Abstract
Erythrocyte antioxidant enzymes are major circulating antioxidant enzymes in the oxidative stress defense system. Few prospective studies have assessed the association between these enzymes and the risk of coronary heart disease (CHD) in generally healthy adults. We conducted a prospective nested case-control study of CHD among 32,826 women at baseline with 15 years of follow-up from 1989 to 2004 in the Nurses' Health Study. We investigated the association of baseline erythrocyte superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) activities with the risk of CHD. A total of 365 cases and 728 controls were included in the analysis. Overall, the relative risks of CHD associated with 1–standard deviation higher SOD, GPx, and CAT activities were 1.07 (95% confidence interval (CI): 0.94, 1.22), 1.04 (95% CI: 0.91, 1.18), and 1.04 (95% CI: 0.92, 1.17), respectively. Multivariable adjustments did not change the associations appreciably. Fasting status did not modify the associations, with the exception that SOD activity was positively associated with the risk of CHD among participants who provided blood samples within 12 hours of fasting. Overall, activities of SOD, GPx, and CAT were not associated with CHD among women who were generally healthy at the time of blood collection.
Keywords: antioxidant enzymes, catalase, coronary heart disease, glutathione peroxidase, superoxide dismutase
Oxidative stress is generated as a result of an imbalance between reactive oxygen species and the antioxidant defense system. The oxidative stress pathway has been suggested to be an important etiological factor for the development of atherosclerosis and coronary heart disease (CHD) in experimental (1–4) and prospective human studies (5–7).
Superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) are 3 major antioxidant enzymes in humans (8). In the past few decades, there have been extensive studies of superoxide and other reactive oxygen species, including hydrogen peroxide, in biological systems and of how these free radicals are controlled by SOD, GPx, and CAT (9, 10). Measurement of the activity of the 3 antioxidant enzymes in erythrocytes is a noninvasive way to assess the circulating antioxidant defense in humans.
A recent meta-analysis consisting of 42 case-control and 3 cohort studies suggested that lower activity of SOD, GPx, or CAT was associated with higher risk of CHD (11). However, the majority of the included studies measured the activity of the 3 antioxidant enzymes in blood samples after nonfatal acute coronary events and had small sample sizes. Among existing prospective studies (12–14), only 1 study (12) has examined the association between GPx and myocardial infarction (MI) in a generally healthy population, but the associations of SOD and CAT with the risk of MI were not investigated. Thus, high-quality prospective studies are needed to examine the relationships of SOD, GPx, and CAT with the incidence of CHD within generally healthy free-living populations.
The use of fasting blood samples improves the prediction for CHD for some biochemical measurements (5, 15), because these biomarkers are influenced by postprandial factors (16, 17). Animal interventional studies have shown that prolonged starvation or food restriction leads to an inhibition of the synthesis of hepatic antioxidant enzymes, which may be due to suppressed production of insulin and other factors (18, 19). Although the influence of fasting on antioxidant enzymes has been suggested in animal models, the ways in which fasting status and fasting duration influence the antioxidant enzymes and their ability to predict CHD have not been investigated in large human epidemiologic studies. Thus, we used a prospective study design to 1) examine the associations of CAT, GPx, and SOD activities with future CHD events and 2) determine whether the association is influenced by the duration of fasting before sample collection.
METHODS
Population and study design
The Nurses’ Health Study (NHS) enrolled 121,701 women aged 30–55 years in 1976 (20). Baseline questionnaires on dietary and lifestyle information and self-reported disease information were administered to participants. Participants were followed biennially via questionnaire (20). Between 1989 and 1991, we collected blood samples from 32,826 NHS participants. We requested that participants provide fasting blood samples, and approximately 77% of the returned samples were collected at least 8 hours after the last meal. The present nested case-control study was conducted among participants aged 43–70 years who provided blood samples and who were free of cardiovascular diseases and cancers at the time of blood collection. The study protocol was approved by the institutional review boards of the Brigham and Women's Hospital (Boston, Massachusetts), the Harvard School of Public Health (Boston, Massachusetts), and the University of Cincinnati (Cincinnati, Ohio).
Case and control selection
Participants were followed from 1989 to 2004 for a median of 8 years (maximum, 15 years) after initial blood collection. During follow-up, we identified 474 newly diagnosed cases of CHD, including nonfatal MI and fatal CHD. Nonfatal MI was defined according to the World Health Organization (Geneva, Switzerland) criteria, which include MI symptoms plus either diagnostic electrocardiographic changes or elevated levels of cardiac enzymes. The documentation process was conducted by physicians using medical records. All physicians were blinded to baseline risk factors reported by study participants. Fatal CHD was documented if it was confirmed by hospital or autopsy records, if it was the cause of death listed on the death certificate, if CHD was the underlying and most plausible cause, or if evidence of previous CHD was available.
