Abstract
Iron deficiency affects 40%–50% of pregnancies. Iron is critical for early neurodevelopmental processes that are dysregulated in autism spectrum disorder (ASD). We examined maternal iron intake in relation to ASD risk in California-born children enrolled in a population-based case-control study (the Childhood Autism Risks from Genetics and the Environment (CHARGE) Study) from 2003 to 2009 with a diagnosis of ASD (n = 520) or typical development (n = 346) that was clinically confirmed using standardized assessments. Mean maternal daily iron intake was quantified on the basis of frequency, dose, and brands of supplements and cereals consumed each month from 3 months before pregnancy through the end of pregnancy and during breastfeeding (the index period), as reported in parental interviews. Mothers of cases were less likely to report taking iron-specific supplements during the index period (adjusted odds ratio = 0.63, 95% confidence interval: 0.44, 0.91), and they had a lower mean daily iron intake (51.7 (standard deviation, 34.0) mg/day) than mothers of controls (57.1 (standard deviation, 36.6) mg/day; P = 0.03). The highest quintile of iron intake during the index period was associated with reduced ASD risk compared with the lowest (adjusted odds ratio = 0.49, 95% confidence interval: 0.29, 0.82), especially during breastfeeding. Low iron intake significantly interacted with advanced maternal age and metabolic conditions; combined exposures were associated with a 5-fold increased ASD risk. Further studies of this link between maternal supplemental iron and ASD are needed to inform ASD prevention strategies.
Keywords: autism, case-control studies, child development, dietary supplements, iron, pregnancy, primary prevention, risk factors
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by the presence of social deficits, language impairments, and stereotyped or repetitive behaviors and interests (1–3). ASD affects 1 in every 68 children in the United States, and prevalence is increasing (4). Combinations of multiple genetic and environmental factors likely play an etiological role in ASD. Evidence supports the hypothesis of prenatal origins for autism (5) and an influence of gestational nutrition (6, 7).
Iron deficiency, with its resultant anemia, is the most commonly measured nutrient deficiency, and it is especially common during pregnancy, affecting 40%–50% of women and their infants (8–10). The fetus depends on maternal iron as his or her only source of iron (11), and severe maternal iron deficiency can induce fetal and infant iron deficiency (12, 13). Iron is crucial to early neurodevelopment. In the brain, iron contributes to neurotransmitter production, myelination, and immune function (14); dysregulation of all 3 of these pathways has been associated with ASD. Iron deficiency early in life has been shown to impair cognition, motor development, social orientation and engagement, and language development, with improvements being observed upon iron supplementation (15–19). Poor iron status is more prevalent in children with ASD (20–24) and does not necessarily correlate with low iron intake (22), suggesting that these children could absorb and/or metabolize iron less efficiently. However, to our knowledge, no study to date has examined gestational iron status in relation to development of ASD. We examined maternal intake of supplemental iron in relation to ASD risk.
METHODS
Study population
Participants in the Childhood Autism Risks from Genetics and the Environment (CHARGE) Study, a population-based case-control study (25), who had undergone interviews from the start of the study in 2003 until the questionnaire was revised in September 2011 were included in these analyses. Eligible children included those who were aged 24–60 months, had been born in California, were living with at least 1 biological parent who spoke English or Spanish, and resided in one of the catchment areas on a specified list of California Department of Developmental Services regional centers that coordinate services for children with autism and developmental delay. Children were excluded if they had impairments that would preclude a valid developmental assessment. Children with genetic syndromes were not excluded if they met other inclusion criteria.
Children with autism and developmental delays were identified through the Department of Developmental Services’ regional centers, clinics and providers, self-referrals by parents, and public outreach. A stratified random sample of children from the general population, identified from state birth files, was generated by frequency-matching to the projected distribution of autism cases on age, sex, and regional center catchment area. The CHARGE Study protocol was approved by institutional review boards at the University of California, Davis, and the University of California, Los Angeles, and by the State of California Committee for the Protection of Human Subjects. Written informed consent was obtained before participation.
Diagnostic confirmation
All children were assessed at the Medical Investigation of Neurodevelopmental Disorders (MIND) Institute clinic (Sacramento, California) for confirmation of their diagnosis. Children were assessed for cognitive function using the Mullen Scales of Early Learning (26) and for adaptive function using the Vineland Adaptive Behavior Scales (27). The Autism Diagnostic Interview–Revised (28, 29) and the Autism Diagnostic Observation Schedule–Generic (30, 31) were used to confirm autism diagnoses. The children of families recruited from the general population or with developmental delays were screened for evidence of ASD using the Social Communication Questionnaire (32); if children scored above 14, they were evaluated for autism. Autism case status was defined as meeting criteria in the communication, social, and repetitive-behavior domains of the Autism Diagnostic Interview–Revised and scoring at or above the total cutoff point for autistic disorder on the Autism Diagnostic Observation Schedule–Generic, module 1, 2, or 3. A broader definition of impairment encompasses ASD as defined by Risi et al. (33). Because autism and ASD represent different symptom severities along the continuum of the disorder (33), we present results for the combined ASD group in accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (34). All interviews and clinical assessments were conducted in English or Spanish by bilingual staff.
Maternal supplemental iron intake
As previously described (35), trained telephone interviewers collected information from the mother on her intake of multivitamins, prenatal vitamins, iron-specific vitamins, cereals, and other supplements (including whether or not each item had been consumed and, if so, what brand and dose had been consumed, how frequently, and in which months) during a defined period (the index period) beginning 3 months before pregnancy and continuing throughout each month of pregnancy and while breastfeeding. From this information, we calculated average daily intake of iron (and other nutrients) for each product and summed these values to a total value for each month for each woman. Iron amounts were assigned to each brand/product based on information obtained from the manufacturer; if this information was not available, a standard amount was assigned based on the amount most commonly found in similar products.
Statistical analyses
Data were reviewed for outliers using univariate descriptive analyses. Logistic regression was used to calculate odds ratios (with 95% confidence intervals) as measures of association between iron intake categories and case status, using SAS software, version 9.3 (SAS Institute Inc., Cary, North Carolina). Stratified analyses and interaction terms were used to examine effect modification of maternal iron intake by child sex, interpregnancy interval (defined as elapsed time from the last livebirth or previous pregnancy with a gestational age of ≤20 weeks to the start of the current pregnancy), maternal and child race/ethnicity (non-Hispanic white, Hispanic, or other), maternal age, and maternal metabolic conditions (prepregnancy obesity, defined as body mass index (weight (kg)/height (m)2 ≥30), hypertension, and/or diabetes), as defined previously (36). The above variables were also examined as potentially confounding factors because of their relationship with iron status, as were the following variables: child's birth year, paternal age, home ownership, month in which prenatal care began, number of prenatal-care visits, parity, maternal birthplace, education, folic acid intake during the first month of pregnancy (<600 µg/day or ≥600 µg/day), cigarette smoking, residing with a smoker, and alcohol consumption. Our analyses started with a full model containing potential confounders identified in the bivariate analyses as being broadly associated (P < 0.2) with both ASD and quintile categories of iron intake (based on control intake). Variables were then excluded using backward selection, retaining in the model any variables that caused at least a 10% change in the exposure parameter estimates. Maternal folic acid intake, home ownership, and child's birth year were the only variables meeting the confounder criteria.
