Abstract
The fruit fly Drosophila melanogaster (Dm) mutant for PTEN-induced putative kinase 1 (PINK1B9) gene is a powerful tool to investigate physiopathology of Parkinson's disease (PD). Using PINK1B9 mutant Dm we sought to explore the effects of Mucuna pruriens methanolic extract (Mpe), a L-Dopa-containing herbal remedy of PD. The effects of Mpe on PINK1B9 mutants, supplied with standard diet to larvae and adults, were assayed on 3–6 (I), 10–15 (II) and 20–25 (III) days old flies. Mpe 0.1% significantly extended lifespan of PINK1B9 and fully rescued olfactory response to 1-hexanol and improved climbing behavior of PINK1B9 of all ages; in contrast, L-Dopa (0.01%, percentage at which it is present in Mpe 0.1%) ameliorated climbing of only PINK1B9 flies of age step II. Transmission electron microscopy analysis of antennal lobes and thoracic ganglia of PINK1B9 revealed that Mpe restored to wild type (WT) levels both T-bars and damaged mitochondria. Western blot analysis of whole brain showed that Mpe, but not L-Dopa on its own, restored bruchpilot (BRP) and tyrosine hydroxylase (TH) expression to age-matched WT control levels. These results highlight multiple sites of action of Mpe, suggesting that its effects cannot only depend upon its L-Dopa content and support the clinical observation of Mpe as an effective medication with intrinsic ability of delaying the onset of chronic L-Dopa-induced long-term motor complications. Overall, this study strengthens the relevance of using PINK1B9 Dm as a translational model to study the properties of Mucuna pruriens for PD treatment.
Introduction
Parkinson’s disease (PD) is, after the Alzheimer’s disease, the second most prevalent neurodegenerative disease first affecting medulla oblongata, olfactory bulb and substantia nigra [1]. Loss of olfaction is a very consistent marker of PD occurring in 95% of patients early before the onset of motor symptoms [2]. Olfactory dysfunction is observed in PTEN-induced putative kinase 1 (PINK1B9) Parkinsonism, both in humans [3] and in animal models of PD [4]. The Drosophila melanogaster (Dm) PINK1B9 mutant model recapitulates several of the essential features of PD [5] and has been used to study neuronal dysfunction and molecular aspects of neurodegeneration [6]. In particular, PINK1B9 model provides major information regarding pathogenic molecular basis of early onset PD and mitochondrial dysfunction [5]. Accordingly, it was recently reported that PINK1 mutation enhances mitochondrial stress-induced neurodegeneration in mice [7].
L-Dopa is the most effective symptomatic medication of PD and is still considered the gold standard in its treatment, although other drugs such as dopamine (DA) agonists, DA uptake and mono amino oxidase-B inhibitors are commonly used in the clinical management of PD patients [8] [9] [10]. Besides, other drugs such as adenosine A2A antagonists used as adjunct might be effective in the symptomatic treatment of PD [11]. In addition, the involvement of non-dopaminergic neurotransmitters such as noradrenaline, serotonin, glutamate, and acetylcholine in different brain areas like cortex, brainstem and basal ganglia has prompted many researchers to investigate the effects of non-dopaminergic drugs [12] indicating the involvement of multiple targets in treatment of PD.
Several reports on antiparkinsonian activity of Mucuna pruriens (Mp) [13] [14] endorse the use of Mp seeds in PD. In addition to L-Dopa, Mp seeds contain genistein and polyunsaturated fatty acids which support its antiparkinsonian and neuroprotective actions [15]. Furthermore, phytic acid, another Mp constituent with antioxidant and iron sequestrant activity, has been reported to suppress methyl-phenyl-tetrahydropyridine (MPTP) induced hydroxyl radical generation [16]. Hence, in view of multiple phytoconstituents supporting antiparkinsonian activity of Mp, the present study was aimed at verifying if Mpe’s ability to ameliorate symptoms in this PD model might be attributable to L-Dopa only or to the Mp extract as a whole in which L-Dopa is present along with other ingredients. On these bases we evaluated the antiparkinsonian profile of the standardized methanolic extract of the seeds of Mp (Mpe) on lifespan, climbing activity and olfactory function in PINK1B9 as compared to either wild type (WT) and untreated PINK1B9 Dm. In addition, in order to gain mechanistic insights on the neuroprotective and neuro-rescue properties of Mpe, we also evaluated the expression of bruchpilot protein and tyrosine hydroxylase, as well as the morphology of presynaptic active zones and mitochondria in flies’antennal lobes, i.e. the olfactory bulbs-equivalent structure, and thoracic ganglia, of both WT as well as untreated and Mpe-treated PINK1B9 mutants.
