Abstract
Background
Semaphorins are guidance proteins implicated in several processes such as angiogenesis, organogenesis, cell migration, and cytokine release. Experimental studies showed that semaphorin-3a (SEMA3A) administration induces transient massive proteinuria, podocyte foot process effacement and endothelial cell damage in healthy animals. While SEMA3A signaling has been demonstrated to be mechanistically involved in experimental diabetic glomerulopathy and in acute kidney injury, to date its role in human chronic kidney disease (CKD) has not been investigated.
Methods
To test the hypothesis that SEMA3A may play a role in human CKD, we performed a cross-sectional, nested, case–control study on 151 matched hypertensive patients with and without CKD. SEMA3A was quantified in the urine (USEMA) by ELISA. Glomerular filtration rate was estimated (eGFR) by the CKD-EPI formula and albuminuria was measured as albumin-to-creatinine ratio (ACR).
Results
USEMA levels were positively correlated with urine ACR (p = 0.001) and serum creatinine (p < 0.001). USEMA was higher in patients with both components of renal damage as compared to those with only one and those with normal renal function (p < 0.007 and <0.001, respectively). The presence of increased USEMA levels (i.e. top quartile) entailed a fourfold higher risk of combined renal damage (p < 0.001) and an almost twofold higher risk of macroalbuminuria (p = 0.005) or of reduced eGFR, even adjusting for confounding factors (p = 0.002).
Conclusions
USEMA is independently associated with CKD in both diabetic and non diabetic hypertensive patients. Further studies may help clarify the mechanisms underlying this association and possibly the pathogenic changes leading to the development of CKD.
Keywords: Albuminuria, Chronic kidney disease, Diabetes, Hypertension, Semaphorin-3A
Introduction
Greater knowledge of the cellular and molecular mechanisms underlying the development and progression of hypertensive and diabetic renal damage may lead to the identification of new biomarkers of disease progression and eventually to more effective treatment. Semaphorins are a large family of guidance proteins that have been implicated in several cellular developmental processes [1, 2]. Semaphorin-3a (SEMA3A) has been shown to be involved in a number of rather different biological mechanisms. Indeed, SEMA3A is known to regulate immune response by suppressing both T and B cell autoimmunity [1]. SEMA3A levels have been inversely related to disease activity and to the degree of renal damage in systemic lupus erythematosus and rheumatoid arthritis (RA) patients [3, 4]. Interestingly, both SEMA3A and its receptor neuropilin-1 are expressed in the developing kidney [5–7], and SEMA3A remains expressed in adult podocytes and collecting tubules [8]. While SEMA3A inhibits ureteric bud branching by downregulating the glial cell-line-derived neurotrophic factor [6], the regulation of SEMA3A expression in the kidney and its pathophysiological role are unknown.
In animal studies, administering SEMA3A induces acute and transient massive proteinuria [9]. Furthermore, SEMA3A is secreted into the urine in response to hypoxia, and preliminary studies suggest that urine SEMA (USEMA) could be a promising acute kidney injury (AKI) biomarker in critically ill patients [10]. While it has been hypothesized that SEMA3A signaling may be implicated in microvascular lesions and mesangiolysis in experimental diabetic nephropathy [11], to date its role in chronic kidney disease (CKD) has never been investigated. We therefore decided to investigate the relationship between USEMA and the presence and degree of renal damage in a cross-sectional, case–control study on hypertensive patients with or without diabetes.
Subjects and methods
Study design and selection of patients
The cohort object of the present study was derived from the Italy-Developing Education and awareness on MicroAlbuminuria in patients with hypertensive Disease (I-DEMAND) study. Details on the design of the study, inclusion criteria and study procedures have been previously published [12, 13]. In brief, participants included patients between 18 and 80 years of age recruited in 87 centers of specialized care (Internal Medicine, Cardiology, Nephrology, Diabetology) with treated or untreated hypertension documented for at least 1 year. Regarding renal involvement, exclusion criteria were acute renal failure or rapid deterioration of renal function in patients with chronic renal failure, serum creatinine more than 3 mg/dl, secondary hypertension (with the exception of nephroparenchymal hypertension) and clinical signs of urinary tract infection. Glomerular filtration rate was estimated (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [14]. Reduced GFR (eGFR+) was defined as GFR <60 ml/min/1.73 m2. Albuminuria was evaluated by measuring the urinary albumin-to-creatinine ratio (ACR) from a single urine specimen. Albuminuria was measured by immunonephelometry on an Immage Immunochemistry System (Beckman Coulter Inc., Fullerton, CA, USA) using the manufacturer’s reagents. Macroalbuminuria (AlbU+) was defined as ACR ≥35 mg/mmol [15]. CKD was defined as either macroalbuminuria or GFR<60 ml/min/1.73 m2.
