger pool if they had been administered the Family History Interview (which assesses clinical diagnostic history) and either of the gold standard diagnostic tools, all described below. Participants were ascertained through medical facilities and parent groups, and through the Research Participant Registry Core of the Carolina Institute for Developmental Disabilities at the University of North Carolina at Chapel Hill. Recruitment was based in the Eastern and Midwestern regions of the United States. As inclusionary criteria for the larger study, all participants were verbal (using three-word phrases) and were native speakers of English. See Table 2 for demographic characteristics.
Table 2.
Participant Characteristics
| Full sample (N = 86) | Males (n = 51) | Females (n = 35) | |
|---|---|---|---|
| Age in years | |||
| M (SD) | 10.77 (3.64) | 11.75 (3.11) | 9.38 (3.91) |
| Range | 4.23–17.90 | 6.47–17.90 | 4.23–15.87 |
| Mental age in years | |||
| M (SD) | 5.79 (1.90) | 5.11 (0.60) | 6.77 (2.60) |
| Range | 3.50–14.92 | 3.50–6.67 | 3.92–6.77 |
| FSIQ | |||
| M (SD) | 64.22 (24.05) | 49.94 (12.99) | 84.63 (21.25) |
| Range | 36.00–124.00 | 36.00–79.00 | 38.00–124.00 |
| Maternal education | |||
| M (SD) | 15.72 (2.06) | 15.79 (2.13) | 15.62 (2.00) |
| Race | |||
| White | 79.1% | 84.3% | 71.4% |
| African American | 5.8% | 3.9% | 8.6% |
| Other | 9.3% | 7.8% | 11.4% |
| Household Income | |||
| <20k | 7.7% | 5.7% | 7.7% |
| 40–79k | 20.5% | 22.9% | 21.5% |
| >80k | 70.8% | 71.4% | 70.8% |
Procedures
Assessments were administered within the context of the broader research protocol. While all child-based measures were administered concurrently, it was not always possible to administer the caregiver-report measures (the Family History Interview [Bolton et al., 1994] and the Autism Diagnostic Interview-Revised [ADI-R; Lord et al., 1994], described below) concurrently with the child assessments. An average of 11 months passed between administration of the Family History Interview and the child assessments, and an average of six months between the ADI-R and child assessments. Procedures were approved by the institutional review boards of the University of North Carolina at Chapel Hill and Northwestern University.
ASD Diagnostic Procedures
Gold standard ASD assessments
Two ASD diagnostic assessments were administered: the Autism Diagnostic Interview-Revised (ADI-R) and the Autism Diagnostic Observation Schedule (ADOS). In the ADI-R, an examiner leads the caregiver through an interview designed to elicit descriptions of the child’s social, communicative, and repetitive/restricted/stereotyped behaviors. The diagnostic algorithm is based on symptoms exhibited during early childhood (focused on 4–5 years of age), and yields a diagnosis of “autism” or “no autism”. In order to meet diagnostic criteria for “autism” participants must meet thresholds for four symptom domains: social interaction, communication, restricted/repetitive/stereotyped behavior, and onset before three years. The ADI-R was coded by examiners who had achieved research reliability per the standards of the instrument developers.
The second gold standard diagnostic instrument, the ADOS, bases diagnoses on direct observation of behaviors during a play-based interaction. All examiners were trained to reliably code the ADOS either through training with the developers of the ADOS or through intra-lab reliability, in accordance with the standards of the instrument developers. Classification of “autism”, “autism spectrum” and “no autism” was determined using the revised ADOS algorithms (Gotham et al. 2007), which consider information from all three domains of the autism triad in determining diagnostic status (whereas the original algorithms did not take into account restricted/repetitive features). Individuals who met diagnostic thresholds for either “autism” or “autism spectrum” were considered “positive” for an ASD diagnosis. This strategy was chosen as it is consistent with the DSM-5 diagnostic criteria for ASD, which collapse autistic disorder and PDD-NOS into the single category, “autism spectrum disorder” (American Psychiatric Association 2013). ADOS diagnostic information was missing for one male, who was unable to complete the assessment due to problem behaviors.
Clinical diagnoses of ASD
Clinical diagnoses of ASD were determined via caregiver report within the context of an abbreviated Family History Interview (Bolton et al. 1994), which was most often completed by the participant’s mother prior to the ADI-R. Within the context of a broader interview about family structure and medical histories, caregivers were asked whether anyone in their family (including their child) had a diagnosis of autism or autism spectrum disorder. While the Family History Interview is a well-established method for evaluating autism family history, reports are based solely on caregiver recollection of whether the child had been evaluation and what the outcome was. Therefore, two circumstances might apply to children who were reported to be negative for a clinical diagnosis: (1) the child had been evaluated but did not meet criteria for an ASD diagnosis or (2) the child had never been referred/evaluated for ASD diagnostics. Children whose caregivers reported a diagnosis of autism, autism spectrum disorder, or PDD-NOS were considered “positive” for a clinical diagnosis of ASD.