In sensitivity analyses, we assessed the impact of missing data using multiple imputation via the Markov chain Monte Carlo algorithm (37). To ensure that the results represented the study base, we used survey research methods to fit the logistic regression models, with participants being assigned weights equal to the inverse of the estimated participation probability in strata defined by the entry case group and demographic factors (38). Estimates were also stratified by maternal folic acid intake during the first month of pregnancy to further control for folic acid's correlated association. To assess the effect of recall bias using the length of time elapsed before mothers were asked to recall their intake, associations were also examined for children who were under the median age of controls at the start of the interview (completion of the interview could require multiple sessions) compared with those who were older at the start of the interview.
RESULTS
Characteristics of participants with ASD and those with typical development (TD) are shown in Table 1. Children with ASD were more likely to have been born earlier than children with TD, and their mothers were significantly more likely to have some college education but no bachelor's or higher degree, to have smoked cigarettes, and to have taken a multivitamin during the index period and less likely to have eaten cereal during the index period. Their mothers also tended to be older, were more likely to have been born outside of the United States, and were less likely to have private medical insurance. Parents of children with ASD were significantly less likely to own their homes.
Table 1.
Characteristics of Children With Autism Spectrum Disorder or Typical Development and Their Mothers, CHARGE Study, California, 2003–2009
| TD (n = 346) |
ASD (n = 520) |
P Valuea | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Child sex | |||||
| Male | 285 | 82.4 | 445 | 85.6 | 0.20 |
| Female | 61 | 17.6 | 75 | 14.4 | |
| Child race/ethnicity | |||||
| Non-Hispanic white | 179 | 51.7 | 265 | 51.0 | 0.12 |
| Hispanic | 103 | 29.8 | 166 | 31.9 | |
| Non-Hispanic black | 8 | 2.3 | 12 | 2.3 | |
| Asian | 9 | 2.6 | 28 | 5.4 | |
| Mixed or other | 47 | 13.6 | 49 | 9.4 | |
| Child's birth year | |||||
| 1998–2001 | 62 | 17.9 | 238 | 45.8 | <0.0001 |
| 2002–2004 | 190 | 54.9 | 181 | 34.8 | |
| 2005–2006 | 65 | 18.8 | 91 | 17.5 | |
| 2007–2009 | 29 | 8.4 | 10 | 1.9 | |
| Maternal age at child's birth, years | |||||
| <20 | 12 | 3.5 | 10 | 1.9 | 0.09 |
| 20–25 | 49 | 14.2 | 63 | 12.1 | |
| 25–29 | 75 | 21.7 | 138 | 26.5 | |
| 30–34 | 130 | 37.6 | 165 | 31.7 | |
| 35–39 | 63 | 18.2 | 122 | 23.5 | |
| ≥40 | 17 | 4.9 | 22 | 4.2 | |
| Maternal birthplace | |||||
| United States | 283 | 81.8 | 394 | 75.8 | 0.10 |
| Mexico | 23 | 6.7 | 41 | 7.9 | |
| Other | 40 | 11.6 | 85 | 16.4 | |
| Maternal education | |||||
| High school graduation or less | 54 | 15.6 | 75 | 14.4 | 0.04 |
| Some college, vocational school, or associate's degree | 109 | 31.5 | 208 | 40.0 | |
| Bachelor's degree or higher | 183 | 52.9 | 237 | 45.6 | |
| Home ownership | |||||
| No | 78 | 22.7 | 173 | 33.5 | 0.001 |
| Yes | 266 | 77.3 | 344 | 66.5 | |
| Type of health insurance | |||||
| Private | 49 | 14.2 | 97 | 18.7 | 0.08 |
| Government | 296 | 85.8 | 422 | 81.3 | |
| Intentionality of pregnancy | |||||
| Intended to become pregnant at that time | 233 | 68.1 | 322 | 63.5 | 0.39 |
| Indifferent about becoming pregnant at that time | 42 | 12.3 | 75 | 14.8 | |
| Intended to become pregnant later | 20 | 5.8 | 41 | 8.1 | |
| Did not intend to become pregnant at all | 47 | 13.7 | 69 | 13.6 | |
| Maternal cigarette smokingb | |||||
| No | 322 | 93.9 | 455 | 89.6 | 0.03 |
| Yes | 21 | 6.1 | 53 | 10.4 | |
| Maternal alcohol consumptionb | |||||
| No | 243 | 72.3 | 353 | 69.9 | 0.45 |
| Yes | 93 | 27.7 | 152 | 30.1 | |
| Use of a prenatal vitaminc | |||||
| No | 22 | 6.4 | 31 | 6.0 | 0.83 |
| Yes | 324 | 93.6 | 486 | 94.0 | |
| Use of a multivitaminc | |||||
| No | 304 | 88.6 | 432 | 83.7 | 0.04 |
| Yes | 39 | 11.4 | 84 | 16.3 | |
| Use of an iron-specific vitaminc | |||||
| No | 234 | 68.6 | 380 | 74.5 | 0.06 |
| Yes | 107 | 31.4 | 130 | 25.5 | |
| Consumption of cerealc | |||||
| No | 27 | 7.9 | 70 | 13.5 | 0.01 |
| Yes | 316 | 92.1 | 448 | 86.5 | |
| Use of other supplementsc | |||||
| No | 306 | 88.7 | 456 | 87.9 | 0.71 |
| Yes | 39 | 11.3 | 63 | 12.1 | |
Abbreviations: ASD, autism spectrum disorder; CHARGE, Childhood Autism Risks from Genetics and the Environment; TD, typical development.
a P values were derived from χ2 tests comparing category proportions between the ASD group and the TD group.
b Any reported intake during the period from 3 months before pregnancy through the end of pregnancy.
c Any reported intake during the index period (3 months before pregnancy, during pregnancy, or while breastfeeding).
Of the 520 ASD and 346 TD CHARGE participants eligible for these analyses, 510 (98%) ASD participants and 341 (99%) TD participants had information on maternal use of iron supplements during the index period, and 454 (87%) ASD participants and 307 (89%) TD participants had information on total average maternal iron intake from all sources.
Twenty-five percent of mothers of children with ASD and 31% of mothers of TD children reported taking an iron-specific supplement at any time during the index period (Table 2), producing a significant association between reported iron supplement use and ASD (odds ratio (OR) = 0.63, 95% confidence interval (CI): 0.44, 0.91) after adjustment for maternal folic acid intake, home ownership, and the child's birth year. More mothers reported taking an iron supplement in the latter half of pregnancy (Figure 1). Adjusted odds ratios for associations between ASD and reported use of iron supplements were consistently below the null across the index period but were nonsignificant except during breastfeeding (among those who breastfed) (Figure 2; also see Web Table 1, available at http://aje.oxfordjournals.org/). Adjusted odds ratios also differed by the child's birth year (Web Table 2, Web Figure 1).
