Abstract
Early life stressors are associated with elevated inflammation, a key physiological risk factor for disease. However, the mechanisms by which early stress leads to inflammation remain largely unknown. Using a longitudinal dataset, we examined smoking, alcohol consumption, and body mass index (BMI) as health behavior pathways by which early adversity might lead to inflammation during young adulthood. Contemporaneously measured early adversity predicted higher BMI and increased smoking, but not alcohol consumption, and these effects were partially accounted for by chronic stress in young adulthood. Higher BMI in turn predicted higher levels of soluble TNF receptor type II (sTNF-RII) and C-reactive protein (CRP), and smoking predicted elevated sTNF-RII. Findings establish that early adversity contributes to inflammation in part through ongoing stress and maladaptive health behavior. Given that maladaptive health behaviors portend inflammation in young adulthood, they serve as promising targets for interventions designed to prevent the negative consequences of early adversity.
Keywords: Early adversity, inflammation, depression, smoking, body mass index
Stressful early experiences have been linked to elevated inflammation later in life (e.g., Danese et al., 2007). Although an acute inflammatory response is necessary for fighting infection and wound healing, chronic inflammation is implicated in the development and progression of a number of serious diseases (e.g., Koenig et al., 1999). Thus, chronic inflammation is hypothesized to be a key physiological process by which early stress influences physical health (Miller, Chen, & Parker, 2011). However, the mechanisms by which early stressors lead to later inflammation are largely unknown.
A number of processes, largely neurobiological in nature, have been hypothesized to contribute to the long-term effects of early adversity on inflammation (Miller et al., 2011).The role of health behaviors as potential mediators of the relationship between early stress and inflammation has received less attention. Certain early adversities, such as poverty and maltreatment, have been linked to later smoking, alcohol consumption, and body mass index (BMI) (Goldstein, Flett, & Wekerle, 2010; Midei & Matthews, 2011; Najman, Toloo, & Siskind, 2006). These health behaviors, in turn, have been linked to elevations in systemic inflammation in cross-sectional studies (Ambrose & Barua, 2003; Festa et al, 2001; Imhof et al., 2004).
Previous research on early adversity-inflammation associations has tended to control for the effects of health behaviors, rather than directly examining them as mechanisms of these associations (e.g., Danese et al., 2007; Kiecolt-Glaser et al., 2011); however, several recent studies provide evidence that specific early adversities, such as low socioeconomic status (Brummett et al., 2013; Hagger-Johnson, Mottus, Craig, Starr, & Deary, 2012) and abuse (Matthews, Chang, Thurston, & Bromberger, 2014) affect CRP indirectly through unhealthy behaviors and increased BMI. The current project therefore tested whether maladaptive health behaviors serve as mechanisms of the effects of cumulative early adversity on inflammation in a large community sample of young adults. Moreover, because early adversity has been linked to continued stress throughout development (Hammen, Hazel, Brennan, & Najman, 2012), analyses tested whether early adversity influences health behaviors through more proximal life stress. Our prospective design expanded on prior studies by allowing contemporaneous, rather than retrospective, assessment of a broad range of early life adversities. We hypothesized that early adversity would predict increased smoking, BMI, and alcohol consumption in young adulthood, in part through ongoing chronic stress in young adulthood, and that negative health behaviors would in turn predict elevated inflammation.
Method
Participants
Although most research on early stress and inflammation has been conducted with adults, the current analyses used a sample of young adults, given recent evidence that childhood adversity could influence markers of inflammation as early as adolescence (Slopen, Koenen, & Kubzansky, 2012). Participants were drawn from more than 7,000 children included in the Mater-University of Queensland Study of Pregnancy (MUSP; Keeping et al., 1989), a longitudinal birth cohort study of children’s development. From this birth cohort, 815 mother-child pairs were originally selected at youth age 15 for a follow-up study of children at risk for depressive and other disorders, based on mothers’ reports of depressive symptoms from pregnancy through child age 5. These families were selected to represent a range of symptom presence, chronicity, and severity of maternal depression (see Hammen & Brennan, 2001 for details). The adolescent sample at age 15 was 50.4% male and 49.4% female. Families were largely lower and lower-middle income and predominantly Caucasian (91.4%; 3.6% Asian; 5% other or not reported).