Materials and Methods
Fly Strains
For these experiments we used adult wild type (WT) Oregon-R (Oregon-R-C) and PTEN-induced putative kinase 1 PINK1B9 (w[*] Pink1[B9]) mutant Drosophila melanogaster (Dm) males (from Bloomington Stock Center; Fly Base: http://flybase.bio.indiana.edu). After emergence from pupae, male WT and PINK1B9 mutant flies were separated. WT and mutant flies were reared on a standard cornmeal-yeast-agar medium in controlled environmental conditions (24–25°C; 60% relative humidity; light/dark = 12/12 hours). In detail, four groups of mutant flies were reared on a standard medium supplemented with Mucuna pruriens methanolic extract (Mpe) (Batch no. FMPEX/2012060001; Natural Remedies Ltd., Bangalore, India). PINK1B9 mutants were supplied with Mpe at different concentrations (0 (i.e. untreated PINK1B9 mutants), 0.1, 1 and 10% w/w in their standard diet) both as larvae and adults (L+/A+). In addition, another group was reared on a standard medium supplemented with 0.01% (0.5 mM) L-Dopa (Sigma Aldrich, Milan, Italy), a percentage similar to that at which L-Dopa was supplemented with 0.1% Mpe [15]. The effects of Mpe were assayed at different age steps (I: 3–6; II: 10–15; III: 20–25 days old). A series of experiments on life span, using various concentrations of Mpe (see below in Survival curves) provided the basis for selecting the optimal concentration at which conduct the behavioral, morphological, and protein expression assessments. In particular, based on lifespan results, the olfaction behavior assessments, transmission electron microscopy (TEM) and western blot analyses were restricted to group II flies after 0.1% Mpe administration as L+/A+. Standard genetic procedures were used during the study.
Survival curves
With the aim of selecting the optimal Mpe’s concentration, Dm were grown on standard diet supplemented with different concentrations of Mpe at 25°C. Cohorts of 40 flies (4 flies/tube) from each experimental group (i.e. WT, untreated and Mpe-treated PINK1B9) were monitored every 2 days for their survival. Mortality was analyzed using Kaplan-Meier survival curves and the statistical comparisons were made with a Gehan-Breslow-Wilcoxon test. Experiments were done in duplicate with the exception of those on WT, untreated mutants, 0.1% Mpe- and 0.01% L-Dopa-treated PINK1B9 that were done in triplicate.
Each experiment was conducted with the appropriate control group (i.e. WT, untreated PINK1B9 and treated PINK1B9).
Climbing assay
The climbing assay (negative geotaxis assay) was used to assess locomotor ability [17]. Climbing data were obtained from groups I–III of untreated WT, untreated PINK1B9 and, as L+/A+, 0.1, 1 and 10% Mpe- and 0.01% L-Dopa-treated PINK1B9 mutants. Cohorts of 30 flies from each experimental group were subjected to the assay. Flies were placed individually in a vertically-positioned plastic tube (length 10 cm; diameter 1.5 cm) and tapped to the bottom. Climbing time was recorded upon crossing a line drawn at 6 cm from the bottom. The number of flies that could climb unto, or above, this line within 10 seconds was recorded and expressed as percentage of total flies. Data were expressed as average + SEM from at least three separate experiments. The statistical evaluation was made by two-way ANOVA (p<0.05) followed by HSD post-hoc test.
Electroantennograms (EAGs) recordings
In vivo electroantennogram recordings (EAG) were performed following a previously described protocol [4]. Briefly, live adult WT Dm and untreated, Mpe- and L-Dopa-treated PINK1B9 from group II (n = 12/each strain) were singly positioned under the view of an Olympus BX51WI light microscope (Olympus, Tokyo, Japan). Electrodes were silver wires inserted in glass capillaries filled with a saline solution (NaCl 150 mM).The recording glass electrode was positioned on the tip of the left antenna while the reference was pierced through the compound eye. The EAG signal was amplified with an AC/DC probe and then acquired with an IDAC-4 interface board (Syntech, Hilversum, NL). The antennae were constantly blown by a flow of charcoal purified and humidified air (speed 0.5 m/s) via a glass tube. Odor stimuli were administered by injecting a puff of purified air (0.5 s at 10 mL/s airflow) through the pipette using the stimulus delivery controller (Syntech, Hilversum, NL).
Odor stimuli were prepared in 3 step-dose concentration (0.01, 0.1, and 1% v/v) diluted in hexane. Odor stimulus, 1-hexanol, was chosen according to Fishilevich and Vosshall [20], for its well-known stimulant activity in Dm. Mean values of EAG amplitude were calculated and then analyzed by comparing the results obtained in untreated PINK1B9, Mpe- and L-Dopa-treated flies with matched WT. The significance of differences was tested by one-way ANOVA (followed by HSD post hoc test) with a threshold level of statistical significance set at p<0.05. EAG results are expressed as average values ± S.E.M and represented by histograms.