Predictors of ASD Diagnosis
Cognitive and language ability
Nonverbal cognitive ability was assessed using the Leiter International Performance Scale-Revised Brief IQ (Leiter-R; Roid and Miller 1997). Receptive and expressive language ability were also measured, with the Peabody Picture Vocabulary Test-III (PPVT-III ; Dunn and Dunn 1997) and the Expressive Vocabulary Test (EVT; Williams 1997), respectively. Age equivalent scores were computed for each measure from published norms.
Executive functions
The Behavior Rating Inventory of Executive Function-Preschool Version (Brief-P; Gioia et al. 2000) was used as a caregiver-report scale of executive functions such as inhibition, shifting emotional control, and planning/organizing. The preschool version was administered as the younger items were judged to be more closely aligned with the mental age of the participants. Because a number of participants were thus outside of the age range of the normative sample for the Brief-P, raw scores of the Global Executive Composite were used in analysis.
Behavioral and emotional problems
The Child Behavior Checklist-1 ½–5 years (CBCL; Achenbach and Rescorla 2000) was completed by the primary caregiver to assess child maladaptive behavioral and emotional problems. The total score was examined, which includes a range of symptoms such as social problems, anxiety, and destructive behavior. Like the Brief-P, the younger version of the CBCL was administered as the items were thought to be more applicable to the functioning level of the participants. Raw scores were used, which is recommended by the test publishers (Achenbach and Rescorla 2000).
Socioeconomic factors
Maternal education level (in years) and household income (<39,000, 40,000–79,000, 80,000) were collected using a standard demographic survey.
Analysis strategy
Descriptive statistics were used to examine the rates of ASD diagnoses. Gender differences in the rates of ASD were tested using Fisher’s exact test. The concordance of clinical and gold standard diagnoses was determined by calculating point-to-point “agreement” for each participant. The term “agreement” is used throughout to describe the concordance of the various ASD diagnostic methods. However, it should be noted that the Family History Interview does not include extensive clinical evaluation. As such, reports of clinical diagnostic status were based solely on caregiver recollection of whether children had been evaluated and what the outcome was. Therefore, using the Family History Interview we were unable to determine whether a “negative” agreement might in some cases reflect children never having been evaluated for ASD. Finally, the contribution of the predictor variables to diagnostic outcomes was examined with a series of binary logistic regression models. Because children who are older may have had more opportunities to have been evaluated for ASD (Bailey et al. 2008), chronological age was covaried in the models to facilitate comparison across genders.
Results
Rates of ASD Across Diagnostic Methods
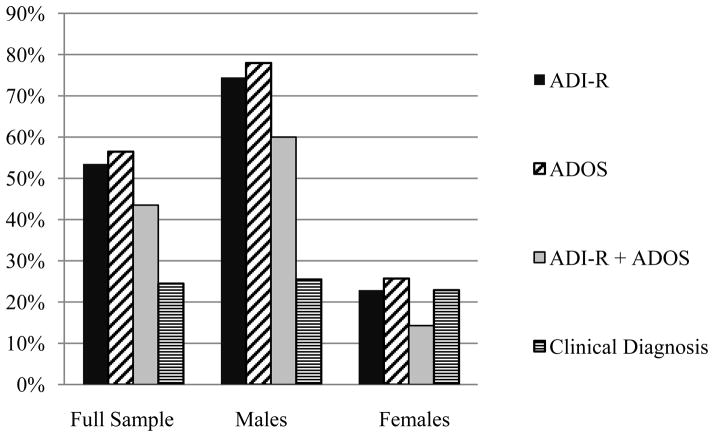
Using the stringent criterion of meeting cut-off on both the ADI-R and the ADOS, 43.5% of participants met criteria for ASD overall. Sixty percent of the males met the stringent criteria, and 14.3% of the females (see Figure 1). Using the ADI-R only, just over half of the sample (53.5%) met criteria for ASD. Among the males, 74.5% met ADI-R cut-offs, while 22.9% of the females did. Slightly more participants (56.5%) scored above ADOS diagnostic cut-offs. Seventy-eight percent of the males and 25.7% of the females met ADOS criteria. Significant gender effects were detected for the rate of diagnoses per the ADOS, ADI-R, and ADI-R+ADOS (ps < .001), with more males diagnosed with ASD than females. Twenty-five percent of children (25.5% of males and 22.9% of females) had been diagnosed with ASD clinically, and no gender effects were detected (p = .494).
Figure 1.
Percent of Children Meeting Criteria for ASD
Agreement Among Diagnostic Methods
Agreement between clinical diagnosis and the ADI-R+ADOS
Using stringent research classification (meeting cut-offs for both the ADI-R and the ADOS), the agreement with clinical diagnosis was 68% in the full sample. More individuals were identified as positive for ASD on the ADI-R+ADOS than by clinical diagnosis. Sixty percent of individuals who met criteria on the ADI-R+ADOS did not have a clinical diagnosis, whereas 30% who had a clinical diagnosis did not meet ADI-R+ADOS criteria.