In the present case–control study, a group of 151 age- (±5 years), gender-, diabetes-, and body mass index (BMI) (±1 point)-matched hypertensive patients were extracted from the original I-DEMAND database (n = 4,151) and pair-matched with a nested, case–control methodology to form four groups on the basis of the presence/absence of eGFR reduction, macroalbuminuria, both or neither.
Urinary biomarker measurement
Urine samples were spun at 10,000 rpm for 5 min and 50 µl supernatant was used for USEMA quantification by enzyme-linked immunosorbent assay (ELISA) (Cat # MBS732622, My Biosource, San Diego, CA, USA). Briefly, USEMA standard and samples were added to antibody-coated 96-well plates, after which 100 µl of conjugate was placed in each well. The plates were incubated at 37 °C for 1 h after mixing thoroughly, then washed and incubated with tetramethylbenzidine (TMB) substrate for 20 min and reaction was arrested by adding sulfuric acid. The color change was measured using a plate reader (BioTek Synergy HT, BioTek Instruments Inc., Winooski, VT, USA) at a wavelength of 450 nm. The concentration of SEMA3A in the samples was then determined by comparing the optical density of the samples to the standard curve. The minimum detectable level of SEMA3A is typically <0.1 ng/ml. All measurements were made in duplicate and in a blinded fashion.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) or median with interquartile range as appropriate, and categorical variables are expressed as a percentage. Data that were not normally distributed, such as urinary SEMA3A/creatinine (USemaCR), ACR and triglycerides, were logarithmically transformed before analysis. Comparisons among groups were made by analysis of variation (ANOVA) for continuous variables and χ2 test for categorical variables. Logistic regression analysis was used to describe the relationship between SEMA3A and the presence of CKD and its components. Odds ratios (OR) and 95 % confidence intervals (CI) were calculated by exponentiation of logistic regression coefficients. Statistical analyses were performed using Statview for Windows (SAS Institute Inc., version 5.0.1, Cary, NC, USA). A p value <0.05 was considered statistically significant.
Results
The study sample was composed of 151 patients aged 60 ± 6 years, 48 % males, and 49 % with type II diabetes. The main clinical characteristics of the study population as a whole and when analyzed on the basis of GFR and ACR values are shown in Table 1. Due to the study design, anthropometric, clinical and hemato-chemical characteristics were similar among the four groups of patients, except for waist circumference, serum uric acid and USEMA which increased along with the severity of renal damage.
Table 1.