Using risk set sampling (18), we randomly selected controls at the time of case diagnosis at a 2:1 ratio. The matching factors were age (within 2 years), smoking status (never, past <15 cigarettes/day, past ≥15 cigarettes/day, current <15 cigarettes/day, or current ≥15 cigarettes/day), month of blood collection (within 2 months), and fasting status (<8 or ≥8 hours). We included 1,093 participants in the final analysis (365 cases and 728 controls; 290 of the original matched case-control sets).
Blood collection
Blood samples were collected in heparin anticoagulant tubes. Participants were asked to place tubes on ice packs in Styrofoam containers and return the sample kits to our central laboratory by overnight courier. The blood samples were processed within 48 hours (most within 36 hours).
Measurement of the activities of SOD, GPx, and CAT
Assay procedure
Erythrocyte activity was measured using SOD assay kit number 706002 and GPx assay kit number 703102 (Cayman Chemical Company, Ann Arbor, Michigan). Briefly, the packed erythrocytes were added to ice-cold water and centrifuged at 10,000 × g for 15 minutes at 4°C to obtain erythrocyte lysate. The samples were subsequently measured at different wavelengths (for GPx, 340 nm; for SOD, 440–460 nm). Erythrocyte CAT activity was tested using the method of Beers et al. (19), which was later modified by Aebi (21). Briefly, erythrocyte supernatant was diluted with a phosphate buffer. A standard solution was prepared using CAT from bovine liver (product C1345, Sigma-Aldrich Corp., St. Louis, Missouri). The samples and standard were mixed with 20 mM of hydrogen peroxide. The wavelength absorbance for CAT samples was 240 nm. GPx, CAT, and SOD activities were expressed as U/g of hemoglobin. The average within-run coefficients of variation for SOD, GPx, and CAT measurements were 14%, 10%, and 6%, respectively.
Assay stability in blood samples with delayed processing
Because the NHS blood samples were stored on ice until they were processed between 24 and 48 hours after blood collection, we needed to evaluate the stability of these assays in this type of sample. We found that the measurements of activity of the 3 antioxidant enzymes taken after 48 hours were not significantly different from those taken immediately after collection (P = 0.7, 0.3, and 0.5 for SOD, GPx, and CAT, respectively). The overall intraclass correlation coefficients (ICCs) for the 3 time points (0, 24, and 48 hours) were 0.2, 0.7, and 0.6 for SOD, GPx, and CAT measurements, respectively. Though the ICC for SOD was low, this was probably due to relatively low between-person variation in this set of samples and the lower number of participants (n = 7); as noted below, the ICC over 1 year was excellent.
Within-person assay reproducibility over a 1- to 2-year period
We measured the 3 enzymes in 40 NHS participants who donated 2 blood samples each at an average interval of 1.4 years. The ICCs for repeated measurements 1.4 years apart were 0.7 for SOD, 0.8 for GPx, and 0.9 for CAT.
Statistical analysis
We used both conditional and unconditional logistic regression models to examine the associations of SOD, GPx, and CAT activities with CHD. Covariates in unconditional models included matching factors (age, smoking status, time and month of blood collection) and body mass index (weight (kg)/height (m)2), physical activity, alcohol intake, menopausal status, family history of MI, history of diabetes, history of hypertension, hormone replacement therapy, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and C-reactive protein. Conditional models included the covariates mentioned above except for the matching factors. Because the results from the 2 models were similar, we present the results from the unconditional logistic models; this analysis resulted in fewer exclusions (i.e., all cases/controls were included when a matched case/control had missing information).
To determine the potential influence of fasting duration, we performed the following exploratory analyses: 1) we created 6 groups using 5 cutpoints of fasting duration (8, 10, 12, 14, and 16 hours); 2) we used unconditional logistic regression models to examine the associations of SOD, GPx, and CAT activities with CHD in every stratum of fasting duration; and 3) we tested interactions between SOD, GPx, and CAT activities and fasting duration by including the main effects of the 3 antioxidant enzymes as continuous variables and fasting duration as a categorical variable using the final selected cutpoint (described below). The Wald P value for the interaction term was used for assessing significance. We collapsed fasting strata that had similar trends and then decided on the cutpoint. For instance, the associations between SOD and CHD in participants in each fasting stratum of less than 12 hours were similar, but they were different from the fasting strata of 12 or more hours. Therefore, we used 12 hours as a cutpoint. We did not observe different trends of the associations of GPx and CAT with CHD in any stratum of fasting, irrespective of the cutpoints. All P values were 2-sided. All analyses were performed using SAS, version 9.3, software (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Baseline characteristics
At baseline, cases had higher body mass index values, low-density lipoprotein cholesterol, triglycerides, and C-reactive protein; they had greater proportions of family history of MI, history of diabetes, and history of hypertension; and they had lower high-density lipoprotein cholesterol and alcohol intake than controls (Table 1). Overall, the SOD, GPx, and CAT activities were not significantly different between cases and controls.