Olfactory behavior
Free-walking bioassay was performed following the experimental procedures used by Dekker et al. [18]. In particular, group II WT, untreated PINK1B9 and, as L+/A+, 0.1% Mpe- and 0.01% L-Dopa-treated PINK1B9 mutants were given the opportunity to choose between vials containing water with or without odor. Two 4 mL glass vials were placed symmetrically in a large petri dish (arena) and then fitted with truncated pipette tips. The vials were filled with 300 µL of water with 0.25% Triton X (Sigma-Aldrich, Milan, Italy) with or without the odorant (0.1% (v/v) 1-hexanol; Sigma-Aldrich, Milan, Italy). As mentioned above, in order to allow detection of possible Mpe’s effects independently from the circuit, octopaminergic -for appetitive- and dopaminergic -for aversive stimuli [19], 1-hexanol was chosen, according to Fishilevich and Vosshall [20], because the mechanism(s) of olfactory transduction signal involve several glomeruli and complex neural pathways. Flies were starved for 8 hours prior to starting the experiments. These, done in triplicate, were performed in controlled environmental conditions (n = 12 bioassays/each experimental group of flies; n = 20 flies/arena). The assays lasted 18 hours, a streamlined range of time to overtake the possible influence of motor impairment in mutants. The dehydration of flies was prevented by placing a cotton ball with 3 mL of water in the arenas. Data obtained were expressed as average of percentages of flies reaching the 1-hexanol or water trap and statistically evaluated by one-way ANOVA (p<0.05) followed by HSD post-hoc test.
Electron microscopy analysis
Group II WT, untreated PINK1B9 and, as L+/A+, 0.1% Mpe-treated PINK1B9 mutants were anesthetized using carbon dioxide and carefully decapitated. The brains and the thoracic ganglia, once rapidly removed, were fixed in a mixture of 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M cacodylate buffer, washed several times in the same buffer, post-fixed in 1% osmium tetroxide in distilled water for 2 hours, and stained overnight at 4°C in an aqueous 0.5% uranyl acetate solution. After several washes in distilled water, the samples were dehydrated in a graded ethanol series, and embedded in SPURR resin. To identify the antennal lobes (ALs), semi-thin coronal sections of the whole brains were cut with a Leica EM UC6 ultramicrotome, stained with toluidine blue and observed with a Leica DM2700 P light microscope. Sections of about 70 nm corresponding to portions of the ALs and thoracic ganglia were cut with a diamond knife on a Leica EM UC6 ultramicrotome. Transmission electron microscopy (TEM) images were collected with a FEI Tecnai G2 F20 (FEI Company, The Netherlands) and a Jeol JEM 1011 (Jeol, Japan) electron microscopes, working respectively at an acceleration voltage of 80 and 100 kV, and recorded with a 1 and 2 Mp charge-coupled device (CCD) camera (Gatan BM Ultrascan and GatanOrius SC100, respectively). T-bars density (expressed as number of T-bars/m2) in both ALs and thoracic ganglia presynaptic boutons was assessed on a total of ten animals (three WT, three untreated PINK1B9 and four 0.1% Mpe-treated PINK1B9 mutants). 459 and 683 T-bars were randomly sampled respectively in the ALs and the thoracic ganglia on a total 496 non-overlapping micrographs at a final magnification of 6000, corresponding to a total sampled area of more than 6000 µm2. T-bars were unambiguously identified at presynaptic active zones by the presence of T-shaped electron-dense projections typically tethered by a large number of presynaptic vesicles.
The number of damaged mitochondria within ALs (expressed as percentage of the total number of mitochondria/sampled area) was evaluated in WT, untreated PINK1B9 and 0.1% Mpe-treated PINK1B9 mutants. More than 3000 mitochondria were randomly sampled on 191 non-overlapping micrographs at a final magnification of 4000, corresponding to a total sampled area of more than 5000 µm2. Damaged mitochondria were recognized for the presence of swollen external membrane, clearly fragmented cristae and inhomogeneous electron transparent mitochondrial matrix. The mean differences were tested using a two tailed t-test and a p<0.01 level was considered statistically significant.
Protein extraction and western blot analysis
Group II WT, untreated PINK1B9 and, as L+/A+, 0.1% Mpe- and 0.01% L-Dopa-treated PINK1B9 mutants flies were collected and immediately stored at −80°C. Head lysate preparations of adult males were performed by homogenization in RIPA buffer (9.1 mmol/L dibasic sodium phosphate, 1.7 mmol/L monobasic sodium phosphate, 150 mmol/L sodium chloride, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecylsulfate [pH adjusted to 7.4]) containing fresh protease inhibitor cocktails (Sigma-Aldrich, St. Louis, MO, USA). Two centrifugations were performed at 4°C at 10,000 g for 15 minutes, before protein quantification by DC Protein assay (Biorad, Hercules, CA, USA). 20 µg of proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis using the mini-PROTEIN 3-electrophoresis module assembly (Biorad, Hercules, CA, USA) and then transferred to immobilon-polyvinylidenedifluoride membranes (Amersham Biosciences). The membranes were incubated with primary antibodies overnight at 4°C. Immune complexes were detected with horseradish peroxidase–conjugated secondary antibodies and chemiluminescence reagents (ECL, Amersham Biosciences) and visualized by Image Quant LAS 4000. Densitometric analysis was performed by Image Studio Lite software for quantitative assessment.
Primary antibodies used in this study were against nc82 (1∶100 dilution, DSHB); Tyrosine Hydroxylase (1∶1000 dilution, MAB 318 Merk Millipore); actin (1∶100, sc1616 Santa Cruz Biotechnology); Horseradish-peroxidase–conjugated secondary antibodies were purchased from Life Technologies. Statistical significance of the results was evaluated by one-way ANOVA (p<0.05) followed by a HSD post-hoc test.