Table 2.
Use of Iron Supplements During the Index Perioda by Mothers of Children With Autism Spectrum Disorder and Mothers of Children With Typical Development, CHARGE Study, California, 2003–2009
| Iron-Specific Vitamin Useb |
TD (n = 341) |
ASD (n = 510) |
Odds Ratioc |
95% Confidence Interval |
P Value | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| No | 234 | 68.6 | 380 | 74.5 | 1 | Reference | |
| Yes | 107 | 31.4 | 130 | 25.5 | 0.63 | 0.44, 0.91 | 0.01 |
Abbreviations: ASD, autism spectrum disorder; CHARGE, Childhood Autism Risks from Genetics and the Environment; TD, typical development.
a The index period was defined as the period from 3 months before pregnancy through breastfeeding.
b Use at any time during the index period.
c Adjusted for maternal periconceptional folic acid intake, child's year of birth, and home ownership.
Figure 1.
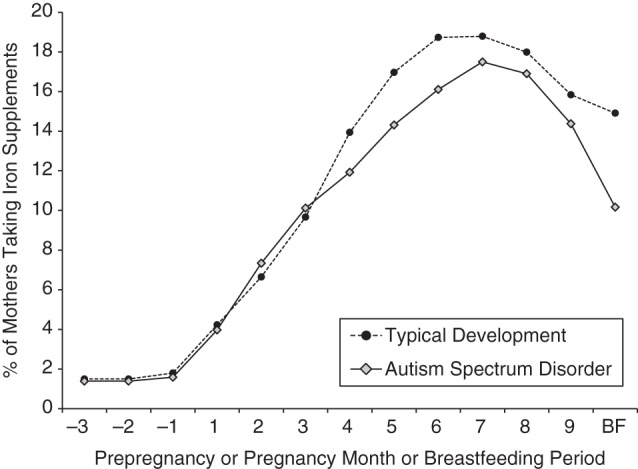
Percentages of mothers of children with autism spectrum disorder and mothers of children with typical development who took iron supplements during the index period (from 3 months before pregnancy through the end of pregnancy and during breastfeeding), Childhood Autism Risks from Genetics and the Environment (CHARGE) Study, California, 2003–2009. A trend towards a significant difference between groups was found during the breastfeeding (BF) period (P = 0.06).
Figure 2.
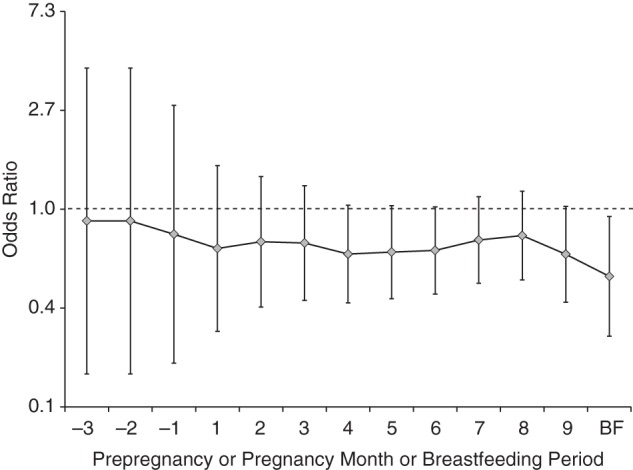
Adjusted odds ratios for associations between reported maternal iron supplement intake during the index period (from 3 months before pregnancy through the end of pregnancy and during breastfeeding) and autism spectrum disorder, Childhood Autism Risks from Genetics and the Environment (CHARGE) Study, California, 2003–2009. Odds ratios were adjusted for maternal folic acid intake during the first month of pregnancy, child's year of birth, and home ownership. Vertical bars represent 95% confidence intervals. Frequencies and P values are presented in Web Table 2. There was a significant association with autism spectrum disorder during the breastfeeding (BF) period (P < 0.05) and a borderline-significant association during months 4, 5, 6, and 9 of pregnancy (P < 0.10).
Prenatal vitamins were the greatest source of iron for mothers in both diagnostic groups during the index period (Table 3). Mean daily maternal iron intake from cereal and total iron intake from all collected sources during the index period were significantly lower for children with ASD than for children with TD, while maternal intake of iron from multivitamins was higher (Table 3).
Table 3.
Mean Maternal Iron Intake During the Index Period,a by Source, CHARGE Study, California, 2003–2009
| Source of Iron | Maternal Iron Intake,b mg/day |
Difference, mg/day | P Valuec | |
|---|---|---|---|---|
| TD | ASD | |||
| Prenatal vitamins | 32.2 (20.9) | 29.5 (15.8) | −2.7 | 0.39 |
| Iron supplements | 15.9 (28.5) | 13.6 (28.4) | −2.4 | 0.08 |
| Cereal | 7.0 (7.5) | 6.3 (7.6) | −0.6 | 0.047 |
| Multivitamins | 1.7 (6.7) | 2.3 (6.0) | 0.6 | 0.04 |
| Other supplements | 0.2 (0.8) | 0.3 (1.2) | 0.1 | 0.30 |
| Total | 57.1 (36.6) | 51.7 (34.0) | −5.5 | 0.03 |
Abbreviations: ASD, autism spectrum disorder; CHARGE, Childhood Autism Risks from Genetics and the Environment; SD, standard deviation; TD, typical development.
a The index period was defined as the period from 3 months before pregnancy through breastfeeding.
b Values are presented as mean (standard deviation).
c Wilcoxon 2-sample test.
Mean total iron intake was highest in the second half of pregnancy for both groups (Figure 3). Mean intake reported by case mothers was lower than that reported by control mothers, especially for the months before pregnancy and during early pregnancy (Figure 3). During this time (from 3 months before pregnancy through the second month of pregnancy), nonsignificantly more case mothers (74%) than control mothers (69%) (P = 0.13) had iron intakes below the Dietary Reference Intake for iron established by the Institute of Medicine for nonpregnant (18 mg/day) and pregnant (27 mg/day) women aged 19–50 years (39).
Figure 3.