Table 1.
Baseline Characteristics and Levels of Biochemical Variables in Cases and Controls in the Nurses’ Health Study, 1989–2004
| Variable | Cases (n = 365) |
Controls (n = 728) |
P Value | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | No. | % | Mean (SD) | No. | % | ||
| Age, years | 60.3 (6.5) | 60.0 (6.5) | 0.53 | ||||
| BMIa | 26.5 (5.4) | 25.0 (4.2) | <0.001 | ||||
| Physical activity, METs/week | 10.9 (3.8, 25.2)b | 12.7 (5.4, 25.2)b | 0.23 | ||||
| Alcohol intake, g/day | 1.1 (0, 5.8)b | 1.8 (0, 8.6)b | 0.03 | ||||
| Fasting duration, hours | 11 (9, 12)b | 12 (8, 13)b | 0.18 | ||||
| Current smoker | 90 | 25 | 176 | 24 | 0.86 | ||
| Postmenopause | 310 | 85 | 610 | 84 | 0.89 | ||
| Family history of MI | 77 | 21 | 90 | 12 | <0.001 | ||
| History of diabetes | 54 | 15 | 41 | 6 | <0.001 | ||
| History of hypertension | 187 | 51 | 211 | 29 | <0.001 | ||
| Hormone replacement therapy | 127 | 34.8 | 289 | 39.7 | 0.12 | ||
| Biomarkers | |||||||
| LDL cholesterol, mg/dL | 143.0 (37.3) | 136.2 (37.3) | <0.001 | ||||
| HDL cholesterol, mg/dL | 52.1 (15.4) | 59.4 (16.5) | <0.001 | ||||
| Triglycerides, mg/dL | 124 (87, 178)b | 105 (77, 146)b | <0.001 | ||||
| CRP, mg/dL | 0.26 (0.11, 0.55)b | 0.19 (0.08, 0.39)b | 0.03 | ||||
| GPx activity, U/mg of hemoglobin | 16.34 (3.96) | 16.19 (4.01) | 0.58 | ||||
| CAT activity, U/mg of hemoglobin | 236 (56) | 234 (52) | 0.57 | ||||
| SOD activity, U/mg of hemoglobin | 8.93 (1.61) | 8.83 (1.54) | 0.29 | ||||
Abbreviations: BMI, body mass index; CAT, catalase; CRP. C-reactive protein; GPx, glutathione peroxidase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MET, metabolic equivalent; MI, myocardial infarction; SD, standard deviation; SOD, superoxide dismutase.
a Weight (kg)/height (m)2.
b Values with skewed distributions are expressed as medians (interquartile ranges).
SOD, GPx, and CAT activities and baseline factors in controls
SOD activity was inversely correlated with high-density lipoprotein cholesterol (P = 0.01) and physical activity (P = 0.03) (Web Table 1, available at http://aje.oxfordjournals.org/). Higher quintiles of SOD activity were associated with greater proportions of current smokers (P = 0.01). GPx activity was positively associated with history of diabetes (P = 0.03) and hormone replacement therapy (P < 0.01) and was negatively associated with low-density lipoprotein cholesterol (P =0.04) and proportion of current smokers (P = 0.02). CAT activity was positively associated with age (P = 0.04), body mass index value (P = 0.04), and proportion of postmenopausal women (P = 0.02) and negatively associated with alcohol intake (P < 0.01) and proportion of current smokers (P = 0.02).
SOD, GPx, and CAT activities and CHD
Overall, we did not find that these markers were associated with CHD risk (Table 2). The relative risks of CHD associated with 1–standard deviation higher SOD, GPx, and CAT activities were 1.07 (95% confidence interval (CI): 0.94, 1.22), 1.04 (95% CI: 0.91, 1.18), and 1.04 (95% CI: 0.92, 1.17), respectively. Multivariable adjustments did not change the associations appreciably. We did not find significant associations of SOD, GPX, and CAT with CHD when we alternatively classified these enzymes into quintiles (Table 2).
Table 2.