For males, the ADI-R+ADOS classification was concordant with clinical diagnoses 56% of the time. Of the 30 males who were positive on the ADI-R+ADOS, 66.6% had not received a clinical diagnosis. Two males had received a clinical diagnosis without also meeting diagnostic criteria on the ADI-R+ADOS. For females, agreement was 85.7%. One of the five females who were identified with the ADI-R+ADOS did not have a clinical diagnosis (20%), and 50% of the females had a clinical diagnosis yet did not meet thresholds on both the ADI-R and ADOS.
Agreement between clinical diagnosis and the ADI-R
Agreement between the ADI-R and caregiver reports of clinical diagnoses was 68.6% overall (see Table 3). More individuals were identified as positive on the ADI-R than by clinical diagnosis; 55.6% of the individuals who were identified with the ADI-R did not have a clinical diagnosis (whereas 5% of those who had a clinical diagnosis did not meet ADI-R thresholds). For males, clinical diagnoses agreed with the ADI-R in 51% of cases, with discrepancies related to higher identification with the ADI-R than clinical diagnosis (65.8% of the males who were identified with the ADI-R did not have a clinical diagnosis). For females, overall agreement was 94.3%; 12.5% were positive on the ADI-R but not clinically and 12.5% had a clinical diagnosis yet did not meet ADI-R criteria.
Table 3.
Number of Children Identified as Positive or Negative for ASD via Gold Standard Diagnostic Tools in Comparison to Clinical Diagnoses
| Clinical Diagnosis
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Full Sample
|
Males
|
Females
|
|||||||
| Pos. | Neg. | Total | Pos. | Neg. | Total | Pos. | Neg. | Total | |
|
|
|
||||||||
| ADI-R+ADOS | |||||||||
| Positive | 14 | 21 | 35 | 10 | 20 | 30 | 4 | 1 | 5 |
| Negative | 6 | 44 | 50 | 2 | 18 | 20 | 4 | 26 | 30 |
| Total | 20 | 65 | 85 | 12 | 38 | 50 | 8 | 27 | 35 |
|
| |||||||||
| ADI-R | |||||||||
| Positive | 20 | 26 | 46 | 13 | 25 | 38 | 7 | 1 | 8 |
| Negative | 1 | 39 | 40 | 0 | 13 | 13 | 1 | 26 | 27 |
| Total | 21 | 65 | 86 | 13 | 38 | 51 | 8 | 27 | 35 |
|
| |||||||||
| ADOS | |||||||||
| Positive | 14 | 34 | 48 | 10 | 29 | 39 | 4 | 5 | 9 |
| Negative | 6 | 31 | 37 | 2 | 9 | 11 | 4 | 22 | 26 |
| Total | 20 | 65 | 85 | 12 | 38 | 50 | 8 | 27 | 35 |
Note: ADI-R=Autism Diagnostic Interview-Revised. ADOS=Autism Diagnostic Observation Schedule
Agreement between clinical diagnosis and the ADOS
Within the full sample, 52.9% of the caregiver-reported clinical diagnoses agreed with ADOS classification. Agreement was lower among males than females (40% versus 74.3%, respectively). Diagnostic disagreements were most often related to higher identification rates with the ADOS (70.8% of children who met ADOS criteria did not have a clinical diagnosis). Among males, 74.4% met criteria on the ADOS but did not have a clinical diagnosis, whereas 16% had a clinical diagnosis but did not reach ADOS thresholds. Of the females, 55.6% were positive on the ADOS yet did not have a clinical diagnosis. Fifty percent of the females who had received a clinical diagnosis did not meet ASD criteria (see Table 3).
Agreement between ADI-R and ADOS
The ADI-R and ADOS agreed 76.5% of the time in the full sample. Twenty-two percent of children who were positive on the ADI-R were not identified with the ADOS, and 27.1% of those who were positive on the ADOS were negative on the ADI-R. ADOS and ADI-R agreement was lower for males (68%) than females (80%). Discrepancies did not appear to be related to one instrument over-identifying ASD in comparison to the other; see Table 4).
Table 4.
Number of Individuals Identified as Positive or Negative for ASD According to the ADI-R and ADOS.
| ADI-R
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Full Sample | Males | Females | |||||||||
|
| |||||||||||
| Pos. | Neg. | Total | Pos. | Neg. | Total | Pos. | Neg. | Total | |||
|
|
|||||||||||
| ADOS | Positive | 35 | 13 | 48 | 30 | 9 | 39 | 5 | 4 | 9 | |
| Negative | 10 | 27 | 37 | 7 | 4 | 11 | 3 | 23 | 26 | ||
| Total | 45 | 40 | 85 | 37 | 13 | 50 | 8 | 27 | 35 | ||
Note: ADI-R=Autism Diagnostic Interview-Revised; ADOS=Autism Diagnostic Observation Schedule
Predictors of ASD Diagnosis
Cognitive and language predictors
In the full sample, nonverbal mental age, receptive vocabulary, and expressive vocabulary were significant predictors of ADI-R and ADOS diagnoses (with the odds of having ASD decreasing as each of these skills increased). Mental age, receptive vocabulary, and expressive vocabulary did not predict clinical diagnoses (see Table 5). No significant predictors of ASD emerged among the subgroups of males or females, although there was a trend for nonverbal mental age as a predictor of ADI-R status among females (OR= .47, p = .088).