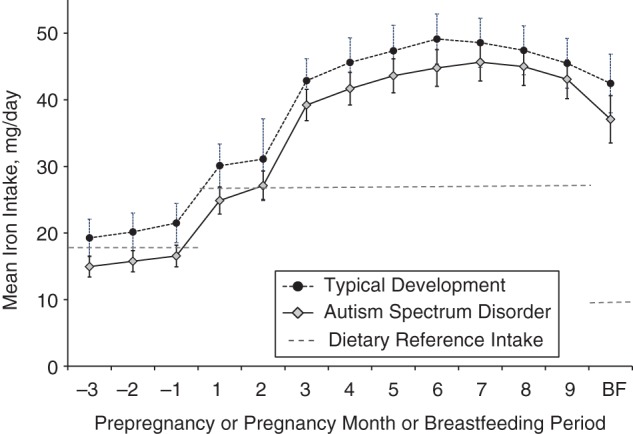
Mean iron intakes of mothers of children with autism spectrum disorder and mothers of children with typical development during the index period (from 3 months before pregnancy through the end of pregnancy and during breastfeeding), Childhood Autism Risks from Genetics and the Environment (CHARGE) Study, California, 2003–2009. Vertical bars represent 95% confidence intervals. Dietary Reference Intakes for iron in females aged 19–50 years are 18 mg/day for all women, 27 mg/day during pregnancy, and 9 mg/day during lactation (39). Significant differences between mothers of children with autism spectrum disorder and mothers of children with typical development were found for the 3 months before and the first month of pregnancy (P < 0.01) and for the second month of pregnancy (P < 0.05). A borderline-significant association was observed for months 3, 4, and 6 of pregnancy and during the breastfeeding (BF) period (P < 0.10).
The highest category of maternal iron intake (≥86 mg/day) during the index period was associated with significantly reduced risk of ASD in the child, before and after adjustment for supplemental periconceptional folic acid intake, child's birth year, and home ownership (OR = 0.49, 95% CI: 0.29, 0.82) (Table 4). ASD risk decreased as mean maternal iron intake increased (Ptrend = 0.01) (Table 4). These findings remained after imputation of missing values and when using survey weights (Table 4).
Table 4.
Odds Ratios for Associations Between Quintile of Mean Maternal Iron Intake During the Index Perioda and Child's Risk of Autism Spectrum Disorder, CHARGE Study, California, 2003–2009
| Quintile of Mean Iron Intake, mg/day | TD (n = 307) |
ASD (n = 454) |
Crude ORb | 95% CI | P Value | Adjusted ORc | 95% CI | P Value | Imputed-data ORc,d | 95% CI | P Value | Weighted ORc,e | 95% CI | P Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | |||||||||||||
| <30 | 60 | 19.5 | 116 | 25.6 | 1 | Reference | 1 | Reference | 1 | Reference | 1 | Reference | ||||
| 30–<36 | 61 | 19.9 | 92 | 20.3 | 0.78 | 0.50, 1.23 | 0.28 | 0.92 | 0.57, 1.51 | 0.75 | 0.87 | 0.55, 1.39 | 0.57 | 1.08 | 0.64, 1.83 | 0.75 |
| 36–<52 | 63 | 20.5 | 88 | 19.4 | 0.72 | 0.46, 1.14 | 0.16 | 0.80 | 0.48, 1.33 | 0.38 | 0.82 | 0.51, 1.33 | 0.43 | 0.85 | 0.49, 1.49 | 0.38 |
| 52–<86 | 60 | 19.5 | 92 | 20.3 | 0.79 | 0.50, 1.25 | 0.32 | 0.76 | 0.46, 1.24 | 0.27 | 0.74 | 0.46, 1.19 | 0.21 | 0.76 | 0.45, 1.30 | 0.27 |
| ≥86 | 63 | 20.5 | 66 | 14.5 | 0.54 | 0.34, 0.87 | 0.01 | 0.49 | 0.29, 0.82 | 0.01 | 0.52 | 0.32, 0.84 | 0.01 | 0.46 | 0.27, 0.79 | 0.01 |
| 2-sided P for trend | 0.02 | 0.02 | 0.01 | 0.01 | 0.004 | |||||||||||
Abbreviations: ASD, autism spectrum disorder; CHARGE, Childhood Autism Risks from Genetics and the Environment; CI, confidence interval; OR, odds ratio; TD, typical development.
a The index period was defined as the period from 3 months before pregnancy through breastfeeding.
b Unadjusted.
c Adjusted for maternal periconceptional folic acid intake, child's birth year, and home ownership.
d Missing values were imputed.
e To account for unequal sampling and response probabilities, estimated weights based on case group and demographic factors were used.
Adjusted odds ratios for the association between ASD and each quintile of maternal iron intake differed across the index period: Odds ratios were near the null during the months before pregnancy; above the null for the first 2 months of pregnancy (after adjustment for the highly correlated folic acid intake during this time); consistently below the null for the highest quintile from the third month of pregnancy onward; and below the null during breastfeeding (Figure 4, Web Table 3). Unadjusted odds ratios were more consistent across time (Web Figure 2). Post-hoc analysis showed that estimates for iron-specific supplement intake and the highest quintiles of iron intake during breastfeeding were not meaningfully different after adjustment for folic acid intake during breastfeeding (Web Table 4).
Figure 4.
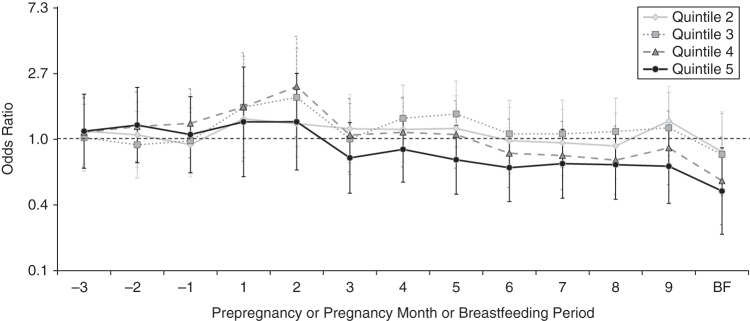
Adjusted odds ratios for associations between mean maternal iron intake during the index period (from 3 months before pregnancy through the end of pregnancy and during breastfeeding) and autism spectrum disorder, Childhood Autism Risks from Genetics and the Environment (CHARGE) Study, California, 2003–2009. Odds ratios were adjusted for maternal folic acid intake during the first month of pregnancy, child's year of birth, and home ownership. Vertical bars represent 95% confidence intervals. Frequencies and P values are presented in Web Table 3. There was a significant inverse association with autism spectrum disorder for the highest iron quintile (P = 0.02) during the breastfeeding (BF) period; a significant positive association for the fourth quintile (P = 0.04) and a borderline-significant association for the third quintile (P = 0.10) during the second month of pregnancy; and a borderline-significant association for the fourth quintile (P = 0.06) during breastfeeding.
There were no significant differences in mean maternal iron intake on the basis of ASD-related characteristics such as regression, delayed or atypical development, seizure, or verbal status (Web Table 5); however, children with ASD who had experienced early-onset symptoms and delayed or atypical development had larger differences from TD children in mean maternal iron intake than children with ASD who had experienced regression and who did not have delayed or atypical development.
No significant interactions were observed between maternal iron intake and child sex, child or maternal race/ethnicity, or interpregnancy interval. A significant multiplicative interaction (P = 0.002) was found between low maternal iron intake and older maternal age at delivery, with more than a 5-fold increased risk of ASD for mothers aged 35 years or older with iron intake in the lowest quintile as compared with younger mothers with iron intake in the highest quintile (OR = 5.01, 95% CI: 1.98, 12.69) (Table 5). The estimate for the combination of older maternal age and low iron intake was more than 5 times that expected from adding (OR = 0.98) or multiplying (OR = 0.80) their independent associations. There was also a significant multiplicative interaction (P = 0.01), with higher ASD risk being associated with the combination of mothers having metabolic conditions and iron intake in the lowest quintile (OR = 4.72, 95% CI: 1.69, 13.15), which was over twice that expected from adding (OR = 2.07) or multiplying (OR = 2.36) their independent associations (Table 6).