Multivariable Logistic Regression Analysis of the Association of Superoxide Dismutase, Glutathione Peroxidase, and Catalase Activities With Coronary Heart Disease in the Nurses’ Health Study, 1989–2004
| Quintile, by Enzymea | No. of Cases | No. of Controls | Mean (SD) | Unadjusted RR | 95% CI | Adjusted RRb | 95% CI |
|---|---|---|---|---|---|---|---|
| SOD activityc | |||||||
| 1 | 76 | 143 | 6.80 (0.96) | 1.00 | Referent | 1.00 | Referent |
| 2 | 66 | 148 | 8.11 (0.21) | 0.84 | 0.56, 1.25 | 0.88 | 0.57, 1.36 |
| 3 | 66 | 145 | 8.78 (0.20) | 0.86 | 0.57, 1.28 | 0.94 | 0.61, 1.45 |
| 4 | 68 | 145 | 9.48 (0.24) | 0.88 | 0.59, 1.32 | 0.85 | 0.55, 1.31 |
| 5 | 89 | 147 | 11.00 (1.00) | 1.14 | 0.78, 1.67 | 1.12 | 0.73, 1.71 |
| Overall (per 1-SD increase) | 365 | 728 | 8.86 (1.56) | 1.07 | 0.94, 1.22 | 1.06 | 0.92, 1.22 |
| GPx activityc | |||||||
| 1 | 74 | 145 | 11.37 (1.44) | 1.00 | Referent | 1.00 | Referent |
| 2 | 76 | 146 | 14.07 (0.53) | 1.02 | 0.69, 1.51 | 0.87 | 0.57, 1.34 |
| 3 | 62 | 145 | 15.74 (0.52) | 0.84 | 0.56, 1.26 | 0.75 | 0.48, 1.17 |
| 4 | 63 | 146 | 17.68 (0.66) | 0.85 | 0.56, 1.27 | 0.79 | 0.51, 1.24 |
| 5 | 90 | 146 | 21.97 (3.10) | 1.21 | 0.82, 1.77 | 1.07 | 0.70, 1.63 |
| Overall (per 1-SD increase) | 365 | 728 | 16.24 (3.99) | 1.04 | 0.91, 1.18 | 1.02 | 0.88, 1.17 |
| CAT activityc | |||||||
| 1 | 83 | 149 | 168 (23) | 1.00 | Referent | 1.00 | Referent |
| 2 | 67 | 145 | 208 (7) | 0.83 | 0.56, 1.23 | 0.76 | 0.49, 1.17 |
| 3 | 57 | 149 | 230 (7) | 0.69 | 0.46, 1.03 | 0.73 | 0.47, 1.14 |
| 4 | 73 | 142 | 258 (9) | 0.92 | 0.63, 1.36 | 0.92 | 0.60, 1.41 |
| 5 | 85 | 143 | 310 (36) | 1.07 | 0.73, 1.56 | 0.98 | 0.64, 1.48 |
| Overall (per 1-SD increase) | 365 | 728 | 235 (53) | 1.04 | 0.92, 1.17 | 1.03 | 0.91, 1.18 |
Abbreviations: CAT, catalase; CI, confidence interval; GPx, glutathione peroxidase; RR, relative risk; SD, standard deviation; SOD, superoxide dismutase.
a Quintiles of SOD, GPx, and CAT activities were calculated using the distributions in controls.
b Adjusted for age, smoking, time and month of blood collection, body mass index (weight (kg)/height (m)2), physical activity, alcohol intake, menopausal status, family history of myocardial infarction, history of diabetes, history of hypertension, hormone replacement therapy, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and C-reactive protein.
c Measured in U/mg of hemoglobin.
After testing the interactions of SOD, GPx, and CAT activities with age, history of diabetes, and body mass index value, we found an interaction only for CAT activity with diabetes (P = 0.01). We further tested the association between CAT activity and risk of CHD stratified by history of diabetes (54 cases and 41 controls in women with diabetes; 311 cases and 687 controls in women without diabetes) and found that higher levels of CAT activity (per 1–standard deviation increase) were associated with lower risk of CHD in women with diabetes (relative risk (RR) = 0.49, 95% CI: 0.27, 0.88), but not in those without diabetes (RR = 1.10, 95% CI: 0.96, 1.27).
SOD, GPx, and CAT activities and CHD, stratified by fasting duration
We observed different trends of the association between SOD and CHD in fasting strata of less than 12 hours compared with fasting strata of 12 hours or more (Figure 1). In multivariable adjusted models, the relative risk of CHD for a 1–standard deviation greater level of SOD activity was 1.25 (95% CI: 1.03, 1.52) for all participants in fasting strata of less than 12 hours (Figure 1); in contrast, there was no association with CHD in fasting strata of 12 hours or more (RR = 0.85, 95% CI: 0.68, 1.05). When we classified SOD into quintiles among fasting strata of less than 12 hours, the relative risk between the extreme quintiles (top vs. bottom quintile) was 2.34 (95% CI: 1.26, 4.33). The interaction between SOD activity and fasting duration was marginally significant (P = 0.08). Neither GPx nor CAT activity was associated with CHD in the fasting strata of less than 12 hours or 12 hours or more (Figures 2 and 3).We did not observe different patterns of the associations between GPx and CAT activities and CHD when we used other cutpoints of fasting duration (Appendix Table 1).
Figure 1.