These 815 youth were contacted for additional follow-ups at ages 20 and 21 during which they completed questionnaires about depressive symptoms and health behavior, as well as interviews about recent stressful experiences. At one final time point between ages 22 and 25, participants were recruited to contribute blood samples, resulting in a reduced sample of 444 youth who completed all follow-ups and provided blood samples at ages 22 to 25. Some participants were excluded from analyses based on extreme values for certain study measures, as detailed below. This left a final sample of 389 (57% female, 43% male) participants for the current study. The final 389 participants did not differ from the rest of the original sample of 815 youth in terms of maternal depression history (χ2 (1, 681) = .98, p = .32), family income (t(782) = .89, p = .38), cigarette smoking (t(573) = .77, p = .44), depressive symptoms (t(693) = .78, p = .44), or alcohol consumption rates (t(575) = −1.82, p = .07). However, participants included in the final sample had slightly lower BMI scores (t(427) = 2.42, p = .02), and were more likely to be female (χ2 (1, 815) = 15.04, p < .001).
Procedure
Mothers completed measures about the child’s early rearing environment at 4 time points during the first five years of the child’s life: at their first prenatal visit—typically in the first trimester of pregnancy, 3–4 days after the child’s birth, 6 months after birth, and age 5. Youth completed questionnaires about health behaviors and depressive symptoms at ages 20 and 21, completed interviews about experiences with stress at age 20, and provided blood samples at one time point between ages 22 and 25. Participants all gave informed consent (assent) and the institutional review/ethics panels of the University of Queensland, Emory University, and the University of California, Los Angeles approved the research protocol.
Measures
Early adversity
Early adversity was assessed using mothers’ reports on five indicators of adversity measured in the first five years of the child’s life. These specific indicators were selected based on a review of studies that have examined links between cumulative indices of early adversity and various physical and emotional health outcomes (e.g., Felitti et al., 1998; Kiecolt-Glaser et al., 2011). Maternal psychopathology was coded as present if mothers reported any Axis I diagnosis (excluding specific phobia) between the child’s birth and child age 5 on the Structured Clinical Interview for DSM-IV for lifetime disorders (SCID; First, Spitzer, Gibbon, & Williams, 1995) administered to mothers at youth age 15. The most common diagnoses were major depressive disorder (n = 78), dysthymic disorder (n = 68), and social phobia (n = 34). Parental discord was coded as present if mothers consistently endorsed high levels of quarreling across the early childhood assessments. Harsh discipline was coded as present if mothers consistently reported that they would hit or smack their child as a method of discipline at the age 5 assessment. Family income was measured using an average of maternal ratings of the family’s annual income on a 7-point scale completed at 3 of the early childhood assessments: pregnancy, 6 months after birth, and 5 years after birth. Individuals falling within the lowest third of family income were coded as having this early adversity. Finally, parental criminal behavior was scored as present or absent based on mothers’ reports about whether she or her partner had been arrested or spent time in prison at any point during the first five years of the child’s life.
An early adversity composite was created given that risk for poor mental and physical health has been shown to increase with increasing numbers of early adversities (e.g., Felitti et al., 1998). In the present study, a count of the number of early adversities present for each child was used. Due to the fact that few participants had all 5 adversities, participants with 4 or more adversities were combined to create a composite with a more normal distribution (range 0 to 4).
Body mass index (BMI)
BMI was calculated from youth self-report of height and weight at the blood draw between ages 22 and 25. If these data at the blood draw were missing, youth height and weight at the age 21 assessment were used to calculate BMI. One participant was excluded from the current analyses due to an extreme BMI value (59.9), which was more than 6 standard deviations above the mean for males in this sample.
Smoking
Youth completed a self-report measure of the number of cigarettes smoked per day at the age 21 assessment (0 = did not smoke, 1 = 1–9 cigarettes per day, 2 = 10–19 cigarettes per day, 3 = 20–29 cigarettes per day, 4 = 30+ cigarettes per day). Due to the fact that few participants endorsed the highest smoking category, participants who reported smoking 20 or more cigarettes per day were combined to create a measure with a more normal distribution (range 0 to 3).