Results
Effects of Mucuna pruriens and L-Dopa on life span of PINK1B9 mutants
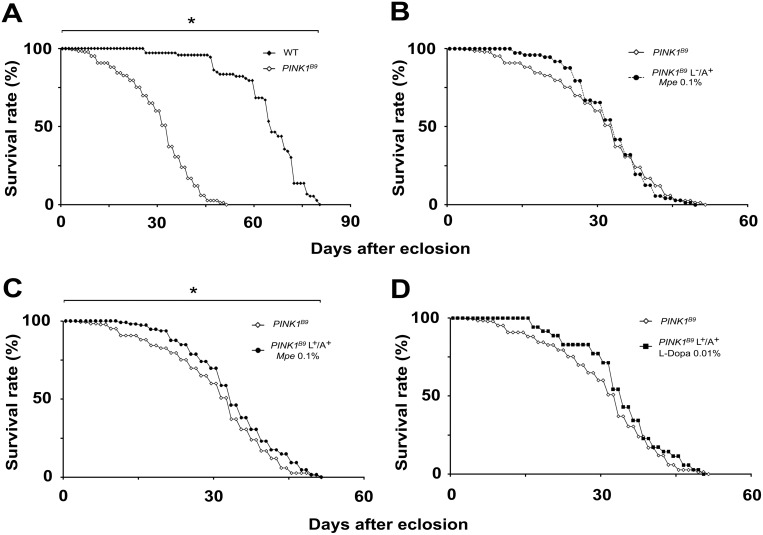
As shown in Fig. 1A, in agreement with our previous report [4], PINK1B9 mutants displayed a significantly shorter lifespan with respect to WT flies. To assess the ability of Mpe to affect lifespan of PINK1B9 mutants, they were supplied Mpe at different concentrations (0 (untreated), 0.1, 1 and 10% w/w in their standard diet) both as adults only (L−/A+) (Fig. 1B and Fig. S1A), and as larvae and adults (L+/A+) (Fig. 1C and Fig. S1B). The effects of L-Dopa (supplied as L+/A+ (at the concentration, 0.01%, at which is present in the Mpe 0.1%) on life span of PINK1B9 are also reported in Fig. 1D. The comparison between untreated and Mpe-treated PINK1B9, as shown by Kaplan-Meier survival curves, revealed a statistically significant effect of Mpe on lifespan of PINK1B9 mutants only when L+/A+ flies were fed 0.1% Mpe (Fig. 1C, p<0.05 by Gehan-Breslow-Wilcoxon test). No effect was observed following the L-Dopa administration in L+/A+. As shown in Fig. S1A, no significant effects were detected in in L−/A+ flies, no matter the concentration tested, nor in L+/A+ flies fed 1% or 10% Mpe enriched standard diet (Fig. S1B).
Figure 1. Effects of Mpe and L-Dopa on lifespan.
(A): Lifespan, expressed as % survival rates, of wild type (WT) and PINK1B9 flies. (B) and (C): Lifespan of PINK1B9 treated with Mucuna pruriens extract (Mpe) 0.1%,only when adults (L−/A+) (panel B) or from their larval stage to the end of their life-cycle (L+/A+) (panel C), respectively, as compared to lifespan of untreated PINK1B9 flies. (D): Lifespan of PINK1B9 flies treated with L-Dopa (L+/A+) 0.01%. *indicates p<0.05 at Kaplan-Meier survival curves (Gehan-Breslow–Wilcoxon - GraphPad Prism 5.01) between WT and untreated PINK1B9 (A) and between untreated PINK1B9 and PINK1B9 fed Mpe 0.1% (C).
Mucuna pruriens rescues impaired climbing behavior of PINK1B9 mutants
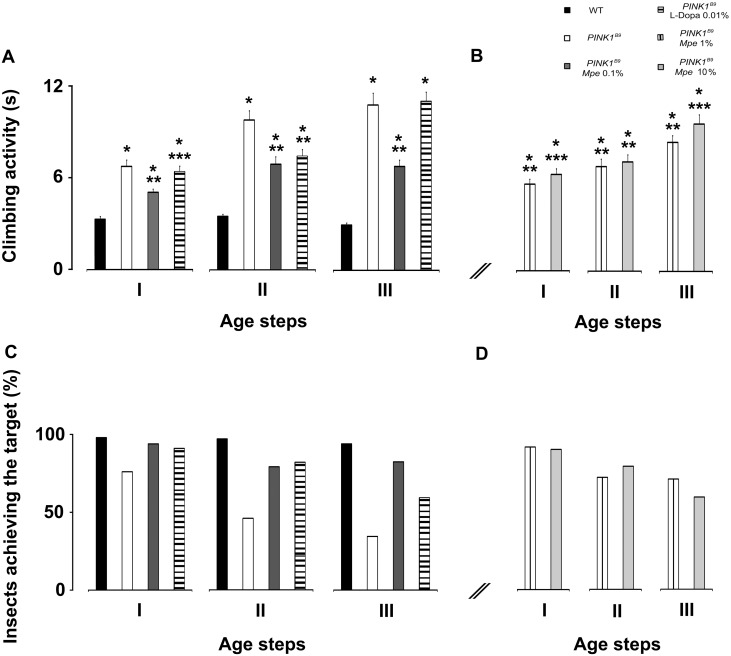
To investigate the locomotor ability the negative geotaxis assay, as described previously [17], was used. An impairment of climbing behavior was observed in untreated PINK1B9 at different age steps (I: 3–6; II: 10–15; III: 20–25 days old) with a worsening trend with aging, while WT flies fulfilled the evaluation criterion without differences among age groups. As shown in Fig. 2A, the mutants took longer times to accomplish the task than the WT (p<0.001).