Table 5.
Regression Coefficients Showing Cognitive and Language Factors as Predictors of ASD Classification
| Leiter-R
|
PPVT-III
|
EVT
|
||||
|---|---|---|---|---|---|---|
| B (SE) | OR (95% CI) | B (SE) | OR (95% CI) | B (SE) | OR (95% CI) | |
| ADI-R | ||||||
| Full Sample | −1.03 (0.32)** | 0.36 (0.19–0.67) | −0.42 (0.13)** | 0.66 (0.50–0.86) | −0.44 (0.14)** | 0.64 (0.49-.847) |
| Males | −0.96 (0.63) | 0.38 (0.11–1.32) | −0.26 (0.25) | 0.77 (0.48–1.25) | −0.12 (0.25) | 0.99 (0.62–1.58) |
| Females | −0.76 (0.44) † | 0.47 (0.20–1.12) | −0.21 (0.22) | 0.81 (0.53–1.24) | −0.48 (0.32) | 0.62 (0.33–1.14) |
| ADOS | ||||||
| Full Sample | −0.77 (0.24)** | 0.46 (0.28–0.74) | −0.40 (0.13)** | 0.67 (0.52–0.87) | −0.38 (0.13)** | 0.69 (0.53–0.89) |
| Males | −0.82 (0.66) | 0.44 (0.12–1.61) | −0.12 (0.27) | 0.89 (0.52–1.50) | −0.11 (0.26) | 0.89 (0.54–1.47) |
| Females | −0.43 (0.28) | 0.65 (0.38–1.12) | −0.24 (0.19) | 0.79 (0.54–1.15) | −0.16 (0.18) | 0.86 (0.61–1.21) |
| Clinical Diagnosis | ||||||
| Full Sample | −0.29 (0.23) | 0.75 (0.47–1.18) | −1.00 (0.13) | 0.91 (0.71–1.16) | −0.09 (0.12) | 0.91 (0.72–1.15) |
| Males | −0.12 (0.60) | 0.89 (0.27–2.89) | −0.23 (0.25) | 0.79 (0.49–1.29) | 0.11 (0.24) | 1.12 (0.70–1.79) |
| Females | −0.55 (0.36) | 0.58 (0.28–1.18) | −0.09 (0.19) | 0.92 (0.63–1.33) | −0.30 (0.26) | 0.74 (0.44–1.23) |
Note:
p < .01,
p < .09.
All models adjusted for chronological age. ADI-R=Autism Diagnostic Interview-Revised; ADOS=Autism Diagnostic Observation Schedule; PPVT-III=Peabody Picture Vocabulary Test; EVT=Expressive Vocabulary Test; Leiter-R=Leiter International Performance Scale-Revised.
Behavioral predictors
Executive functions and behavioral/emotional control were not significant predictors of diagnostic outcome on the ADOS or ADI-R; however, there were trends for increased likelihood of a diagnosis on the ADI-R as behavioral/emotional problems increased, and for increased likelihood of ADOS diagnosis with greater executive impairment (see Table 6). Executive function significantly predicted caregiver-reported clinical diagnoses, with increased odds of having a diagnosis with greater executive impairment after controlling for age (OR = 1.04, p = .013). In the gender subgroups, executive function significantly predicted clinical diagnoses in males but not females. Behavioral/emotional problems did not significantly predict ADOS or ADI-R outcomes, although a trend was detected for increased odds of a clinical diagnosis with more significant behavioral/emotional difficulties (OR = 1.02, p = .062).
Table 6.
Regression Coefficients Showing Executive Function and Behavioral Control as Predictors of ASD Classification
| Brief-P Global Executive Composite
|
CBCL Total Score
|
|||
|---|---|---|---|---|
| B (SE) | OR (95% CI) | B (SE) | OR (95% CI) | |
| ADI-R | ||||
| Full Sample | 0.01 (0.01) | 1.01 (0.99–1.03) | 0.02 (0.01) | 1.02 (1.00–1.05) |
| Males | 0.01 (0.01) | 1.01 (0.98–1.03) | 0.02 (0.19) | 1.02 (0.99–1.06) |
| Females | 0.02 (0.02) | 1.02 (0.98–1.06) | 0.02 (0.02) | 1.02 (0.98–1.07) |
| ADOS | ||||
| Full Sample | 0.02 (0.01) † | 1.02 (1.00–1.04) | 0.01 (0.01) | 1.01 (0.99–1.04) |
| Males | 0.02 (0.02) | 1.02 (0.99–1.05) | −0.01 (0.02) | 1.00 (0.96–1.03) |
| Females | 0.02 (0.02) | 1.02 (0.98–1.06) | 0.03 (0.02) | 1.03 (0.99–1.07) |
| Clinical Diagnosis | ||||
| Full Sample | 0.02 (0.09)* | 1.04 (1.01–1.07) | 0.02 (0.01) † | 1.02 (1.00–1.05) |
| Males | 0.05 (0.02)* | 1.05 (1.01–1.10) | 0.02 (0.02) | 1.02 (0.99–1.06) |
| Females | 0.02 (0.02) | 1.02 (0.98–1.07) | 0.02 (0.02) | 1.02 (0.98–1.07) |
Note:
p < .05,
p < .09.