Alcohol consumption
At age 21, youth reported how many alcoholic beverages they typically consumed per day on a scale ranging from 1 (0 drinks per day) to 5 (3.5 or more drinks per day).
Depressive symptoms
Youth depressive symptoms at age 20 were assessed using the Beck Depression Inventory—II (BDI-II; Beck, Steer, & Brown, 1996), a well-validated, self-report questionnaire. Coefficient alpha in the current sample was .93.
Young adulthood chronic stress
Target youth experiences with chronic stress in the past 6 months were measured at age 20 using the UCLA Life Stress Interview, a semi-structured interview that probes stressful ongoing conditions across a number of life domains (Hammen & Brennan, 2001). The interview probes several developmentally appropriate domains: social life, close friendship, romantic relationships, family relationships, financial, work, academic, and health problems of close family. For each domain, trained advanced graduate student interviewers used standard probes and semi-structured follow-up queries to make an objective rating of chronic stress on a 5-point scale (from “1” superior/exceptional functioning to “5” severe difficulties), using behaviorally anchored descriptors. Total chronic stress levels were computed by summing across all domains. Evidence of the convergent and predictive validity of the UCLA Life Stress Interview in the current sample is reported in Hammen, Brennan, & Keenan-Miller (2008). In the current sample, the mean intraclass correlation across all domains at age 20 was r = .81.
Inflammatory markers
Plasma levels of two inflammatory markers, C-reactive protein (CRP) and soluble tumor necrosis factor receptor II (sTNF-RII) were assessed between ages 22 and 25. CRP is an acute phase protein produced by the liver in response to the proinflammatory cytokine IL-6 and serves as a reliable marker of chronic inflammation. The soluble receptor sTNF-RII is shed from a cell surface after stimulation of the cell by the proinflammatory cytokine TNF-α and serves as a marker of TNF activity.
Blood samples were collected in EDTA plasma and stored at −80°C for subsequent batch testing. Plasma levels of sTNF-RII were determined by ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocols, with a lower limit of detection of 234 pg/ml,. CRP levels were determined by a high sensitivity ELISA (Immundiagnostik, ALPCO Immunoassays, Salem, NH) according to the manufacturer’s protocol, but with an extended standard curve to a lower limit of detection of 0.2 mg/L. All samples were run in duplicate. Due to non-normality, CRP values were log transformed for all analyses. Participants were excluded from the current study if they had CRP values ≥ 10 or sTNF-RII values ≥ 400 (n = 54), given that such levels may be indicative of an acute inflammatory response rather than chronic inflammation (Pearson et al., 2003).
Data Analytic Procedures
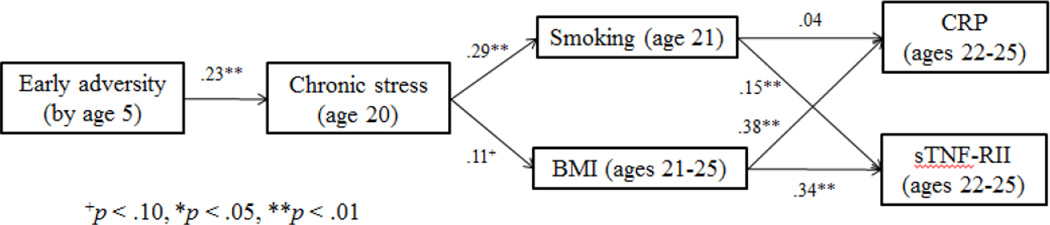
A structural equation modeling (SEM) framework was chosen to examine direct and indirect paths among variables, given its ability to test complex mediational models. The hypothesized model examined the effects of early adversity on the two inflammatory markers via health behaviors (see Figure 1). Gender was controlled for in all paths of this model, due to increased rates of depression in females across adolescence and adulthood (Hankin et al., 1998). We also ran the model including depressive symptoms as another mechanism of the effects of early adversity on later inflammation to control for the effects of depression in the model, given the close links between depression and early life stress (Hammen, 2005), negative health behaviors (e.g., Allgower, Wardle, & Steptoe, 2001), and inflammation (Kiecolt-Glaser & Glaser, 2002). Finally, we also examined a second model, in which early adversity contributed to health behaviors indirectly via more proximal chronic stress at age 20.