Figure 2. Effects of Mpe and L-Dopa on climbing activity.
(A): Climbing activity of adult males wild-type (WT), untreated PINK1B9, PINK1B9 treated with Mucuna pruriens extract (Mpe) 0.1% and PINK1B9 treated with L-Dopa 0.01% (L-Dopa 0.01%). (B): Climbing activity of PINK1B9 adult males treated with Mpe 1 and 10% as compared with WT and untreated PINK1B9. (A) and (B): Treatments were administered to flies from their larval stage to the end of their life-cycle (L+/A+) and their effects were assayed at three different age steps (I: 3–6; II: 10–15; III: 20–25 days) of flies’ life-span. Values are average + SEM. *indicates p<0.05 at two-way ANOVA followed by HSD post-hoc test as compared to WT; **indicates p<0.05 at two-way ANOVA followed by HSD post-hoc test as compared to PINK1B9; ***indicates p<0.05 at two-way ANOVA followed by HSD post-hoc test as compared to PINK1B9 Mpe 0.1%. (C) and (D): Percentages of adult males WT, PINK1B9, Mpe 0.1%, L-Dopa 0.01% (C) and Mpe 1 and 10% (D) that could climb unto, or above, the line drawn at 6 cm from the bottom of the tube within 10 seconds.
The Mpe 0.1% treatment significantly ameliorated the climbing activity in mutants and also reduced the worsening trend with aging although the score obtained by treated mutants still remained higher than that measured in WT. Interestingly, the climbing time of L-Dopa-treated mutants from groups I and III did not significantly differ with respect to age-matched untreated PINK1B9, the performance of only group II flies being significantly ameliorated.
As shown in Fig. 2B, L+/A+ 1% Mpe-treated mutants reached similar rescue of climbing activity as observed in 0.1% Mpe-treated ones only when tested at early ages (groups I and II). On the other hand, 10% Mpe administration failed to significantly ameliorate motor behavior in groups I and III with respect to untreated PINK1B9 mutants, while a significant effect was detected in treated flies from group II. We also considered the percentages of flies that were able to complete the test and the results are depicted in histograms shown in Fig. 2C and D. In this respect, most of WTs of all age steps (97–98%) were able to complete the test, while only 76% of PINK1B9 from group I, 46% from group II and 36% from group III accomplished it, showing a clear age-dependent worsening. Administration of 0.1% Mpe, as L+/A+, greatly rescued PINK1B9 mutants (groups I–III) from motor impairment and restored to WT values the percentages (86–94%) of flies able to accomplish the task according to the evaluation criterion (10 sec). Furthermore, at variance with the above results, the effects of L-Dopa worsened over time. In particular, 0.01% L-Dopa administration determined a decrease of the number of flies able to complete the task showing a negative trend with aging. In fact, percentages of flies were 91%, 82% and 62%, in groups I, II and III, respectively.
Mucuna pruriens and L-Dopa effects on the EAG amplitude
As expected, the olfactory stimulations of flies’ antennae elicited responses with the typical EAG wave form, i.e. a rapid depolarization followed by a slower recovery phase, ending with the hyperpolarized wave before complete reversal to the baseline.
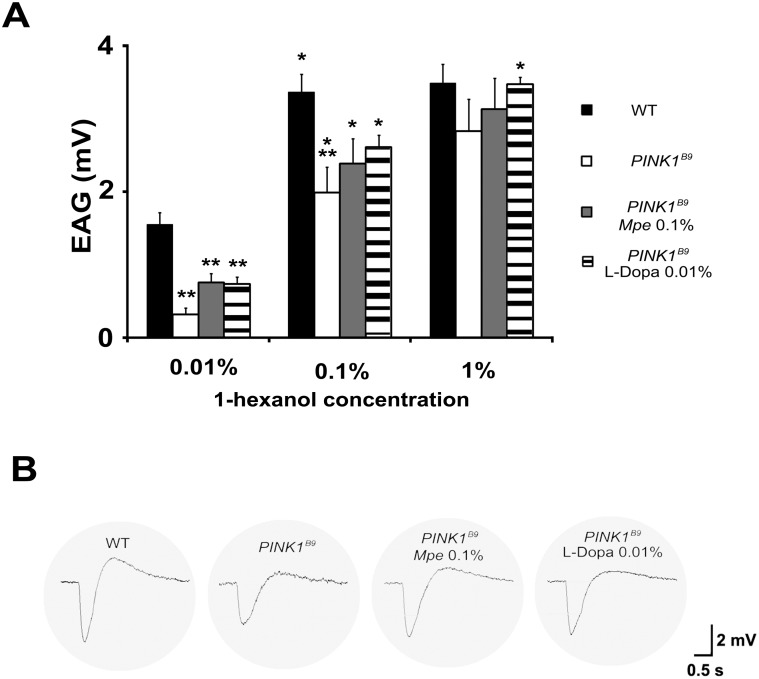
The results, summarized in Fig. 3A and 3B, show the olfactory response to 1-hexanol (0.01, 0.1 and 1%) elicited in WT, untreated PINK1B9, 0.1% Mpe- and 0.01% L-Dopa-treated mutants from age group II. In details, the average EAG signal amplitudes evoked by stimuli were significantly lower in PINK1B9 specimens in respect to WT thus substantially confirming data previously reported [4].