Clinical characteristics of the study patients according to albuminuria and eGFR
| Variable | All | eGFR− AlbU− |
eGFR− AlbU+ |
eGFR+ AlbU− |
eGFR+ AlbU+ |
p for trend |
|---|---|---|---|---|---|---|
| N | 151 | 39 | 32 | 41 | 39 | |
| eGFR (ml/min) | 62 ± 23 | 82 ± 13 | 83 ± 16 | 50 ± 8 | 38 ± 12 | <0.001 |
| ACR | 66.8 ± 114.1 | 1.0 ± 0.6 | 101.7 ± 83.0 | 0.8 ± 0.6 | 173.3 ± 154.7 | <0.001 |
| Diabetes (%) | 49 | 49 | 44 | 49 | 51 | 0.938 |
| Sex (% males) | 48 | 49 | 44 | 49 | 49 | 0.969 |
| Age (years) | 60 ± 6 | 60 ± 1 | 60 ± 10 | 61 ± 2 | 61 ± 7 | 0.831 |
| BMI (kg/m2) | 28.6 ± 4.8 | 27.7 ± 3.9 | 28.9 ± 6.0 | 28.7 ± 4.0 | 29.2 ± 5.4 | 0.672 |
| WC (cm) | 100 ± 12 | 96 ± 9 | 98 ± 14 | 100 ± 11 | 104 ± 13 | 0.042 |
| Systolic BP (mmHg) | 141 ± 18 | 138 ± 15 | 140 ± 21 | 140 ± 17 | 147 ± 20 | 0.143 |
| Diastolic BP (mmHg) | 83 ± 10 | 83 ± 9 | 81 ± 10 | 83 ± 8 | 86 ± 12 | 0.185 |
| Mean BP (mmHg) | 103 ± 12 | 101 ± 10 | 100 ± 13 | 102 ± 10 | 106 ± 13 | 0.133 |
| Pulse pressure (mmHg) | 58 ± 14 | 55 ± 10 | 59 ± 17 | 57 ± 15 | 61 ± 13 | 0.269 |
| Fasting serum glucose (mg/dl) | 130 ± 50 | 118 ± 37 | 148 ± 68 | 124 ± 47 | 134 ± 45 | 0.081 |
| Serum creatinine (mg/dl) | 1.3 ± 0.5 | 0.9 ± 0.2 | 0.9 ± 0.2 | 1.4 ± 0.3 | 1.8 ± 0.5 | <0.001 |
| Serum uric acid (mg/dl) | 5.8 ± 1.7 | 5.1 ± 1.6 | 5.3 ± 1.4 | 5.9 ± 1.6 | 6.8 ± 1.6 | 0.006 |
| Triglycerides (mg/dl) | 165 ± 99 | 167 ± 128 | 158 ± 80 | 152 ± 60 | 182 ± 114 | 0.599 |
| Cholesterol (mg/dl) | 208 ± 43 | 204 ± 35 | 214 ± 39 | 206 ± 40 | 208 ± 57 | 0.803 |
| HDL-cholesterol (mg/dl) | 50 ± 13 | 51 ± 12 | 52 ± 11 | 46 ± 12 | 50 ± 16 | 0.222 |
| LDL-cholesterol (mg/dl) | 126 ± 39 | 122 ± 33 | 136 ± 41 | 131 ± 37 | 115 ± 43 | 0.147 |
| USEMA (pg/ml) | 61 ± 42 | 46 ± 32 | 53 ± 34 | 58 ± 30 | 85 ± 54 | <0.001 |
| USemaCR (pg/mg) | 90 ± 91 | 69 ± 95 | 71 ± 61 | 93 ± 96 | 125 ± 97 | 0.0250 |
| LogSEMA | 1.66 ± 0.38 | 1.51 ± 0.41 | 1.62 ± 0.32 | 1.65 ± 0.40 | 1.87 ± 0.27 | <0.001 |
| LogUSemaCR | 4.02 ± 1.07 | 3.60 ± 1.13 | 3.93 ± 0.84 | 3.97 ± 1.20 | 4.60 ± 0.75 | <0.001 |
| Antihypertensive treatment (%) | 97 | 95 | 97 | 95 | 100 | 0.561 |
| RAAS inhibitors (%) | 86 | 75 | 87 | 90 | 87 | 0.551 |
| Diuretics (%) | 42 | 31 | 37 | 51 | 46 | 0.262 |
| Antiplatelet therapy (%) | 39 | 36 | 44 | 37 | 41 | 0.889 |
| Statins (%) | 42 | 49 | 34 | 36 | 49 | 0.442 |
| Glucose lowering drugs (%) | 46 | 41 | 56 | 41 | 46 | 0.556 |
Data are mean ± standard deviation (SD) or percentage
eGFR estimated glomerular filtration rate, eGFR− eGFR ≥60 ml/min, eGFR+ eGFR <60 ml/min, AlbU− normoalbuminuria, AlbU+ macroalbuminuria, ACR urinary albumin creatinine ratio, BMI body mass index, WC waist circumference, BP blood pressure, HDL high density lipoprotein, LDL low density lipoprotein, USEMA urinary Semaphorin-3A, UsemaCR urinary semaphorin 3A creatinine ratio, RAAS renin–angiotensin–aldosterone system