Figure 1.
Path model examining cigarette use and BMI as pathways from early adversity to levels of CRP and sTNF-RII.
All analyses were carried out in Mplus v5 (Muthén & Muthén, 1998–2007), using full information maximum likelihood methods to accommodate missing data. Due to univariate and multivariate non-normality of the data, robust maximum likelihood procedures were used to estimate standard errors. Overall model fit was evaluated using several standard fit indices, including the likelihood ratio chi-square test, the comparative fit index (CFI; Bentler, 1990), the root-mean-square error of approximation (RMSEA; Browne & Cudeck, 1993), and the standardized root mean-square residual (SRMR; Hu & Bentler, 1998).
Results
Descriptive statistics for all main study variables, as well as Pearson correlations among these variables, are presented in Table 1. Alcohol consumption at age 21 was not significantly correlated with experiences of early adversity by age 5 or the two inflammatory markers. As a result, only smoking behavior and BMI were included in subsequent analyses testing health behaviors as pathways from early adversity to inflammation.
Table 1.
Descriptive Statistics and Pearson Correlations among Early Adversity, Health Behaviors, Depressive Symptoms, Young Adulthood Stress, and Inflammatory Markers
| Study Variable | Mean | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Early adversity | 1.23 | 1.07 | -- | ||||||
| 2. Smoking | 1.62 | 0.96 | .12* | -- | |||||
| 3. BMI | 24.51 | 4.8 | .09+ | 0.02 | -- | ||||
| 4. Alcohol consumption | 2.88 | 1.14 | 0.06 | .20** | −0.03 | -- | |||
| 5. Depressive symptoms | 7.27 | 8.46 | .15** | .18** | 0.08 | 0.04 | -- | ||
| 6. Age 20 chronic stress | 20.23 | 4.12 | .23** | .29** | 0.09 | 0.01 | .46** | -- | |
| 7. CRP (mg/L) | 2.22 | 2.21 | −0.04 | 0.01 | .36** | −0.04 | 0.07 | 0.03 | -- |
| 8. sTNFRII (pg/ml) | 2571.13 | 511.92 | 0.06 | .13* | .34** | 0.01 | 0.02 | .10* | .26** |
Note.
p < .10,
p < .05,
p < .01
Path model analyses were used to test the indirect effects of early adversity by age 5 on CRP and sTNF-RII levels at age 25, via smoking behavior and BMI at age 21. Standardized betas for all direct paths are displayed in Figure 1. Fit indices showed that this model provided an overall good fit to the data χ2 (df= 3, N=389) = 3.25, p = .36; CFI = .99; RMSEA = .02 (90% CI .00, .09); SRMR = .02. Cumulative experiences of early adversity by age 5 predicted elevated cigarette smoking, and were marginally significant in predicting elevated BMI, in young adulthood. Higher BMI in turn predicted higher levels of CRP and sTNF-RII, and more cigarette smoking predicted higher levels of sTNF-RII, but not CRP. Early adverse experiences had significant indirect effects on each of the inflammatory markers through the proposed health behaviors (β = .05, p < .05 for sTNF-RII; β = .04, p < .05 for CRP).1
When youth depressive symptoms at age 20 were included as a third path from early adversity to the inflammatory markers, the model showed similar fit χ2 (df= 5, N=389) = 15.02, p = .01; CFI = .94; RMSEA = .07 (90% CI .03, .12); SRMR = .04 and identical patterns of significance. Early adversity strongly predicted youth depressive symptoms at age 20, in addition to cigarette use and BMI. However, youth depressive symptoms did not have a relationship with inflammatory markers over and above the effects of smoking and BMI.