Table 5.
Odds Ratios for Autism Spectrum Disorder According to Maternal Age at the Child's Birth and Category of Mean Maternal Iron Intake During the Index Period,a CHARGE Study, California, 2003–2009
| Category of Mean Iron Intake, mg/day | Maternal Age <35 Years |
Maternal Age ≥35 Years |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. With TD | No. With ASD | ORb | 95% CI | P Value | No. With TD | No. With ASD | ORb | 95% CI | P Value | |
| Highest quintile (≥86) | 49 | 56 | 1 | Reference | 14 | 10 | 0.57 | 0.19, 1.72 | 0.31 | |
| Middle 3 quintiles (30–<86) | 140 | 204 | 1.46 | 0.89, 2.38 | 0.13 | 44 | 68 | 1.85 | 0.98, 3.48 | 0.06 |
| Lowest quintile (<30) | 50 | 75 | 1.41 | 0.79, 2.51 | 0.24 | 10 | 41 | 5.01c | 1.98, 12.69 | 0.001 |
Abbreviations: ASD, autism spectrum disorder; CHARGE, Childhood Autism Risks from Genetics and the Environment; CI, confidence interval; OR, odds ratio; TD, typical development.
a The index period was defined as the period from 3 months before pregnancy through breastfeeding.
b Adjusted for maternal periconceptional folic acid intake, child's birth year, and home ownership.
c The P value for multiplicative interaction was 0.002 for the lowest quintile of iron intake. The expected odds ratios for the combination of the lowest quintile of iron intake and maternal age ≥35 years were 0.98 and 0.80 in the additive and multiplicative models, respectively.
Table 6.
Odds Ratios for Autism Spectrum Disorder According to Maternal Metabolic Conditions Present During Pregnancya and Category of Maternal Iron Intake During the Index Period,b CHARGE Study, California, 2003–2009
| Category of Mean Iron Intake, mg/day | No Maternal Metabolic Condition |
Maternal Metabolic Condition |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. With TD | No. With ASD | ORc | 95% CI | P Value | No. With TD | No. With ASD | ORc | 95% CI | P Value | |
| Highest quintile (≥86) | 53 | 54 | 1 | Reference | 7 | 9 | 1.51 | 0.39, 5.84 | 0.55 | |
| Middle 3 quintiles (30–<86) | 148 | 195 | 1.60 | 0.98, 2.61 | 0.06 | 23 | 41 | 2.21 | 1.01, 4.31 | 0.048 |
| Lowest quintile (<30) | 48 | 71 | 1.56 | 0.87, 2.78 | 0.13 | 6 | 27 | 4.72d | 1.69, 13.15 | 0.003 |
Abbreviations: ASD, autism spectrum disorder; CHARGE, Childhood Autism Risks from Genetics and the Environment; CI, confidence interval; OR, odds ratio; TD, typical development.
a Metabolic conditions included obesity (prepregnancy body mass index (weight (kg)/height (m)2) ≥30), hypertension, and/or diabetes.
b The index period was defined as the period from 3 months before pregnancy through breastfeeding.
c Adjusted for maternal education, race/ethnicity, and periconceptional folic acid intake; child's birth year and sex; home ownership; type of health insurance; and regional center catchment area.
d The P value for multiplicative interaction was 0.01 for the lowest quintile of iron intake. The expected odds ratios for the combination of the lowest quintile of iron intake and maternal metabolic conditions were 2.07 and 2.36 in the additive and multiplicative models, respectively.
In analysis conducted to assess recall bias, the associations between reduced ASD risk and both use of an iron-specific supplement during the index period (OR = 0.52, 95% CI: 0.30, 0.90) and highest quintile of maternal iron intake versus the lowest (OR = 0.38, 95% CI: 0.18, 0.83; Ptrend = 0.03) were stronger when data were limited to children who were under 3.6 years of age (the median age of controls) at the time of interview start than in children who were older (for taking an iron-specific supplement, OR = 0.74, 95% CI: 0.45, 1.20; for highest quintile of maternal iron vs. lowest, OR = 0.65, 95% CI: 0.31, 1.37 (Ptrend = 0.11)) (Web Table 6). There were no significant differences in associations for taking an iron-specific supplement during the index period or higher maternal iron intake when data were stratified by maternal folic acid intake during the first month of pregnancy (<600 µg/day, ≥600 µg/day) (Table 7). Finally, adjusted odds ratios for the association between quintile of iron intake and ASD differed somewhat by the child's birth year, but not for the highest quintile of iron intake compared with the lowest, for which odds ratios were consistently below the null (Web Table 7, Web Figure 3).
Table 7.
Odds Ratios for Autism Spectrum Disorder According to Folic Acid Intake During the First Month of Pregnancy, Use of an Iron-Specific Supplement During the Index Period,a and Quintile of Mean Maternal Iron Intake During the Index Period, CHARGE Study, California, 2003–2009
| Folic Acid Intake <600 µg/dayb |
Folic Acid Intake ≥600 µg/dayb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. With TD | No. With ASD | ORc | 95% CI | P Value | No. With TD | No. With ASD | ORc | 95% CI | P Value | |
| Maternal use of iron-specific supplementd | ||||||||||
| No | 73 | 155 | 1 | Reference | 124 | 173 | 1 | Reference | ||
| Yes | 38 | 54 | 0.58 | 0.34, 0.99 | 0.046 | 47 | 49 | 0.68 | 0.41, 1.12 | 0.13 |
| Quintile of mean iron intake, mg/daye | ||||||||||
| <30 | 29 | 61 | 1 | Reference | 26 | 44 | 1 | Reference | ||
| 30–<36 | 16 | 32 | 0.86 | 0.40, 1.86 | 0.71 | 38 | 55 | 0.98 | 0.50, 1.92 | 0.10 |
| 36–<52 | 19 | 32 | 0.72 | 0.34, 1.54 | 0.40 | 38 | 48 | 0.88 | 0.43, 1.80 | 0.73 |
| 52–<86 | 20 | 46 | 0.85 | 0.41, 1.75 | 0.65 | 33 | 40 | 0.72 | 0.35, 1.48 | 0.37 |
| ≥86 | 22 | 27 | 0.48 | 0.22, 1.01 | 0.054 | 33 | 30 | 0.51 | 0.25, 1.07 | 0.07 |
| 2-sided P for trend | 0.09 | 0.04 | ||||||||
Abbreviations: ASD, autism spectrum disorder; CHARGE, Childhood Autism Risks from Genetics and the Environment; CI, confidence interval; OR, odds ratio; TD, typical development.
a The index period was defined as the period from 3 months before pregnancy through breastfeeding.
b The Dietary Reference Intake established by the Institute of Medicine for folic acid during pregnancy is 600 µg/day (39).
c Adjusted for child's birth year and home ownership.
d P for interaction = 0.63.
e P for interaction: second quintile, P = 0.81; third quintile, P = 0.74; fourth quintile, P = 0.83; fifth quintile, P = 0.84.