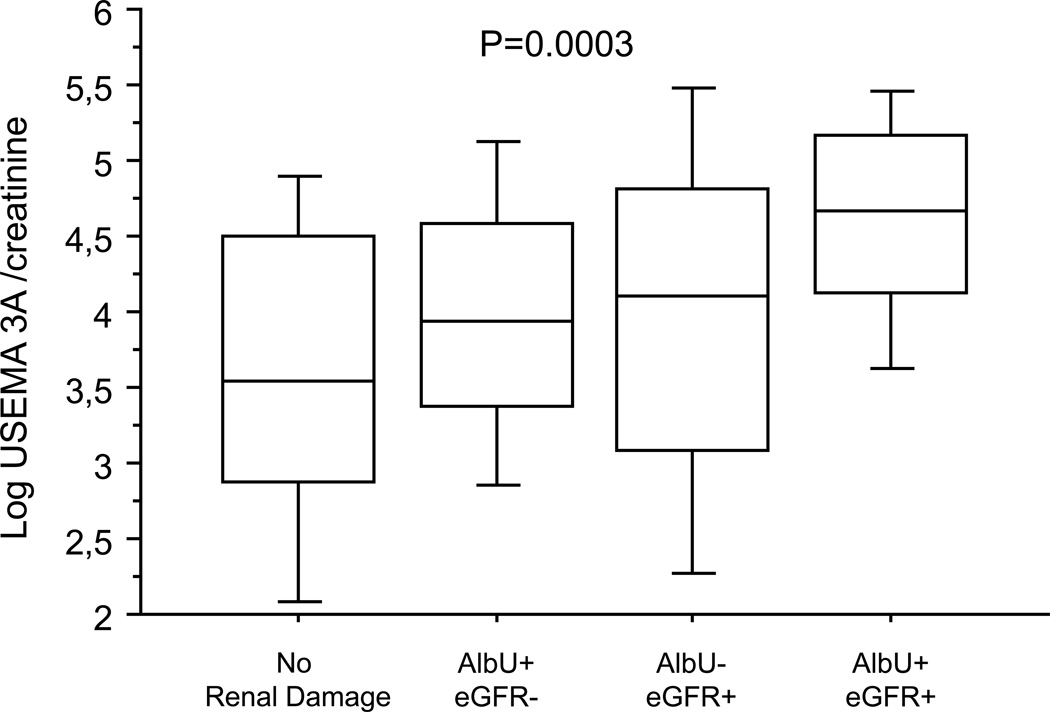
Of note, USEMA increased significantly in patients with renal dysfunction even after adjustment for urinary creatinine excretion (USemaCR) (Fig. 1). USemaCR levels were positively correlated with urinary ACR (r 0.26, p = 0.001), and serum creatinine (r 0.27, p < 0.001), and inversely related to eGFR (r −0.29, p < 0.001). Patients with USemaCR above the median (i.e. >59.77 pg/mg) revealed a tendency to higher ACR levels (83 ± 131 vs. 50 ± 91, p = 0.065), significantly higher serum creatinine levels (1.41 ±0.54 vs. 1.11 ± 0.40, p < 0.001) and lower eGFR (54 ± 21 vs. 70 ± 22, p < 0.001) as compared to those with USemaCR below the median. The concomitant occurrence of both components of CKD was found more frequently in patients with USEMA above the median (39 vs. 12 %, p < 0.001).
Fig. 1.
USEMA3A in hypertensive patients according to the presence/absence of macroalbuminuria and reduced GFR. To provide more detailed information about data distribution, the results are presented as box-and-whisker plots. The central box encloses the middle 50 % of the data; the horizontal line inside the box represents the median. Vertical lines (whiskers) extend from each end of the box and cover the distance between the 10th and 90th percentiles. eGFR estimated glomerular filtration rate, eGFR− eGFR ≥60 ml/min, AlbU− normoalbuminuria, eGFR+ eGFR <60 ml/min, AlbU+ macroalbuminuria. Data were logarithmically transformed before statistical analysis. *p < 0.007 as compared to those with only one marker of renal damage. **p < 0.0001 as compared to those with normal renal function
Patients with either feature of renal damage showed significantly higher USEMA levels as compared with those without CKD. In particular, logarithmically transformed USemaCR was higher in patients with increased albuminuria (4.29 ± 0.86 vs. 3.78 ± 1.18 pg/mg, p = 0.003), or eGFR below 60 ml/min (4.27 ± 1.05 vs. 3.74 ± 1.02 pg/mg, p = 0.002). Conversely, there were no significant differences in USEMA levels on the basis of gender or presence/absence of diabetes. Moreover, the risk of being male, or of having diabetes was similar in all four USemaCR quartiles (data not shown). The presence of increased USEMA levels was significantly related to the occurrence of each component of renal damage we investigated. In particular, being in the USemaCR top quartile (i.e. ≥123.61 pg/mg; logarithmically transformed ≥4.817 pg/mg) entailed a fourfold (OR 4.15, 95 % CI 2.02–8.54, p < 0.001) higher risk of combined renal damage and an almost twofold higher risk of having macroalbuminuria or reduced eGFR even after taking into consideration potentially confounding factors such as serum uric acid and waist circumference (Table 2).