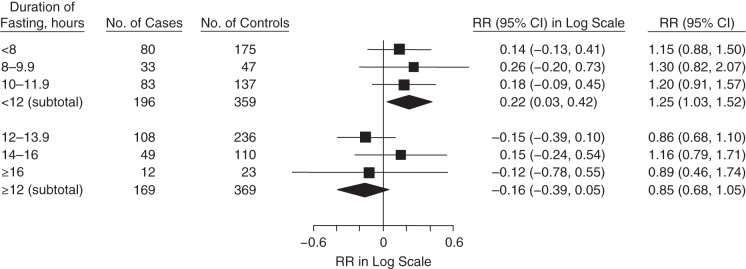
Association between superoxide dismutase activity (per 1–standard deviation increase) and coronary heart disease, stratified by fasting duration in the Nurses’ Health Study, 1989–2004. The relative risk (RR) subtotals were adjusted for age, smoking, time and month of blood collection, body mass index (weight (kg)/height (m)2), physical activity, alcohol intake, menopausal status, family history of myocardial infarction, history of diabetes, history of hypertension, hormone replacement therapy, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and C-reactive protein. Squares represent point estimates of relative risk. Diamonds represent subtotal point estimates of relative risk. Bars, 95% confidence intervals (CIs).
Figure 2.
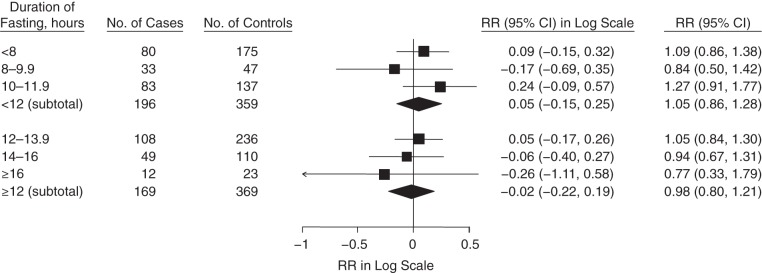
Association between glutathione peroxidase activity (per 1–standard deviation increase) and coronary heart disease, stratified by fasting duration in the Nurses’ Health Study, 1989–2004. The relative risk (RR) subtotals were adjusted for age, smoking, time and month of blood collection, body mass index (weight (kg)/height (m)2), physical activity, alcohol intake, menopausal status, family history of myocardial infarction, history of diabetes, history of hypertension, hormone replacement therapy, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and C-reactive protein. Bars, 95% confidence intervals (CIs).
Figure 3.
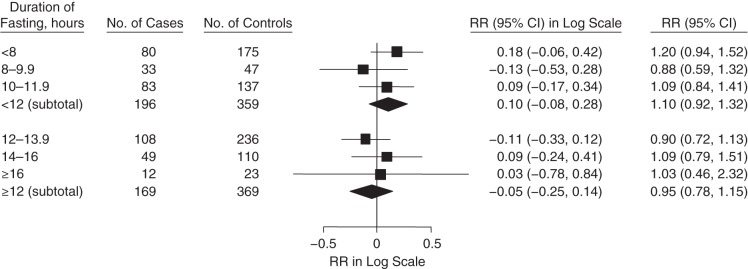
Association between catalase activity (per 1–standard deviation increase) and coronary heart disease, stratified by fasting duration in the Nurses’ Health Study, 1989–2004. The relative risk (RR) subtotals were adjusted for age, smoking, time and month of blood collection, body mass index (weight (kg)/height (m)2), physical activity, alcohol intake, menopausal status, family history of myocardial infarction, history of diabetes, history of hypertension, hormone replacement therapy, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and C-reactive protein. Squares represent point estimates of relative risk. Diamonds represent subtotal point estimates of relative risk. Bars, 95% confidence intervals (CIs).
DISCUSSION
Many studies have investigated the associations of SOD, GPx, and CAT activities with CHD among individuals who already have CHD (22–26); however, few prospective studies have examined the associations of SOD, GPx, and CAT activities in erythrocytes with future CHD events within generally healthy, free-living populations. In this prospective study of women who were free of cardiovascular disease at the time of blood collection, SOD, GPx, and CAT activities were not associated with risk of CHD. The findings suggest that activities of erythrocyte antioxidant enzymes do not predict the risk of CHD among healthy women.
The null association between GPx and CHD that we observed in the current study is in agreement with the results of 1 prospective study (12), but not with the results of 2 others (13, 14). Indeed, the prospective studies that found inverse associations were all performed in participants who had CHD at baseline (13, 14). Therefore, this discrepancy is likely due to the fact that the activity of GPx may be different in participants with CHD versus those without CHD at baseline. In an overall healthy population, GPx may not be the major antioxidant enzyme to neutralize free radicals. However, in individuals with existing CHD, the negative association between GPx and CHD suggests that GPx may play an important role in protecting heart tissue from oxidative damage after a cardiovascular event (13, 14). In participants with either angina or coronary artery disease, the level of oxidative stress can be exceptionally high and, thus, an exhaustion of GPx activity can result in recurrent CHD events. An alternative explanation to the findings in a population with existing cardiovascular disease is reverse causation. In line with the work of Blankenberg et al. (13) and Schnabel et al. (14), several case-control studies found lower GPx activity in CHD cases than in controls (27–29). These studies measured GPx after CHD was diagnosed. Collectively, GPx may have predictive value for CHD recurrence in people with CHD but not in those without existing CHD.