Figure 3. Electroantennogram responses to 1-hexanol.
Histograms in (A) show the dose-response relationship and their differences in signal for olfactory stimulations in WT, untreated PINK1B9 and in Mpe (0.1%)- and L-Dopa (0.01%)-treated PINK1B9, recorded in flies from group II. As odor stimuli, the 1-hexanol was administered in a 3-step dose from 0.01 to 1% in hexane. Values are average + SEM. *indicates p<0.05 at one-way ANOVA followed by HSD post hoc test as compared to the previous concentration of the stimulus. **indicates p<0.05 at one-way ANOVA followed by HSD post-hoc test as compared to WT. (B) Samples of EAGs recordings in response to 1-hexanol 0.1%.
The stimulation with 1-hexanol at 1% did not elicit a significant increase in the EAG amplitude as compared with the stimulation at 0.1% in all strains of flies with the exception of mutants flies treated with L-Dopa 0.01%. This result indicates that at the highest odor concentrations (0.1 and 1%) a saturation of response was reached by all groups but by the L-Dopa treated mutants. Besides, we observed that the responses to stimuli in WTs elicited a greater hyperpolarized phase in the EAGs (Fig. 3B and S2).
Even if a positive trend in treated mutants exists in the signal amplitude in response to 1-hexanol, a statistical difference between untreated, Mpe- and L-Dopa-treated PINK1B9 was not detected. The lowest odor concentration tested elicited a significantly higher response in WT as compared to the all strains of mutants (p<0.05). This difference shrank when the 0.1% concentration of odor was administered. A reduced response, although not statistically significant (p>0.05), was still detected in untreated mutants with respect to WTs (p<0.05), while treated flies showed on average an increased response with respect to untreated flies. The response measured in treated flies was therefore halfway between the highest of WTs and the lowest of untreated PINK1B9. Samples of EAGs responses are shown in Fig. 3B and Fig. S2.
Mucuna pruriens rescues impaired olfactory behavior
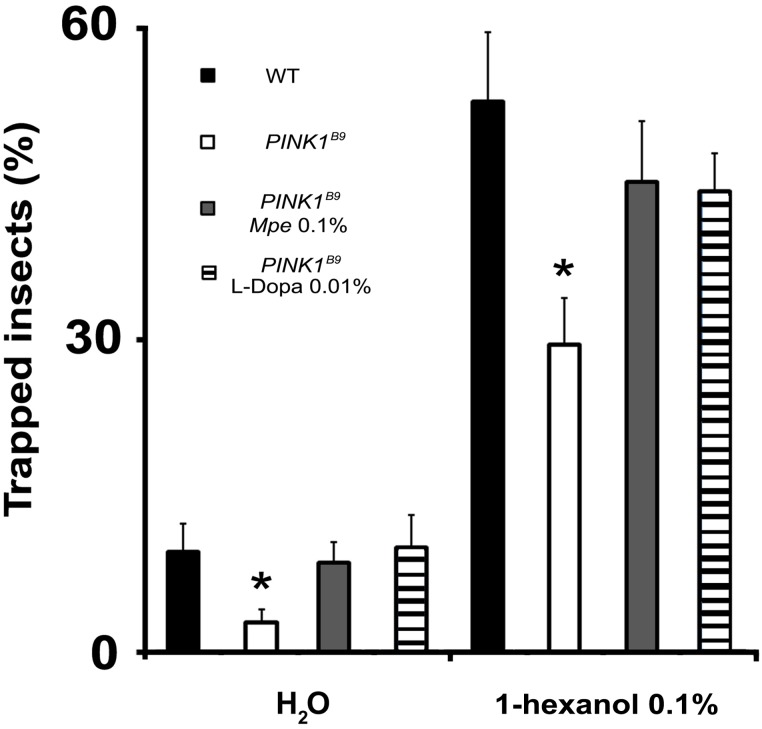
The olfactory behavior assay was restricted to flies of group II, by testing the responses to 1-hexanol (0.1% v/v) of WT, untreated PINK1B9, 0.1% Mpe- and 0.01% L-Dopa-treated, as L+/A+, PINK1B9 mutants. As expected, the analysis of the result, shown in Fig. 4, confirmed the olfactory behavioral impairment in PINK1B9 flies [4]. In fact, only 29.6±4.4% of mutant flies were odor-trapped, while the percentage of baited WT (52.9±6.6%) was significantly higher (p<0.004). PINK1B9 flies treated with 0.1% Mpe and 0.01% L-Dopa were able to reach the stimuli as WT controls (p>0.05). In fact, percentages of trapped flies were 45.2±5.8% and 44.2±3.6% for 0.1% Mpe- and 0.01% L-Dopa-treated mutants, respectively. Similar results were obtained concerning the numbers of trapped flies in the blank bait (H2O) (p<0.05 between untreated PINK1B9with respect to WT, 0.1% Mpe- and 0.01% L-Dopa-treated mutants).