Table 2.
Odds ratios of renal damage for USEMA increase
| Variable | eGFR+ | AlbU+ | Combined renal damage | |||
|---|---|---|---|---|---|---|
| OR (95 % CI) | p | OR (95 % CI) | p | OR (95 % CI) | p | |
| Model 1 | 1.63 (1.18–2.26) | 0.0033 | 1.61 (1.16–2.25) | 0.0043 | 2.84 (1.69–4.75) | <0.0001 |
| Model 2 | 3.06 (1.18–7.91) | 0.0213 | 4.56 (1.68–12.37) | 0.0029 | 16.00 (3.24–78.89) | 0.0007 |
| Model 3a | 2.10 (1.30–3.40) | 0.0025 | ||||
| Model 3b | – | – | 1.83 (1.20–2.80) | 0.0052 | – | – |
| Model 3c | – | – | – | – | 4.15 (2.02–8.54) | 0.0001 |
Model 1 for each 1 pg/mg increase of USemaCR
Model 2 USemaCR highest quartile (i.e. ≥123.61 pg/mg, ≥4.817 pg/mg when logarithmically transformed)
Model 3a for each 1 pg/mg increase of USemaCR also in model Log ACR, SUA and WC
Model 3b Model 1 + eGFR, SUA and WC
Model 3c Model 1 + eGFR, Log ACR, SUA and WC
eGFR estimated glomerular filtration rate, eGFR+ eGFR <60 ml/min, AlbU+ macroalbuminuria, USemaCR urinary semaphorin 3A to creatinine ratio, OR odds ratio, CI confidence intervals, WC waist circumference, Log ACR logarithmically transformed albumin-to-creatinine ratio, SUA serum uric acid
Discussion
The main finding of the present report is the relationship between the amount of USEMA and the severity of renal dysfunction in a group of hypertensive patients selected on the basis of presence/absence of diabetes, increased albuminuria and decreased eGFR. While it was recently reported that USEMA levels significantly increased shortly after cardiopulmonary bypass in pediatric patients who developed AKI [10], to the best of our knowledge this is the first time that its presence and degree has been studied in the urine of CKD patients. Our results extend, to the setting of CKD, literature findings which have thus far mostly indicated an association between increased USEMA and AKI both in animals and in humans. In particular, a single injection of recombinant SEMA3A has been shown to cause transient massive proteinuria, and glomerular damage in mice [9]. Recent studies have shown that USEMA levels increase within the first 2 h after the start of cardiopulmonary surgery in pediatric patients [10].
An increase in USEMA excretion during CKD might be due to greater systemic production, or overexpression at the renal level, two mechanisms which may lead to an abnormal leak of SEMA3A into the urine. As a matter of fact, SEMA3A serum levels have been found to be inversely related to disease activity and to the degree of renal damage in systemic lupus erythematosus patients [3, 4]. Moreover, in experimental models of ischemia–reperfusion in mice, circulating SEMA3A levels have been shown to rapidly downregulate after acute insult [10]. On the other hand, podocyte SEMA3A is known to play a crucial role during glomerular development, and in regulating endothelial cell migration and survival [5]. Since SEMA3A remains expressed in adult podocytes and collecting tubules [8], its urinary accumulation during renal pathological changes might be a consequence of increased local production. In fact, enhanced SEMA3A signaling has been shown in the podocyte of mice with advanced diabetic glomerulopathy [11] and it has been hypothesized that it may play a role in the pathogenesis of microvascular lesions and mesangiolysis in diabetic nephropathy.