All models adjusted for chronological age. BRIEF-P= Behavior Rating Inventory of Executive Function-Preschool Version; CBCL=Child Behavior Checklist; ADI-R= Autism Diagnostic Interview-Revised; ADOS=Autism Diagnostic Observation Schedule;
Socioeconomic predictors
Neither maternal education level nor household income significantly predicted ADI-R, ADOS or clinical diagnoses. There was, however, a trend for decreased likelihood of diagnosis per the ADOS as income level increased from the <40k to 40–79k income bracket (OR = 11.37, p = .063).
Discussion
This study examined the agreement between ASD diagnoses derived from clinical and research settings in boys and girls with FXS and examined potential correlates of ASD diagnoses. While high agreement was observed between the ADOS and ADI-R administered as part of a research protocol, there was poor agreement between these measures and caregiver-reported clinical diagnoses; over half of the children who met diagnostic criteria for ASD in the research protocol were not identified as having ASD clinically.
Approximately 65% of the boys and 20% of the girls who met criteria for ASD as part of the research evaluation had not been previously identified as having ASD in other settings, according to caregiver report. This suggests that a significant proportion of children with FXS who would meet diagnostic criteria for ASD are not identified clinically (although it is unknown whether this is due to under-referral of children with FXS for ASD diagnostics versus false negatives in clinical evaluation). Boys in particular were likely to meet criteria for ASD in the research setting despite being negative for a clinical diagnosis, suggesting that under-diagnosis may be common among boys with FXS. While more research is needed to investigate this finding, it is possible that ASD comorbidity is more likely to be overlooked in boys with FXS, who are generally more affected than girls and may be more likely to receive services under a diagnosis of “intellectual disability” as opposed to “ASD”. Because girls with FXS are less affected, the presentation of ASD among girls may also stand out more clearly, drawing the attention of caregivers and health/educational professionals.
Under-diagnosis of ASD in individuals with FXS has important implications for services, given that in many settings diagnostic classification precedes (and often dictates) the type/intensity of services. Because diagnostic classification serves as a guide for health practitioners and educators, professionals who are aware of ASD comorbidity might be better able to tailor treatment towards ASD-related symptoms. For example, health care providers who are aware of ASD comorbidity might be more watchful of secondary medical conditions that are more common in FXS with co-occurring ASD, such as seizures (Garcia-Nonell et al. 2008). Overall, more accurate diagnostic information can only assist in making informed medical/educational decisions.
While findings support a clear trend of higher identification with the ADOS/ADI-R as compared to clinical diagnoses, there was a subset of children who were identified clinically but did not meet criteria for ASD in the research setting. This pattern appeared to be specific to the ADOS, which failed to identify 30% of the children with a clinical ASD diagnosis, rather than the ADI-R (which missed less than 1% of children). In particular, the ADOS appeared to be less sensitive in identifying ASD in females, missing half of the females who had received a clinical diagnosis. Additional research is needed to determine the cause of this pattern. It is possible that females with FXS are adept at compensating for social difficulties during short-lived interactions such as the ADOS and therefore do not show sufficient features to meet diagnostic criteria on this measure, whereas clinical diagnoses and the ADI-R are able to incorporate more comprehensive information regarding the child’s behaviors in everyday settings.
In attempts to inform potential reasons for under-diagnosis, this study explored factors that may make an individual more likely to be referred, evaluated, and eventually diagnosed with ASD. Contrary to expectations, we found that mental age and language skills did not predict clinical diagnoses (although they did predict ADOS/ADI-R diagnoses, as would be expected given that ASD comorbidity in FXS is associated with greater impairment). Perhaps health professionals expect language and cognitive delays as a part of FXS, and therefore are likely to overlook red flags for ASD when a child is also struggling with language and cognitive skills. Rather, clinical diagnoses became more likely as children showed greater deficits in executive functions. This suggests that regulatory management deficits (e.g., impulsivity, cognitive inflexibility) may be salient features that prompt health professionals to assess for ASD. Surprisingly, socioeconomic variables did not predict clinical diagnoses (families with more resources would be expected to have better access to services). Perhaps this study was under-powered to detect effects; or, the income categories may have been too broad to capture subtle trends. Other factors not measured in this study, such as personal beliefs about the nature of ASD in FXS, may also have a substantial influence on diagnostic patterns. Anecdotally, many caregivers commented during the clinical interview that although their child shows symptoms that are consistent with ASD, their child does not have ASD because such symptoms are caused by FXS. The question of whether ASD within FXS represents “true” autism is a question that researchers, clinicians, and families alike have grappled with, and one that has immense implications for diagnosis and intervention. Stakeholders who hold the view that ASD symptoms are not independent from FXS may be less likely to pursue ASD-related services.