No previous prospective study has investigated the association between CAT and CHD. The null association between CAT and CHD in our study conflicts with the results of several case-control studies that found inverse associations between CAT and CHD (25, 26, 30). Similarly, the discrepancy may be due to different study designs (i.e., whether blood was collected before or after CHD diagnoses), as mentioned above.
We observed a significant association between SOD and CHD in women who had fasted for less than 12 hours before blood collection but not in those who had fasted for 12 hours or more. Two hypotheses could explain the differential findings in different fasting strata. First, the positive relationship between SOD and CHD may indicate a compensatory mechanism of the antioxidant defense system against elevated oxidative stress (24, 31). This scenario resembles the prediabetic stage, in which insulin levels increase in response to elevated glucose levels. The significant association observed in the fasting strata of less than 12 hours may indicate greater postprandial oxidative stress in CHD cases than in healthy controls within the 12-hour fasting window. Although this finding may be influenced by diet before blood collection, it may be mainly due to the duration of fasting rather than the actual dietary components. This is similar to the way in which elevated insulin levels are mainly influenced by duration of fasting rather than dietary components in prediabetic (insulin-resistant) patients. Second, SOD offers first-line antioxidant defense against oxidative damage, in contrast to GPx and CAT (32, 33); this may explain why we did not observe similar patterns of association of GPx and CAT with CHD in this population. There were almost equal numbers of cases and controls in each fasting stratum (i.e., <12-hour and ≥12-hour strata). The distributions of major possible confounders (e.g., smoking, physical activity, and high-density lipoprotein cholesterol) among cases and controls were similar across the 2 strata. Thus, both groups had equal power to detect a significant relationship, though residual confounding may still exist. Future research is warranted to confirm this finding and to elucidate the potential underlying mechanisms.
The present study has several strengths, including the relatively large numbers of CHD cases and controls. In addition, previous case-control studies (24, 34) collected blood samples after CHD was diagnosed. Our prospective study design among a generally healthy population reduces the possibility of reverse causation, although an effect of subclinical disease on biomarkers cannot be excluded.
A limitation of this study is that we do not know whether a single blood sample reflects average levels of SOD, GPx, and CAT over a prolonged period of time. The high ICC suggests that a single measurement reflects a chronic condition for at least a 1- to 2-year period. However, repeated measurements over several years would be ideal. Further, because of the observational nature of our study, the findings may be due to chance. We did not measure SOD, GPx, and CAT activities in tissue, which may be more relevant to CHD outcome. However, we used erythrocyte antioxidant enzymes as markers for antioxidant defense, because activities of these antioxidant enzymes can be measured easily in clinical settings and have been shown to be useful in assessing CHD risk among patients with CHD (13). Although we have adjusted for the time of day of blood collection (morning vs. afternoon), we cannot exclude the possibility of residual confounding of the specific time of blood collection on the associations between antioxidant enzymes and the risk of CHD. All blood samples (from cases and controls) were properly stored in liquid-nitrogen freezers (at −130°C or colder) for the same period of time before measurement of antioxidant enzymes. Therefore, we can generally assume that antioxidant enzymes would be affected similarly if there were any influence. However, there is a possibility that activities of antioxidant enzymes in cases and controls were affected differently even under the same duration of storage. This is considered to be a universal concern for biospecimens collected in large long-term cohorts. Lastly, the participants in our study may not be representative of the general population, because the rate of CHD death in the NHS is lower than that in the US population as a whole.
In summary, we found no overall relationship between the activities of SOD, GPx, and CAT and the risk of CHD among a group of generally healthy women. Notably, higher SOD activity was significantly associated with greater risk of CHD among women who provided blood samples within a 12-hour fasting window. This finding merits further confirmation in other prospective studies.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Environmental Health, Division of Epidemiology and Biostatistics, University of Cincinnati Medical Center, Cincinnati, Ohio (Shuman Yang, Tianying Wu); Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts, (Majken K. Jensen, Eric B. Rimm, Walter Willett); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Majken K. Jensen, Eric B. Rimm, Walter Willett); and the Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts (Majken K. Jensen, Eric B. Rimm, Walter Willett).
This study was funded by the American Heart Association (grant 0430202N to T.W.); the National Cancer Institute (grant CA138714 to T.W.) and start-up funds (to T.W.); the National Institute for Environmental Health Sciences (grant P30-ES006096); and the National Institutes of Health (grants HL34594, CA87969, and HL35464).