DISCUSSION
Findings of this study
To our knowledge, this study was the first to examine maternal iron intake in relation to ASD. We found that mothers of children with ASD had significantly lower iron intakes during the index period than mothers of children with TD. The highest quintile of maternal iron intake during the index period was associated with an approximately halved risk of ASD compared with the lowest quintile. The association between higher maternal iron intake and reduced ASD risk was strongest during breastfeeding, after adjustment for folic acid intake. Further, ASD risk associated with low maternal iron intake was much greater when the mother also was older at the time of the child's birth and/or had metabolic conditions during pregnancy.
Average iron intake from cereals and supplements throughout pregnancy for both groups in this study was above the Tolerable Upper Intake Limit for iron (40) but was in line with national estimates of intake from supplements for pregnant women (48 mg/day) (41) and women of childbearing age (18 mg/day) (42). Notably, the lowest iron quintile (<30 mg/day) for the index period included the amounts recommended for pregnant (27 mg/day), nonpregnant (18 mg/day), and lactating (9 mg/day) women (40). If the results of this study are replicated, it would suggest that the current Recommended Daily Allowance for pregnancy and/or lactation could be set too low to prevent adverse outcomes in the child. A randomized, double-blind trial demonstrated that during pregnancy, 40 mg/day of supplemental iron was needed to prevent iron deficiency in the majority of women and that up to 80 mg/day was well-tolerated (43). On the other hand, even though the amount of iron absorbed is regulated by the gut to correspond to needs and avoid accumulation of excess iron in healthy individuals (44), very high iron intake in supplemental or fortified forms is known to be toxic and has been associated with negative health impacts, including adverse neurodevelopmental outcomes, and could be especially harmful for certain genetically susceptible individuals (42, 45). The stronger association for higher maternal iron intake while breastfeeding is intriguing given the fact that maternal iron intake has been shown to not affect iron concentrations in breast milk (46); however, the very low concentrations of iron in breast milk (relative to those in serum) present measurement problems that could have obscured a relationship, and concentrations in the early postnatal period (before 9 months) have not been examined using modern iron indicators. Alternatively, maternal iron intake could influence breast milk production or composition in other ways.
This study examined maternal iron intake, not maternal or child iron status. Maternal circulation constitutes the only source of iron for the developing fetus (11), and maternal iron intake can influence both the mother's iron status and her child's status (47) during brain development. Our findings may reflect compensation through iron supplementation for poorer iron status resulting from inadequate intake, inefficient uptake or metabolism, or increased needs for iron, producing a functional iron deficiency. It has been demonstrated, primarily in animal studies, that reduced iron supply at several stages of development generates enduring changes in dopamine neurotransmission (48–53) that outlast the iron-deficient periods (52, 54). Long-term effects are observed in adulthood, long after iron repletion, in hippocampal structure and function, monoamine metabolism, and myelination (55–60), indicating that early developmental periods are critical and that prevention of iron deficiency might be key for protecting against adverse neurodevelopmental outcomes. Iron deficiency can also impair the function of several enzymes that are directly involved in antioxidant and nucleic acid metabolism, which could affect genomic stability during periods of DNA synthesis and cell proliferation during development (39). In addition, mothers of children with ASD have been shown to have elevated markers of inflammation (61), and prenatal inflammation is an independent risk factor for ASD (62). Prenatal inflammation can produce a cytokine-mediated reduction of circulating nonheme iron, or hypoferremia (48), that can disrupt fetal brain development and lead to persistent structural and functional brain defects (55–60). Maternal iron supplementation has been shown to prevent effects of inflammation-induced hypoferremia (48).
Metabolic conditions like diabetes and obesity lead to iron deficiency (63, 64), and as their prevalence continues to rise dramatically (65), suboptimal iron status during pregnancy can be expected to increase as well. Notably, metabolic conditions are independently associated with a 1.7-fold increased risk of ASD and nearly a 2-fold risk of developmental delays (36). Our study shows that the combination of maternal metabolic conditions and low supplemental iron intake is associated with a nearly 5-fold increased risk of ASD, and that the ASD risk associated with maternal metabolic conditions was nearly null for persons with the highest supplemental iron intake. This interaction effect, if replicated, implies that maternal supplemental iron is associated with prevention of ASD in children of mothers with metabolic conditions during pregnancy.
The significant interaction between older maternal age and lower supplemental iron intake seems biologically plausible given changes in iron metabolism and storage with age, especially in women (66, 67). If this finding is replicated, more work would be needed to delineate the mechanistic pathways behind this interaction.
Limitations and strengths of this study
The retrospective reporting of vitamin and supplement information after the child's developmental status was known, whereby mothers were asked to recall a period several years before the interview, raises the issues of recall accuracy and bias in this study. A scenario in which recall bias explained part of the association between maternal iron intake and ASD would involve case mothers underreporting or control mothers overreporting their intake of supplements containing iron. Notably, the association between iron supplementation and reduced ASD risk was stronger when women recalled the information for a more recent pregnancy versus a less recent pregnancy, which argues against recall bias in this direction. However, we cannot rule out some role for differential recall across case status.
In addition, during these study years data were not collected on other dietary sources of iron, so information was not available with which to completely assess dietary iron intake. However, fortified cereals, which were included in this study, are the largest source of total dietary iron consumed in the United States (68). In addition, the amount of iron in supplements tends to outweigh the amount of iron found in the diet. Finally, iron supplementation is probably more amenable to prevention strategies than diet is.
We did not collect information on why the mothers took iron supplements, which are often taken after physician recommendations when iron deficiency anemia is detected during pregnancy. However, case mothers reported lower intake of iron from sources other than iron-specific supplements (breakfast cereal) that would not have been as likely to increase in response to an anemia diagnosis. In addition, differences in iron intake between groups were observed not only in late pregnancy, when anemia is more likely to be diagnosed, but throughout the index period. This provides evidence that the association was not entirely due to confounding by indication.
Supplemental intakes of folic acid and iron tend to be correlated, and thus it is difficult to examine their independent contributions. Odds ratios for quintiles of iron intake during the months before pregnancy and during early pregnancy were attenuated after adjustment for folic acid intake, as expected given that periconceptional folic acid is associated with reduced ASD risk (35, 69). However, the association with maternal iron intake during the index period remained strong after adjustment for and stratification across folic acid intake. In addition, the association with iron-specific supplements, which was not correlated with folic acid, was consistently below the null and significant during breastfeeding. Differences in associations by year were probably artifactual, given that the associations were not influenced by year in a consistent pattern. Still, other unmeasured confounding factors associated with taking supplements could have played a role in our findings.