Figure 4. Effects of Mpe and L-Dopa on olfactory behavior.
Responses to 1-hexanol 0.1% and water (H2O) of WT, untreated PINK1B9 and in Mpe (0.1%)- and L-Dopa (0.01%)-treated PINK1B9 flies. Values are average + SEM. *indicates p<0.05 at two-way ANOVA followed by HSD post hoc test as compared to WT, PINK1B9 Mpe 0.1%, PINK1B9 L-Dopa 0.01%.
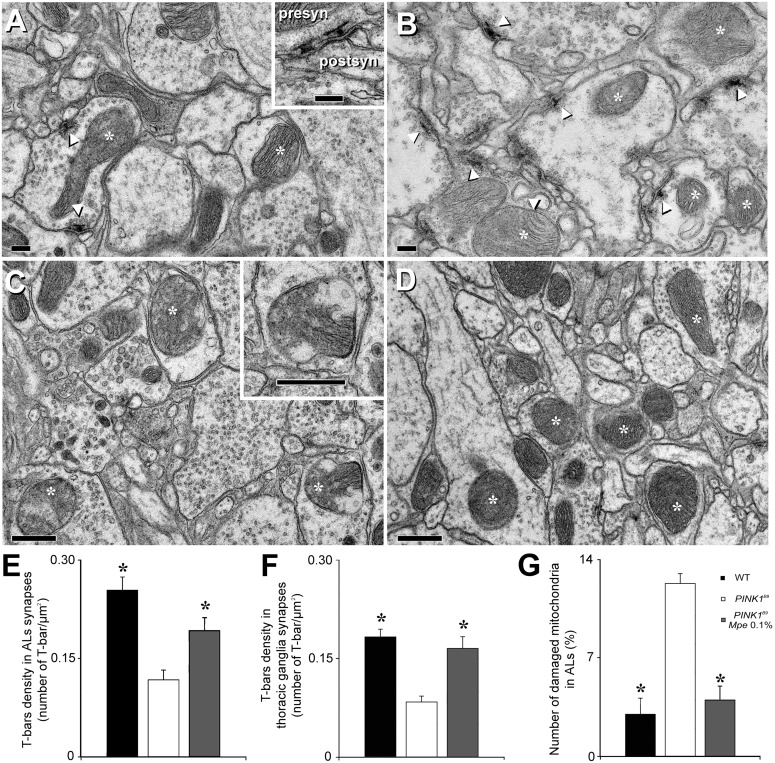
Mucuna pruriens rescues loss of T-bars at active zones of presynaptic terminals and damaged mitochondria in the antennal lobes and thoracic ganglia
Transmission electron microscopy (TEM) analysis was restricted to flies of group II of untreated WT, untreated PINK1B9 and 0.1% Mpe-treated, as L+/A+, PINK1B9 mutants and results are shown in Fig. 5. A significant decrease of T-bars density was observed in the presynaptic bouton active zones of both ALs and thoracic ganglia of PINK1B9 mutants with respect to WT controls (panels A, B, E and F). More importantly, a significant increase of T-bars density was detected in the ALs and thoracic ganglia of PINK1B9 treated with 0.1% Mpe, as L+/A+, with respect to untreated PINK1B9 (panels A, B, E and F). Moreover the number of damaged, swollen and with clearly fragmented cristae, mitochondria was significantly lower in presynaptic boutons of ALs of PINK1B9 mutants treated with 0.1% Mpe, compared with untreated mutants (panels C, D and G).
Figure 5. Effects of Mpe on T-bars and mitochondria in antennal lobes and thoracic ganglia.
Transmission electron microscopy (TEM) images of T-bars and mitochondria inside antennal lobes (ALs) of wild type (WT), untreated PINK1B9 and in Mpe (0.1%)-treated PINK1B9 flies. (A): T-bars in a presynaptic bouton of PINK1B9 ALs (arrowheads). Asterisks indicate mitochondria inside presynaptic boutons and neurites. Inset: high magnification of two T-bar in coronal section. (B): T-bars in presynaptic boutons of ALs of PINK1B9 Mpe 0.1% (arrowheads). Asterisks indicate mitochondria inside presynaptic boutons and neurites. (C): swelling on the external mitochondrial membrane (at high magnification in the inset) and mitochondrial cristae widely degenerated (asterisks) in ALs of PINK1B9. (D): Mitochondria of PINK1B9 Mpe 0.1% (asterisks). (E): Presynaptic T-bar density in ALs of WT, PINK1B9 and PINK1B9 0.1% Mpe flies. Values are average + SEM. *indicates p<0.01 at two tailed t-test with respect to PINK1B9. (F): T-bar density in thoracic ganglia of WT, PINK1B9 and PINK1B9 0.1% Mpe flies. Values are average + SEM. *indicates p<0.01 at two tailed t-test with respect to PINK1B9. (G): Percentages of damaged mitochondria in ALs of WT, PINK1B9 and PINK1B9 0.1% Mpe flies. Values are average + SEM. *indicates p<0.01 at two tailed t-test with respect to PINK1B9. Abbreviations: postsyn: postsynaptic; presyn: presynaptic. Scale bars are 200 µm in A and B and 500 µm in C and D.