It remains to be established whether the increased amount of USEMA we found in our CKD patients merely reflects local injury to glomerular structures or, rather, if it plays a mechanistic role in the development of renal damage. Previous experimental data suggest a pathogenetic role of exogenously administered SEMA3A in the induction of functional and ultrastructural changes at the glomerular filtration barrier level in vivo [9]. In fact, acute systemic SEMA3A injection has been reported to downregulate podocin and nephrin expression and to produce extensive fusion and effacement of podocyte processes, as well as glomerular endothelial cell swelling, thus resulting in massive proteinuria in healthy mice [9]. Furthermore, SEMA3A is thought to regulate integrin function in endothelial cells and has recently been demonstrated to act as a potent vascular permeability factor [16].
In the present study we found a significant relationship between USEMA and clinical signs of CKD such as albuminuria and eGFR. While eGFR is usually taken as a measure of glomerular function, albuminuria is thought to be a marker of both glomerular and tubular damage [17]. Therefore, increased urinary leak of SEMA may be secondary to multifactorial and complex mechanisms of damage. This hypothesis is strengthened by the results of multiple logistic regression, which suggest that the relationship between USEMA, albuminuria and eGFR is independent of each other. Surprisingly, we did not find any differences in USEMA between diabetic and non diabetic patients. While enhanced SEMA3A signaling has been shown in the podocyte of mice with diabetic glomerulopathy [11] there are no data comparing USEMA levels in different renal diseases in the literature.
There are both limitations and strengths to our work that must be acknowledged. Among the former is the cross-sectional nature of the study design which obviously limits our ability to understand the pathogenetic mechanisms underlying the reported relationship. Unfortunately, SEMA3A serum measurements were not performed in our study and therefore we cannot rule out that increased USemaCR is a consequence of a gain in SEMA3A systemic production. Nor were we able to fully characterize the occurrence of increased USEMA with respect to different renal phenotypes since this abnormality seems to cluster with both isolated AlbU+ and eGFR reduction. Undoubtedly, further studies with a different clinical approach might help clarify the pathogenetic mechanisms underlying the reported associations. In this respect our study should be considered as hypothesis-generating. On the other hand, the nested case–control approach we used in our investigation could be considered a powerful means for looking at the association between USEMA and CKD components since it allowed us to compare several subgroups with similar clinical characteristics but different degrees and types of renal involvement from among a large cohort of patients.
In conclusion, for the first time we report an association between CKD components and USEMA, a protein believed to be implicated in cellular developmental processes, cytokine release, immune modulation and AKI. Our data should encourage further experimental and clinical research to clarify the role (pathogenetic and predictive) of USEMA in the context of CKD in diabetic and hypertensive patients.
Acknowledgments
This work was supported by an R01 Grant (1R01DK083379-01A3) to Ganesan Ramesh from NIH-NIDDK.
Footnotes
Conflict of interest Dr. Ganesan Ramesh filed a provisional patent for the discovery of semaphorin 3A as a biomarker of injury and disease. All other authors declared no competing interest.
Contributor Information
Francesca Viazzi, Email: francesca.viazzi@unige.it, Department of Internal Medicine, University of Genoa and IRCCS Azienda Ospedaliera Universitaria San Martino-IST, Istituto Nazionale per la Ricerca sul Cancro, Viale Benedetto XV, 6 CAP 16132 Genoa, Italy.
Ganesan Ramesh, Department of Medicine and Vascular Biology Center, Georgia Health Sciences University, Augusta, GA, USA.
Calpurnia Jayakumar, Department of Medicine and Vascular Biology Center, Georgia Health Sciences University, Augusta, GA, USA.
Giovanna Leoncini, Department of Internal Medicine, University of Genoa and IRCCS Azienda Ospedaliera Universitaria San Martino-IST, Istituto Nazionale per la Ricerca sul Cancro, Viale Benedetto XV, 6 CAP 16132 Genoa, Italy.
Debora Garneri, Department of Internal Medicine, University of Genoa and IRCCS Azienda Ospedaliera Universitaria San Martino-IST, Istituto Nazionale per la Ricerca sul Cancro, Viale Benedetto XV, 6 CAP 16132 Genoa, Italy.
Roberto Pontremoli, Department of Internal Medicine, University of Genoa and IRCCS Azienda Ospedaliera Universitaria San Martino-IST, Istituto Nazionale per la Ricerca sul Cancro, Viale Benedetto XV, 6 CAP 16132 Genoa, Italy.