The efficiency of iron uptake and metabolism differs vastly between individuals on the basis of genetic differences (46). It is likely that genetically determined metabolic efficiency could modify the need for and the effects of iron supplementation. These genetic differences were not considered here, and they deserve further evaluation.
Strengths of this study include detailed information systematically collected on numerous potentially confounding variables, clinical confirmation of all ASD diagnoses, and confirmation of typical social and cognitive development for the population-based controls. Additionally, the study's large sample size allowed for stratification of results by case subgroup and by maternal and child factors likely to modify the association.
Conclusions
This study provides initial evidence for an association between increased maternal supplemental iron intake and reduced risk of ASD. Researchers should attempt to replicate this association in additional studies and to further delineate who is metabolically susceptible, clarify what dose of iron during pregnancy and breastfeeding is ideal for neurodevelopment, and identify and refine strategies for prevention of ASD through supplemental iron intake.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Public Health Sciences, School of Medicine, University of California, Davis, Davis, California (Rebecca J. Schmidt, Paula Krakowiak); Medical Investigation of Neurodevelopmental Disorders (MIND) Institute, University of California, Davis, Sacramento, California (Rebecca J. Schmidt, Robin L. Hansen, Sally Ozonoff); Department of Pediatrics, School of Medicine, University of California, Davis, Sacramento, California (Daniel J. Tancredi, Robin L. Hansen); and Department of Psychiatry and Behavioral Sciences, School of Medicine, University of California, Davis, Sacramento, California (Sally Ozonoff).
The Childhood Autism Risks from Genetics and the Environment (CHARGE) Study has been supported by grants R01-ES015359, P01-11269, and 2K12HD051958-06 from the National Institutes of Health; by grants R-829388 and R833292 from the Environmental Protection Agency's Science to Achieve Results (STAR) Program; and by the Medical Investigation of Neurodevelopmental Disorders (MIND) Institute at the University of California, Davis.
We thank Dr. Irva Hertz-Picciotto, principal investigator of the CHARGE Study, for providing the databases necessary for conducting this research. We appreciate the valuable contributions of the CHARGE investigators and staff. We also thank Dr. Mari Golub for providing helpful feedback on the manuscript.
This research was presented in preliminary form at the 12th Annual International Meeting for Autism Research in San Sebastian, Spain, on May 3, 2013; at the Building Interdisciplinary Research Careers in Women's Health (BIRCWH) Directors and Scholars Meeting in Rockville, Maryland, on October 24, 2013; and at the Tenth Annual Interdisciplinary Women's Health Research Symposium in Bethesda, Maryland, on October 24, 2013. An abstract of this article was published in the Journal of Women's Health in October 2013 (J Womens Health. 2013;22(10):897).
The funders played no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.Volkmar FR, Pauls D. Autism. Lancet. 2003;3629390:1133–1141 [DOI] [PubMed] [Google Scholar]
- 2.Lawler CP, Croen LA, Grether JK, et al. Identifying environmental contributions to autism: provocative clues and false leads. Ment Retard Dev Disabil Res Rev. 2004;104:292–302 [DOI] [PubMed] [Google Scholar]
- 3.Veenstra-Vanderweele J, Christian SL, Cook EH, Jr. Autism as a paradigmatic complex genetic disorder. Annu Rev Genomics Hum Genet. 2004;5:379–405 [DOI] [PubMed] [Google Scholar]
- 4.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;632:1–21 [PubMed] [Google Scholar]
- 5.Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol. 2014;432:443–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheslack-Postava K, Liu K, Bearman PS. Closely spaced pregnancies are associated with increased odds of autism in California sibling births. Pediatrics. 2011;1272:246–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt RJ, Hansen RL, Hartiala J, et al. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology. 2011;224:476–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoltzfus RJ. Defining iron-deficiency anemia in public health terms: a time for reflection. J Nutr . 2001;1312:565S–567S [DOI] [PubMed] [Google Scholar]
- 9.O'Brien KO, Zavaleta N, Abrams SA, et al. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr. 2003;774:924–930 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Iron Deficiency Anaemia: Assessment, Prevention and Control. Geneva, Switzerland: World Health Organization; 2001 [Google Scholar]
- 11.Millard KN, Frazer DM, Wilkins SJ, et al. Changes in the expression of intestinal iron transport and hepatic regulatory molecules explain the enhanced iron absorption associated with pregnancy in the rat. Gut. 2004;535:655–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tchernia G, Archambeaud MP, Yvart J, et al. Erythrocyte ferritin in human neonates: maternofetal iron kinetics revisited. Clin Lab Haematol. 1996;183:147–153 [DOI] [PubMed] [Google Scholar]
- 13.Colomer J, Colomer C, Gutierrez D, et al. Anaemia during pregnancy as a risk factor for infant iron deficiency: report from the Valencia Infant Anaemia Cohort (VIAC) study. Paediatr Perinat Epidemiol. 1990;42:196–204 [DOI] [PubMed] [Google Scholar]
- 14.Beard JL. Effectiveness and strategies of iron supplementation during pregnancy. Am J Clin Nutr. 2000;71(5 suppl):1288S–1294S [DOI] [PubMed] [Google Scholar]
- 15.Sachdev H, Gera T, Nestel P. Effect of iron supplementation on mental and motor development in children: systematic review of randomized controlled trials. Public Health Nutr. 2005;82:117–132 [DOI] [PubMed] [Google Scholar]
- 16.Black MM, Baqui AH, Zaman K, et al. Iron and zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. Am J Clin Nutr. 2004;804:903–910 [DOI] [PubMed] [Google Scholar]
- 17.Siddappa AM, Georgieff MK, Wewerka S, et al. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res. 2004;556:1034–1041 [DOI] [PubMed] [Google Scholar]
- 18.Stoltzfus RJ, Kvalsvig JD, Chwaya HM, et al. Effects of iron supplementation and anthelmintic treatment on motor and language development of preschool children in Zanzibar: double blind, placebo controlled study. BMJ. 2001;3237326:1389–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. N Engl J Med. 1991;32510:687–694 [DOI] [PubMed] [Google Scholar]
- 20.Hergüner S, Keleşog˘lu FM, Tanıdır C, et al. Ferritin and iron levels in children with autistic disorder. Eur J Pediatr. 2012;1711:143–146 [DOI] [PubMed] [Google Scholar]
- 21.Dosman CF, Brian JA, Drmic IE, et al. Children with autism: effect of iron supplementation on sleep and ferritin. Pediatr Neurol. 2007;363:152–158 [DOI] [PubMed] [Google Scholar]
- 22.Reynolds A, Krebs NF, Stewart PA, et al. Iron status in children with autism spectrum disorder. Pediatrics. 2012;130(suppl 2):S154–S159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen D, Van Naarden Braun K, Doernberg NS, et al. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning—Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol. 2014;561:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chauhan A, Chauhan V, Brown WT, et al. Oxidative stress in autism: increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin—the antioxidant proteins. Life Sci. 2004;7521:2539–2549 [DOI] [PubMed] [Google Scholar]