Findings were generally consistent with prior reports. In females, this study detected rates of 20–25% across the ADOS and ADI-R, which is consistent with prior estimates ranging from 3–45% (Hall et al., 2008, Clifford et al., 2007; McDuffie et al., 2010). For males, the rates detected with the ADOS were comparable to existing studies, whereas the rates detected with the ADI-R were elevated, with approximately three-fourths of the boys meeting diagnostic thresholds on the ADI-R, in contrast to 45–50% in prior research (Harris et al., 2008; Rogers et. al. 2001). Differences in participant characteristics may account for discrepant findings; this study excluded nonverbal individuals, which may have led to the inclusion of a subgroup of children who were more likely to meet ADI-R diagnostic thresholds. In fact, Kaufmann et al. (2004) found that better expressive language skills predicted more severe ADI-R scores in boys with FXS. A report by McDuffie et al. (2010) also examined a group of verbal boys with FXS and found that 63% met diagnostic criteria on the ADI-R.
In general, the ADOS and the ADI-R yielded comparable rates of ASD. Within individual participants, agreement between the ADOS and ADI-R was high (~70% for boys and ~80% for girls), supporting their use with individuals with FXS. It is unclear how the ADOS/ADI-R agreement detected in this study compares with that of prior research, as no studies to our knowledge have reported concordance/discordance of the two measures in FXS. That is, prior studies have not reported the diagnostic agreement across tools at the participant level, although a number of studies have compared overall diagnostic rates per instrument. Even if both instruments detected similar group rates of ASD, this does not necessarily indicate that both tools identified the same participants as having ASD.
While this study provides a starting point for understanding ASD diagnostic practices in FXS, it is still unclear why ASD in FXS appears to be under-diagnosed (e.g., whether the low rates of clinical diagnoses were caused by breakdowns in the referral process versus false-negatives in clinical evaluation). As noted previously, the clinical diagnoses did not take into account whether participants had been evaluated for ASD--it is possible that some children were simply never referred for diagnostic evaluation. Along these lines, clinical diagnoses in this study reflect whether caregivers believed that their child had ASD, disregarding the setting from which the child’s diagnosis was received. Future studies investigating the sources of clinical diagnoses (medical, educational, research, or otherwise) would be invaluable in understanding diagnostic practices in FXS. Nonetheless, caregiver beliefs about their child’s ASD status are likely to be clinically meaningful (regardless of the source of the diagnosis), as caregivers who believe that their child has ASD may seek out services that differ in type or intensity. Currently, it is unknown whether knowledge of ASD-status leads to improved educational and medical outcomes for children with FXS. Research investigating the functional implications of clinical ASD diagnoses in FXS is needed to understand the potential consequences of clinical under-diagnosis of ASD.
A limitation of this study is that only verbal individuals with FXS were studied, which might limit generalizability. Findings should also be replicated in different regions of the US and other countries, where diagnostic practices may vary. This study is also limited to examination of a single time point and cannot account for potential changes in the presentation of ASD across the lifespan. Finally, this study operated under the assumption that the ADOS and ADI-R represented the participant’s “true” diagnostic classification. Although these tools are widely used with high sensitivity and specificity, they are not comprehensive and should be used within the context of a broader clinical assessment.
In conclusion, this study identified rates of ASD comorbidity in FXS at about 75% for males and 25% for females, using gold-standard diagnostic tools. Strikingly, a significant proportion of children with FXS who were identified as having ASD in the research setting had not been identified clinically. Results may have implications both for clinical practice and research, in that defining clinically distinct groups of individuals with FXS allows for tailored intervention and contributes to the identification of ASD-associated pathways that become disrupted in the presence of the FMR1 mutation.
Contributor Information
Jessica Klusek, Frank Porter Graham Child Development Institute, 105 Smith Level Road, CB 8180, Chapel Hill, The University of North Carolina at Chapel Hill, United States, 27599.
Gary E. Martin, UNC Chapel Hill, Frank Porter Graham Child Development Institute, Chapel Hill, The University of North Carolina at Chapel Hill, United States
Molly Losh, Email: m-losh@northwestern.edu, Northwestern University, Communication Sciences and Disorders, 2240 Campus Drive, Frances Searle Building 2-340, Evanston, Illinois, United States, 60208.