We thank superoxide dismutase expert, Dr. Daret St. Clair, from the University of Kentucky, for valuable input.
Conflict of interest: none declared.
APPENDIX
Appendix Table 1.
Associations of Superoxide Dismutase, Glutathione Peroxidase, and Catalase Activities With Coronary Heart Disease, Stratified by Different Cutpoints of Fasting Duration in the Nurses’ Health Study, 1989–2004
| Fasting Duration, hours | No. of Cases | No. of Controls | SOD Activitya |
GPx Activitya |
CAT Activitya |
|||
|---|---|---|---|---|---|---|---|---|
| Adjusted RRb Per 1-SD Increase | 95% CI | Adjusted RRb Per 1-SD Increase | 95% CI | Adjusted RRb Per 1-SD Increase | 95% CI | |||
| <8 | 80 | 175 | 1.27 | 0.93, 1.74 | 1.11 | 0.85, 1.47 | 1.11 | 0.84, 1.47 |
| ≥8 | 285 | 553 | 1.01 | 0.86, 1.19 | 0.99 | 0.84, 1.17 | 1.00 | 0.86, 1.16 |
| <10 | 113 | 222 | 1.21 | 0.92, 1.57 | 1.01 | 0.80, 1.29 | 1.13 | 0.89, 1.43 |
| ≥10 | 252 | 506 | 0.98 | 0.83, 1.17 | 1.03 | 0.87, 1.23 | 0.99 | 0.85, 1.17 |
| <12 | 196 | 359 | 1.25 | 1.03, 1.52 | 1.05 | 0.86, 1.28 | 1.10 | 0.92, 1.32 |
| ≥12 | 169 | 369 | 0.85 | 0.68, 1.05 | 0.98 | 0.80, 1.21 | 0.95 | 0.78, 1.15 |
| <14 | 304 | 595 | 1.07 | 0.92, 1.25 | 1.02 | 0.87, 1.18 | 1.02 | 0.88, 1.17 |
| ≥14 | 61 | 133 | 0.91 | 0.60, 1.38 | 1.03 | 0.70, 1.52 | 1.13 | 0.78, 1.65 |
| <16 | 353 | 705 | 1.08 | 0.93, 1.24 | 1.02 | 0.89, 1.17 | 1.03 | 0.90, 1.18 |
| ≥16 | 12 | 23 | NA | NA | NA | |||
Abbreviations: CAT, catalase; CI, confidence interval; GPx, glutathione peroxidase; NA, not available; RR, relative risk; SD, standard deviation; SOD, superoxide dismutase.
a Measured in U/mg of hemoglobin.
b Adjusted for age, smoking, time and month of blood collection, body mass index (weight (kg)/height (m)2), physical activity, alcohol intake, menopausal status, family history of myocardial infarction, history of diabetes, history of hypertension, hormone replacement therapy, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and C-reactive protein.
REFERENCES
- 1.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;27234:20963–20966 [DOI] [PubMed] [Google Scholar]
- 2.Ross R, Glomset JA. The pathogenesis of atherosclerosis. N Engl J Med. 1976;2957:369–377 [DOI] [PubMed] [Google Scholar]
- 3.Stocker R, Keaney JF, Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;844:1381–1478 [DOI] [PubMed] [Google Scholar]
- 4.Quinn MT, Parthasarathy S, Fong LG, et al. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987;849:2995–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu T, Rifai N, Willett WC, et al. Plasma fluorescent oxidation products: independent predictors of coronary heart disease in men. Am J Epidemiol. 2007;1665:544–551 [DOI] [PubMed] [Google Scholar]
- 6.Tsimikas S, Brilakis ES, Miller ER, et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;3531:46–57 [DOI] [PubMed] [Google Scholar]
- 7.Jensen MK, Wang Y, Rimm EB, et al. Fluorescent oxidation products and risk of coronary heart disease: a prospective study in women. J Am Heart Assoc. 2013;25:e000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witztum JL. The oxidation hypothesis of atherosclerosis. Lancet. 1994;3448925:793–795 [DOI] [PubMed] [Google Scholar]
- 9.Matés JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153(1-3):83–104 [DOI] [PubMed] [Google Scholar]
- 10.Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med. 1991;91(3C):14S–22S [DOI] [PubMed] [Google Scholar]
- 11.Flores-Mateo G, Carrillo-Santisteve P, Elosua R, et al. Antioxidant enzyme activity and coronary heart disease: meta-analyses of observational studies. Am J Epidemiol. 2009;1702:135–147 [DOI] [PubMed] [Google Scholar]
- 12.Hallgren CG, Hallmans G, Jansson JH, et al. Markers of high fish intake are associated with decreased risk of a first myocardial infarction. Br J Nutr. 2001;863:397–404 [DOI] [PubMed] [Google Scholar]
- 13.Blankenberg S, Rupprecht HJ, Bickel C, et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;34917:1605–1613 [DOI] [PubMed] [Google Scholar]