- 25.Hertz-Picciotto I, Croen LA, Hansen R, et al. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;1147:1119–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullen EM. Scales of Early Learning. Circle Pines, MN: American Guidance Services, Inc.; 1995 [Google Scholar]
- 27.Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales: Interview Edition, Expanded Form Manual. Circle Pines, MN: American Guidance Services, Inc.; 1984 [Google Scholar]
- 28.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord . 1994;245:659–685 [DOI] [PubMed] [Google Scholar]
- 29.Lord C, Pickles A, McLennan J, et al. Diagnosing autism: analyses of data from the Autism Diagnostic Interview. J Autism Dev Disord. 1997;275:501–517 [DOI] [PubMed] [Google Scholar]
- 30.Lord C, Rutter M, DiLavore PC, et al. The Autism Diagnostic Observation Schedule (ADOS). Los Angeles, CA: Western Psychological Services; 2000 [Google Scholar]
- 31.Lord C, Rutter M, DiLavore PC, et al. Autism Diagnostic Observation Schedule Manual. Los Angeles, CA: Western Psychological Services; 2003 [Google Scholar]
- 32.Rutter M, Bailey A, Lord C, et al. Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003 [Google Scholar]
- 33.Risi S, Lord C, Gotham K, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2006;459:1094–1103 [DOI] [PubMed] [Google Scholar]
- 34.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington, VA: American Psychiatric Association; 2013 [Google Scholar]
- 35.Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;961:80–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krakowiak P, Walker CK, Bremer AA, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;1295:e1121–e1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schafer JL. Analysis of Incomplete Multivariate Data. 1st ed. (CRC Monographs on Statistics and Applied Probability). London, United Kingdom: Chapman & Hall Ltd.; 1997 [Google Scholar]
- 38.Kalton G, Piesse A. Survey research methods in evaluation and case-control studies. Stat Med. 2007;268:1675–1687 [DOI] [PubMed] [Google Scholar]
- 39.Prá D, Franke SI, Henriques JA, et al. Iron and genome stability: an update. Mutat Res. 2012;733(1-2):92–99 [DOI] [PubMed] [Google Scholar]
- 40.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc: A Report of the Panel on Micronutrients. Washington, DC: National Academy Press; 2001 [Google Scholar]
- 41.Branum AM, Bailey R, Singer BJ. Dietary supplement use and folate status during pregnancy in the United States. J Nutr. 2013;1434:486–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanck HM, Cogswell ME, Gillespie C, et al. Iron supplement use and iron status among US adults: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2005;825:1024–1031 [DOI] [PubMed] [Google Scholar]
- 43.Milman N, Bergholt T, Eriksen L, et al. Iron prophylaxis during pregnancy—how much iron is needed? A randomized dose-response study of 20–80 mg ferrous iron daily in pregnant women. Acta Obstet Gynecol Scand. 2005;843:238–247 [DOI] [PubMed] [Google Scholar]
- 44.Beard J. Dietary iron intakes and elevated iron stores in the elderly: Is it time to abandon the set-point hypothesis of regulation of iron absorption? Am J Clin Nutr. 2002;766:1189–1190 [DOI] [PubMed] [Google Scholar]
- 45.Tamura T, Goldenberg RL, Hou J, et al. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;1402:165–170 [DOI] [PubMed] [Google Scholar]
- 46.Domellöf M, Lönnerdal B, Dewey KG, et al. Iron, zinc, and copper concentrations in breast milk are independent of maternal mineral status. Am J Clin Nutr. 2004;791:111–115 [DOI] [PubMed] [Google Scholar]
- 47.Mahomed K. Iron supplementation in pregnancy. Cochrane Database Syst Rev. 2000;2:CD000117. [DOI] [PubMed] [Google Scholar]
- 48.Aguilar-Valles A, Flores C, Luheshi GN. Prenatal inflammation-induced hypoferremia alters dopamine function in the adult offspring in rat: relevance for schizophrenia. PLoS One. 2010;56:e10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58 [DOI] [PubMed] [Google Scholar]
- 50.Youdim MB, Sills MA, Heydorn WE, et al. Iron deficiency alters discrete proteins in rat caudate nucleus and nucleus accumbens. J Neurochem. 1986;473:794–799 [DOI] [PubMed] [Google Scholar]
- 51.Beard JL, Felt B, Schallert T, et al. Moderate iron deficiency in infancy: biology and behavior in young rats. Behav Brain Res. 2006;1702:224–232 [DOI] [PubMed] [Google Scholar]
- 52.Kwik-Uribe CL, Gietzen D, German JB, et al. Chronic marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition. J Nutr. 2000;13011:2821–2830 [DOI] [PubMed] [Google Scholar]
- 53.Unger EL, Paul T, Murray-Kolb LE, et al. Early iron deficiency alters sensorimotor development and brain monoamines in rats. J Nutr. 2007;1371:118–124 [DOI] [PubMed] [Google Scholar]
- 54.Felt BT, Beard JL, Schallert T, et al. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res. 2006;1712:261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lozoff B, Beard J, Connor J, et al. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;645:S34–S43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beard JL, Wiesinger JA, Connor JR. Pre- and postweaning iron deficiency alters myelination in Sprague-Dawley rats. Dev Neurosci. 2003;255:308–315 [DOI] [PubMed] [Google Scholar]
- 57.Carlson ES, Stead JD, Neal CR, et al. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus. 2007;178:679–691 [DOI] [PubMed] [Google Scholar]
- 58.Clardy SL, Wang X, Zhao W, et al. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm Suppl. 2006;71:173–196 [DOI] [PubMed] [Google Scholar]
- 59.Jorgenson LA, Sun M, O'Connor M, et al. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;158:1094–1102 [DOI] [PubMed] [Google Scholar]
- 60.Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci. 2003;256:412–420 [DOI] [PubMed] [Google Scholar]
- 61.Goines PE, Croen LA, Braunschweig D, et al. Increased midgestational IFN-γ, IL-4 and IL-5 in women bearing a child with autism: a case-control study. Mol Autism. 2011;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zerbo O, Iosif AM, Walker C, et al. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord. 2013;431:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sandin S, Lichtenstein P, Kuja-Halkola R, et al. The familial risk of autism. JAMA. 2014;31117:1770–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keil AP, Daniels JL, Hertz-Picciotto I. Autism spectrum disorder, flea and tick medication, and adjustments for exposure misclassification: the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Environ Health. 2014;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simmons D. Diabetes and obesity in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;251:25–36 [DOI] [PubMed] [Google Scholar]
- 66.Volk HE, Kerin T, Lurmann F, et al. Autism spectrum disorder: interaction of air pollution with the MET receptor tyrosine kinase gene. Epidemiology. 2014;251:44–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord. 2014;445:1117–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Subar AF, Krebs-Smith SM, Cook A, et al. Dietary sources of nutrients among US adults, 1989 to 1991. J Am Diet Assoc. 1998;985:537–547 [DOI] [PubMed] [Google Scholar]
- 69.Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;3096:570–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