References
- Abrahams BS, Geschwind DH. Connecting genes to brain in the autism spectrum disorders. Archives of Neurology. 2010;67:395–9. doi: 10.1001/archneurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. University of Vermont Department of Psychiatry, translator. Manual for the ASEBA preschool forms and profiles. Burlington, VT: 2000. [Google Scholar]
- American Psychiatric Association; American Psychiatric Publishing, Incorporated, translator Diagnostic and statistical manual of mental disorders. 2013. [Google Scholar]
- American Psychological Association; Washington, DC, translator Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 1994. [Google Scholar]
- Bagni C, Tassone F, Neri G, Hagerman R. Fragile X syndrome: Causes, diagnosis, mechanisms, and therapeutics. Journal of Clinical Investigation. 2012;122:4314–4322. doi: 10.1172/JCI63141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DB, Hatton DD, Mesiboy G, Ament N, Skinner M. Early development, temperament, and functional impairment in autism and fragile X syndrome. Journal of Autism and Developmental Disorders. 2000;30:49–59. doi: 10.1023/a:1005412111706. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Hatton DD, Skinner M, Mesibov G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with Fragile X syndrome. Journal of Autism and Developmental Disorders. 2001;31:165–174. doi: 10.1023/a:1010747131386. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. American Journal of Medical Genetics: Part A. 2008;146A:2060–9. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nature Neuroscience. 2006;9:1221–1225. doi: 10.1038/nn1765. [DOI] [PubMed] [Google Scholar]
- Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, Bailey A, Rutter M. A Case-Control Family History Study of Autism. Journal of Child Psychology and Psychiatry. 1994;35:877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Budimirovic DB, Bukelis I, Cox C, Gray RM, Tierney E, Kaufmann WE. Autism spectrum disorder in fragile X syndrome: Differential contribution of adaptive socialization and social withdrawal. American Journal of Medical Genetics Part A. 2006;9999:1–13. doi: 10.1002/ajmg.a.31405. [DOI] [PubMed] [Google Scholar]
- Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, Loesch DZ. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. Journal of Autism and Developmental Disorders. 2007;37:738–47. doi: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- Cohen D, Pichard N, Tordjman S. Specific genetic disorders and autism: Clinical contribution towards their identification. Journal of Autism and Developmental Disorders. 2005;35:103. doi: 10.1007/s10803-004-1038-2. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Klann E. The translation of translational control by FMRP: Therapeutic targets for FXS. Nature Neuroscience. 2013 doi: 10.1038/nn.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–61. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rubeis S, Bagni C. Regulation of molecular pathways in the fragile X syndrome: insights into autism spectrum disorders. Journal of Neurodevelopmental Disorders. 2011;3:257–69. doi: 10.1007/s11689-011-9087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake C, Bui Q, Bulhak-Paterson D, Huggins R, Loesch DZ. Behavioural and cognitive phenotypes in idiopathic autism versus autism associated with fragile X syndrome. Journal of Child Psychology and Psychiatry. 2009;50:290–299. doi: 10.1111/j.1469-7610.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. American Guidance Service, translator. Peabody Picture Vocabulary Test. Circle Pines, MN: 1997. [Google Scholar]
- Fernandez-Carvajal I, Walichiewicz P, Xiaosen X, Pan R, Hagerman PJ, Tassone F. Screening for expanded alleles of the FMR1 gene in blood spots from newborn males in a Spanish population. Journal of Molecular Diagnostics. 2009;11:324–329. doi: 10.2353/jmoldx.2009.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Nonell C, Ratera ER, Harris S, Hessl D, Ono MY, Tartaglia N, Marvin E, Tassone F, Hagerman RJ. Secondary medical diagnosis in fragile X syndrome with and without autism spectrum disorder. American Journal of Medical Genetics Part A. 2008;146A:1911–1916. doi: 10.1002/ajmg.a.32290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive functions. Psychological Assessment Resources, Inc; Odessa, FL: 2000. [Google Scholar]
- Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders. 2007;37:613–27. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- Hagerman P. The fragile X prevalence paradox. Journal of Medical Genetics. 2008;45:498–499. doi: 10.1136/jmg.2008.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman R, Hagerman P. Fragile X Syndrome: Diagnosis, Treatment, and Research. Johns Hopkins University Press; Baltimore, MD: 2002. [Google Scholar]
- Hagerman R, Hoem G, Hagerman P. Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Molecular Autism. 2010;1:12. doi: 10.1186/2040-2392-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Narcisa V, Hagerman PJ. Fragile X: A molecular and treatment model for autism spectrum disorders. In: Amaral DG, Geschwind DH, Dawson G, editors. Autism spectrum disorders. Oxford University Press; New York: 2011. [Google Scholar]