- 14.Schnabel R, Lackner KJ, Rupprecht HJ, et al. Glutathione peroxidase-1 and homocysteine for cardiovascular risk prediction: results from the AtheroGene Study. J Am Coll Cardiol. 2005;4510:1631–1637 [DOI] [PubMed] [Google Scholar]
- 15.Sharrett AR, Ballantyne CM, Coady SA, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;10410:1108–1113 [DOI] [PubMed] [Google Scholar]
- 16.Sävendahl L, Underwood LE. Fasting increases serum total cholesterol, LDL cholesterol and apolipoprotein B in healthy, nonobese humans. J Nutr. 1999;12911:2005–2008 [DOI] [PubMed] [Google Scholar]
- 17.Horne BD, Muhlestein JB, Lappé DL, et al. Randomized cross-over trial of short-term water-only fasting: metabolic and cardiovascular consequences. Nutr Metab Cardiovasc Dis. 2013;2311:1050–1057 [DOI] [PubMed] [Google Scholar]
- 18.Prentice RL, Breslow NE. Retrospective studies and failure time models. Biometrika. 1978;651:153–158 [Google Scholar]
- 19.Beers RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;1951:133–140 [PubMed] [Google Scholar]
- 20.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;61:49–62 [DOI] [PubMed] [Google Scholar]
- 21.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126 [DOI] [PubMed] [Google Scholar]
- 22.Adachi T, Yamazaki N, Tasaki H, et al. Changes in the heparin affinity of extracellular-superoxide dismutase in patients with coronary artery atherosclerosis. Biol Pharm Bull. 1998;2110:1090–1093 [DOI] [PubMed] [Google Scholar]
- 23.Wang XL, Adachi T, Sim AS, et al. Plasma extracellular superoxide dismutase levels in an Australian population with coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;1812:1915–1921 [DOI] [PubMed] [Google Scholar]
- 24.Weinbrenner T, Cladellas M, Covas MI, et al. High oxidative stress in patients with stable coronary heart disease. Atherosclerosis. 2003;1681:99–106 [DOI] [PubMed] [Google Scholar]
- 25.Lászlo A, Matkovics B, Varge SI, et al. Changes in lipid peroxidation and antioxidant enzyme activity of human red blood cells after myocardial infarction. Clin Chim Acta. 1991;203(2-3):413–415 [DOI] [PubMed] [Google Scholar]
- 26.Kopff M, Zakrewska I, Fuchs J, et al. Superoxide dismutase and catalase activity in patients with stable angina pectoris. Acta Biochim Pol. 1993;401:158–159 [PubMed] [Google Scholar]
- 27.Porter M, Pearson DJ, Suarez-Mendez VJ, et al. Plasma, platelet and erythrocyte glutathione peroxidases as risk factors in ischaemic heart disease in man. Clin Sci (Lond). 1992;833:343–345 [DOI] [PubMed] [Google Scholar]
- 28.Akkuş I, Sağlam NI, Cağlayan O, et al. Investigation of erythrocyte membrane lipid peroxidation and antioxidant defense systems of patients with coronary artery disease (CAD) documented by angiography. Clin Chim Acta. 1996;2442:173–180 [DOI] [PubMed] [Google Scholar]
- 29.Simić D, Mimić-Oka J, Pljesa M, et al. Time course of erythrocyte antioxidant activity in patients treated by thrombolysis for acute myocardial infarction. Jpn Heart J. 2003;446:823–832 [DOI] [PubMed] [Google Scholar]
- 30.Saha A, Adak S, Chowdhury S, et al. Enhanced oxygen releasing capacity and oxidative stress in diabetes mellitus and diabetes mellitus-associated cardiovascular disease: a comparative study. Clin Chim Acta. 2005;361(1-2):141–149 [DOI] [PubMed] [Google Scholar]
- 31.Zhou X, Zhai X, Ashraf M. Direct evidence that initial oxidative stress triggered by preconditioning contributes to second window of protection by endogenous antioxidant enzyme in myocytes. Circulation. 1996;936:1177–1184 [DOI] [PubMed] [Google Scholar]
- 32.Matés JM, Pérez-Gómez C, Núñez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;328:595–603 [DOI] [PubMed] [Google Scholar]
- 33.Alscher RG, Donahue JL, Cramer CL. Reactive oxygen species and antioxidants: relationships in green cells. Physiol Plant. 1997;1002:224–233 [Google Scholar]
- 34.Bagatini MD, Martins CC, Battisti V, et al. Oxidative stress versus antioxidant defenses in patients with acute myocardial infarction. Heart Vessels. 2011;261:55–63 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