Finally, when early adversity and chronic stress in the 6 months prior to age 20 were both included as predictors of BMI and smoking behavior, recent stress was more strongly predictive of the health behaviors. The best fitting model indicated that early adversity led to higher rates of recent stress, which in turn predicted BMI and smoking behavior χ2 (df= 5, N=389) = 7.55, p = .37; CFI = .99; RMSEA = .01 (90% CI .00, .07); SRMR = .02. All other paths in the model showed identical patterns of significance to the original model.
Discussion
The current study tested the role of maladaptive health behaviors in the link between early adversity and disrupted inflammatory processes. Results indicated that early adversity predicted elevated cigarette use and BMI, but not alcohol consumption, in young adulthood. Further exploration suggested that these effects of early adversity on health behavior were partially accounted for by the relationship between early adversity and chronic stress in young adulthood. Higher BMI in turn predicted higher levels of sTNF-RII and CRP, and cigarette smoking predicted higher levels of sTNF-RII. Although early adverse experiences were a strong predictor of later depressive symptoms, depressive symptoms had negligible effects on inflammatory markers over and above the effects of health behaviors.
Past research on the effects of early adversity on inflammation has tended to control for health behaviors as confounds, and only recently has begun to examine the role of maladaptive health behaviors in these effects. Moreover, few studies have used longitudinal designs to account for the continuity of stress and depressive symptoms in maladaptive health outcomes related to early adversity. Our results highlight negative health behaviors, particularly smoking and high BMI, as important pathways by which continuing life stress “gets under the skin” to contribute to long-term risk for physical illness. Findings are an important complement to studies examining the biological and cognitive impact of early adversity, because they identify potentially modifiable health-related behaviors that predict inflammation during critical developmental periods such as adolescence and early adulthood.
Contrary to predictions, early adversity was not associated with alcohol consumption. This may be because we were not predicting clinical levels of alcohol use and did not directly assess maltreatment, in contrast to most previous research. It is also noteworthy that, in contrast to some previous studies (e.g., Danese et al., 2007), we observed no direct association between early adversity and inflammatory markers. It is possible that the cascade of biological effects that follows exposure to adversity might not yet have had time to result in chronic inflammation because of the relatively young age of our sample. Consistent with this hypothesis, several previous studies have found effects of early adversity on stimulated cytokine production, but not chronic inflammation (e.g., Miller et al., 2009).
Several limitations of the current study should be acknowledged. First, our measure of early adversity did not include some stressors that have been linked to later health (e.g., abuse). In addition, we examined an index of cumulative early risk, but future research would benefit from examining of specific early adversities or clusters of adversity. Second, BMI is a marker of several health behaviors, including diet and exercise. Future research is therefore needed to investigate the specific health behaviors that account for the relationship between BMI and inflammation. Finally, although our final sample did not differ from the original sample on a number of variables, participants with higher BMI and males were more likely to drop out before the final assessment.
Despite these limitations, these results address important questions about the psychological and biological mechanisms by which early adversity confers risk for poor physical health. Findings highlight negative health behaviors as a potentially modifiable pathway by which early adversity leads to inflammation. Research in this area has important implications for our understanding of the psychobiological processes that mediate the long-term pathogenic effects of early life stress, and could inform the development of clinical interventions to offset the physical health consequences of these stressors.
Figure 2.
Path model examining the role of ongoing stress in young adulthood in the effects of early adversity on cigarette use, BMI, and later levels of CRP and sTNF-RII.
Acknowledgements
This work was supported by the National Health and Medical Research Council, Mater Misericordiae Mother’s Hospital, NIMH Grant R01 MH52239, NIMH training grant MH15750, the UCLA Norman Cousins Center for Psychoneuroimmunology, and the UCLA OAIC, NIH/NIA Grant P30-AG028748.
Footnotes
When this model was re-run to examine the health effects of only maternal depressive disorders up to youth age 5 (rather than the early adversity composite), maternal depression predicted more cigarette smoking in youth (β = .17, p < .01), but did not predict BMI. Maternal depression by age 5 had a significant indirect effect on sTNF-RII (β = .02, p < .05), but not CRP, through its effects on cigarette smoking.