- Hall SS, Lightbody AA, Reiss AL. Compulsive, self-injurious, and autistic behavior in children and adolescents with fragile X syndrome. American Journal of Mental Retardation. 2008;113:44–53. doi: 10.1352/0895-8017(2008)113[44:CSAABI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones BL, Ferranti J, Bacalman S, Barbato I, Tassone F, Hagerman PJ, Herman K, Hagerman RiJ, MacLean WE, Jr, Abbeduto L. Autism profiles of males with fragile X syndrome. American Journal on Mental Retardation. 2008;113:427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez RN, Feinberg RL, Vaurio R, Passanante NM, Thompson RE, Kaufmann WE. Autism spectrum disorder in fragile X syndrome: A longitudinal evaluation. American Journal of Human Genetics. 2009;149A:1125–1137. doi: 10.1002/ajmg.a.32848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Reiss AL. Social behavior and cortisol reactivity in children with fragile X syndrome. Journal of child psychology and psychiatry, and allied disciplines. 2006;47:602–610. doi: 10.1111/j.1469-7610.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, Yamrom B, Lee YH, Narzisi G, Leotta A, Kendall J, Grabowska E, Ma B, Marks S, Rodgers L, Stepansky A, Troge J, Andrews P, Bekritsky M, Pradhan K, Ghiban E, Kramer M, Parla J, Demeter R, Fulton LL, Fulton RS, Magrini VJ, Ye K, Darnell JC, Darnell RB, Mardis ER, Wilson RK, Schatz MC, McCombie WR, Wigler M. De Novo Gene Disruptions in Children on the Autistic Spectrum. Neuron. 2012;74:285–99. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau AS, Tierney E, Bukelis I, Stump MH, Kater WR, Trescher WH, et al. Social behavior profile in young males with fragile X syndrome: Characteristics and specificity. American Journal of Medical Genetics. 2004;126A:9–17. doi: 10.1002/ajmg.a.20218. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, Cox C, Capone GT, Stanard P. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. American Journal of Medical Genetics. 2004;129:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Lewis P, Abbeduto L, Murphy M, Richmond E, Giles N, Bruno L, Schroeder S. Cognitive, language and social-cognitive skills of individuals with Fragile X Syndrome with and without autism. Journal of Intellectual Disability Research. 2006;50:532–545. doi: 10.1111/j.1365-2788.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Bui Q, Dissanayake C, Clifford S, Gould E, Bulhak-Paterson D, Tassone F, Taylor A, Hessl D, Hagerman R, Huggins R. Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X. Neuroscience and Behavioral Reviews. 2007;31:315–326. doi: 10.1016/j.neubiorev.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- Lord C, McDuffie A, Abbeduto L, Lewis P, Kover S, Kim JS, Weber A, Brown WT. Autism spectrum disorder in children and adolescents with fragile X syndrome: Within-syndrome differences and age-related changes. American Journal on Intellectual and Developmental Disabilities. 2010;115:307–326. doi: 10.1352/1944-7558-115.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, DeLavore PC, Risi S. Western Psychological Services, translator. Autism Diagnostic Observation Schedule. Los Angeles, CA: 2001. [Google Scholar]
- Losh M, Martin GE, Klusek J, Hogan-Brown A, Sideris J. Pragmatic language in autism and fragile X syndrome: Neuropsychological and genetic links. Frontiers in Psychology: Developmental Psychology. 2012;3:1–12. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: Symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Journal of Developmental Behavioral Pediatrics. 2001;22:409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Roid GH, Miller LJ. Stoelting, translator. Leiter International Performance Scale-Revised. Wood Dale, IL: 1997. [Google Scholar]
- Sabaratnam M, Murthy NV, Wijeratne A, Buckingham A, Payne S. Autistic-like behaviour profile and psychiatric morbidity in fragile X sydnrome: A prospective ten-year follow-up study. European Child and Adolescent Psychiatry. 2003;12:172–177. doi: 10.1007/s00787-003-0333-3. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Ikle DN, Dyer PN, Lampe M, Willemsen R, Oostra BA, Taylor AK. FMRP expression as a potential prognostic indicator in fragile X syndrome. American Journal of Medical Genetics. 1999;84:250–261. [PubMed] [Google Scholar]
- Williams KT. American Guidance Service, translator. Expressive Vocabulary Test. Circle Pines, MN: 1997. [Google Scholar]
- American Psychiatric Association; American Psychiatric Publishing, Incorporated, translator. Diagnostic and statistical manual of mental disorders. Washington, DC: 2013. [Google Scholar]
- Budmirovic reference: Budimirovic DB, Bukelis I, Cox C, Gray RM, Tierney E, Kaufmann WE. Autism spectrum disorder in fragile X syndrome: Differential contribution of adaptive socialization and social withdrawal. American Journal of Medical Genetics Part A. 2006;140:1814–1826. doi: 10.1002/ajmg.a.31405.
- Darnell JC, Klann E. The translation of translational control by FMRP: Therapeutic targets for FXS. Nature Neuroscience. 2013;16:1530–1536. doi: 10.1038/nn.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Narcisa V, Hagerman PJ. Fragile X: a molecular and treatment model for autism spectrum disorders. In: Amaral DG, Geschwind DH, Dawson G, editors. Autism Spectrum Disorders. Oxford University Press; New York: 2011. pp. 801–11. [Google Scholar]