References
- 1.Vadasz Z, Toubi E. Semaphorins: their dual role in regulating immune-mediated diseases. Clin Rev Allergy Immunol. 2013 doi: 10.1007/s12016-013-8360-4. [DOI] [PubMed] [Google Scholar]
- 2.Roth L, Koncina E, Satkauskas S, Crémel G, Aunis D, Bagnard D. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci. 2009;66:649–666. doi: 10.1007/s00018-008-8518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vadasz Z, Haj T, Halasz K, et al. Semaphorin 3A is a marker for disease activity and a potential immunoregulator in systemic lupus erythematosus. Arthritis Res Ther. 2012;14:R146. doi: 10.1186/ar3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vadasz Z, Toubi E. Semaphorin 3A—a marker for disease activity and a potential putative disease-modifying treatment in systemic lupus erythematosus. Lupus. 2012;21:1266–1270. doi: 10.1177/0961203312456753. [DOI] [PubMed] [Google Scholar]
- 5.Reidy KJ, Villegas G, Teichman J, et al. Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development. Development. 2009;136:3979–3989. doi: 10.1242/dev.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tufro A, Teichman J, Woda C, Villegas G. Semaphorin3a inhibits ureteric bud branching morphogenesis. Mech Dev. 2008;125:558–568. doi: 10.1016/j.mod.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reidy K, Tufro A. Semaphorins in kidney development and disease: modulators of ureteric bud branching, vascular morphogenesis, and podocyte–endothelial crosstalk. Pediatr Nephrol. 2011;26:1407–1412. doi: 10.1007/s00467-011-1769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villegas G, Tufro A. Ontogeny of semaphorins 3A and 3F and their receptors neuropilins 1 and 2 in the kidney. Gene Expr Patterns. 2002;2:151–155. doi: 10.1016/s0925-4773(02)00305-2. [DOI] [PubMed] [Google Scholar]
- 9.Tapia R, Guan F, Gershin I, Teichman J, Villegas G, Tufro A. Semaphorin3a disrupts podocyte foot processes causing acute proteinuria. Kidney Int. 2008;73:733–740. doi: 10.1038/sj.ki.5002726. [DOI] [PubMed] [Google Scholar]
- 10.Jayakumar C, Ranganathan P, Devarajan P, Krawczeski CD, Looney S, Ramesh G. Semaphorin 3A is a new early diagnostic biomarker of experimental and pediatric acute kidney injury. PLoS One. 2013;8:e58446. doi: 10.1371/journal.pone.0058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veron D, Bertuccio CA, Marlier A, et al. Podocyte vascular endothelial growth factor (Vegf164) overexpression causes severe nodular glomerulosclerosis in a mouse model of type 1 diabetes. Diabetologia. 2011;54:1227–1241. doi: 10.1007/s00125-010-2034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leoncini G, Viazzi F, Agabiti Rosei E, et al. Chronic kidney disease in hypertension under specialist care: the I-DEMAND study. J Hypertens. 2010;28:156–162. doi: 10.1097/HJH.0b013e328332038c. [DOI] [PubMed] [Google Scholar]
- 13.Leoncini G, Viazzi F, Agabiti Rosei E, et al. Metabolic syndrome and chronic kidney disease in high-risk Italian hypertensive patients: the I-DEMAND study. J Nephrol. 2012;25:63–74. doi: 10.5301/JN.2011.7752. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sciarretta S, Valenti V, Tocci G, et al. Association of renal damage with cardiovascular diseases is independent of individual cardiovascular risk profile in hypertension: data from the Italy-Developing Education and awareness on MicroAlbuminuria in patients with hypertensive Disease study. J Hypertens. 2010;28:251–258. doi: 10.1097/HJH.0b013e3283326718. [DOI] [PubMed] [Google Scholar]
- 16.Acevedo LM, Barillas S, Weis SM, Göthert JR, Cheresh DA. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood. 2008;111:2674–2680. doi: 10.1182/blood-2007-08-110205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gansevoort RT, Nauta FL, Bakker SJ. Albuminuria: all you need to predict outcomes in chronic kidney disease? Curr Opin Nephrol Hypertens. 2010;19:513–518. doi: 10.1097/MNH.0b013e32833e4ce1. [DOI] [PubMed] [Google Scholar]