References
- Allgöwer A, Wardle J, Steptoe A. Depressive symptoms, social support, and personal health behaviors in young men and women. Health Psychology. 2001;20(3):223. [PubMed] [Google Scholar]
- Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular diseasean update. Journal of the American College of Cardiology. 2004;43(10):1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the BDI-II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Brummett BH, Babyak MA, Singh A, Jiang R, Williams RB, Harris KM, Siegler IC. Socioeconomic Indices as Independent Correlates of C-Reactive Protein in the National Longitudinal Study of Adolescent Health. Psychosomatic Medicine. 2013;75(9):882–893. doi: 10.1097/PSY.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventative Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Festa A, D'Agostino R, Jr, Williams K, Karter AJ, Mayer-Davis EJ, Tracy RP, Haffner SM. The relation of body fat mass and distribution to markers of chronic inflammation. International Journal of Obesity and Related Metabolic Disorders. 2001;25(10):1407–1415. doi: 10.1038/sj.ijo.0801792. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCIDI/P. Version 2.0) New York, NY: New York State Psychiatric Institute; 1995. [Google Scholar]
- Goldstein AL, Flett GL, Wekerle C. Child maltreatment, alcohol use and drinking consequences among male and female college students: An examination of drinking motives as mediators. Addictive Behaviors. 2010;35(6):636–639. doi: 10.1016/j.addbeh.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Hagger-Johnson G, Mõttus R, Craig LC, Starr JM, Deary IJ. Pathways from childhood intelligence and socioeconomic status to late-life cardiovascular disease risk. Health Psychology. 2012;31(4):403–412. doi: 10.1037/a0026775. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan PA. Depressed adolescents of depressed and nondepressed mothers: Tests of an interpersonal impairment hypothesis. Journal of Consulting and Clinical Psychology. 2001;69(2):284. doi: 10.1037//0022-006x.69.2.284. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107(1):128. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hazel NA, Hammen C, Brennan PA, Najman J. Early childhood adversity and adolescent depression: the mediating role of continued stress. Psychological Medicine. 2008;38(4):581–590. doi: 10.1017/S0033291708002857. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3(4):424. [Google Scholar]
- Imhof A, Woodward M, Doering A, Helbecque N, Loewel H, Amouyel P, et al. Overall alcohol intake, beer, wine, and systemic markers of inflammation in western Europe: results from three MONICA samples (Augsburg, Glasgow, Lille) European Heart Journal. 2004;25(23):2092–2100. doi: 10.1016/j.ehj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Keeping JD, Najman JM, Morrison J, Western JS, Andersen MJ, Williams GM. A prospective longitudinal study of social, psychological, and obstetrical factors in pregnancy: Response rates and demographic characteristics of the 8,556 respondents. British Journal of Obstetrics and Gynaecology. 1989;96:289–297. doi: 10.1111/j.1471-0528.1989.tb02388.x. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Depression and immune function: Central pathways to morbidity and mortality. Journal of Psychosomatic Research. 2002;53:873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic Medicine. 2011;73(1):16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig W, Sund M, Fröhlich M, Fischer HG, Löwel H, Döring A, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99(2):237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Chang YF, Thurston RC, Bromberger JT. Child abuse is related to inflammation in mid-life women: Role of obesity. Brain, Behavior, and Immunity. 2013;36:29–34. doi: 10.1016/j.bbi.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midei AJ, Matthews KA. Interpersonal violence in childhood as a risk factor for obesity: a systematic review of the literature and proposed pathways. Obesity Reviews. 2011;12(5):e159–e172. doi: 10.1111/j.1467-789X.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137(6):959. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 5th edition. Los Angeles, CA: Author; 1998–2007. [Google Scholar]
- Najman JM, Toloo G, Siskind V. Socioeconomic disadvantage and changes in health risk behaviours in Australia: 1989–90 to 2001. Bulletin of the World Health Organization. 2006;84(12):976–984. doi: 10.2471/blt.05.028928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: A systematic review. Brain, Behavior, & Immunity. 2012;26:239–250. doi: 10.1016/j.bbi.2011.11.003. [DOI] [PubMed] [Google Scholar]