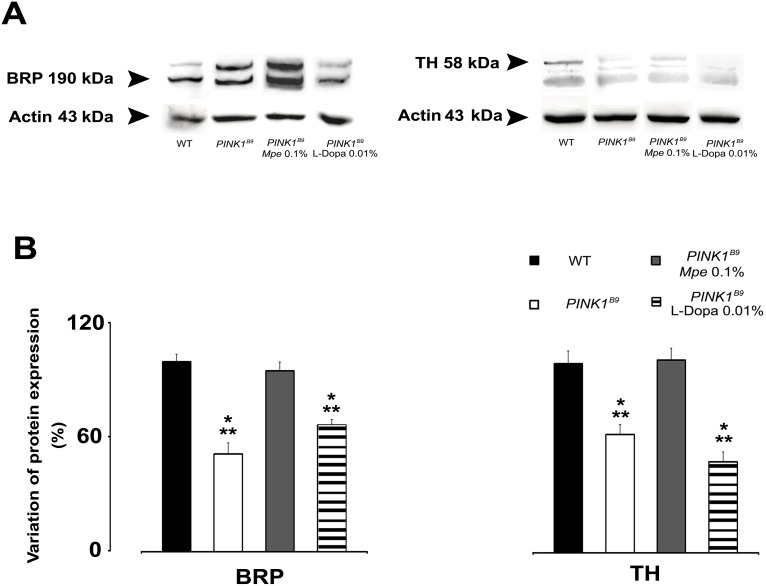
Mucuna pruriens and L-Dopa differentially affect whole brain bruchpilot (BRP) and tyrosine hydroxylase (TH) expression
Fig. 6 shows the results of western blot analysis of whole brain expression of BRP and TH of flies of group II WT, untreated PINK1B9 0.1% Mpe- and 0.01% L-Dopa-treated, as L+/A+, PINK1B9 mutants. As shown in Fig. 6A, the expression of BRP and TH in untreated PINK1B9 mutants was significantly lower (p<0.05) than in WT. Diet supply of 0.1% Mpe to PINK1B9 mutants significantly recovered BRP and TH expression to WT controls levels (p<0.05) and these values did not differ statistically from those of WT. Notably, BRP and TH expression in PINK1B9 mutants fed 0.01% L-Dopa resulted similar to BRP and TH expression in untreated PINK1B9 mutants. ANOVA also revealed that both BRP and TH expression resulted statistically different as compared to their expression of both WT and PINK1B9 mutants fed 0.1% Mpe.
Figure 6. Effects of Mpe and L-Dopa on BRP and TH.
(A): Representative western blot analysis of head homogenates from adult wild type (WT), untreated PINK1B9 and in Mpe (0.1%)- and L-Dopa (0.01%)-treated PINK1B9 flies showing labeled bands of Bruchpilot protein (BRP), of Tyrosine hydroxylase (TH) and of the loading control actin (from top to bottom). (B): Percentage of protein expression variation of BRP and TH in WT, untreated PINK1B9 and in Mpe (0.1%)- and L-Dopa (0.01%)-treated PINK1B9 flies. Values are average + SEM. *indicates p<0.05 at one-way ANOVA with respect to WT; **indicates p<0.05 at one-way ANOVA (HSD post-hoc test) with respect to PINK1B9 Mpe 0.1%.
Discussion
This study was aimed at characterizing the effects of the standardized extract of Mucuna pruriens seeds, known for possible neuroprotective effects in neurotoxin-induced models of PD [21] [22] and reduced risk of dyskinesias [14], in a genetic fly model of PD, the PINK1B9 mutant Dm [23]. Notably, mutations at PINK1 gene model a number of features of early onset PD such as cell energy maintenance [24] and compromised olfactory and mitochondrial function [4] enabling in-depth investigations into physiopathology of PINK1B9-related molecular, morphological and functional bases of PD. The present results show that addition of 0.1% Mpe to the feeding medium of PINK1B9 mutants significantly a) improved climbing ability and olfaction, b) rescued compromised T-bars density and damaged mitochondria in the ALs and thoracic ganglia, c) restored to WT control values the expression of BRP and TH proteins. Moreover, these results suggest that Mpe is an effective medication with intrinsic ability of delaying the onset of chronic L-Dopa-induced long-term motor complications (Fig. 2A and B).
These findings are in general agreement with previous studies reporting antiparkinsonian activity of Mp [14] [25] associated with reduced risk of dyskinesias, both in the clinical [26] and in the experimental [14] setting, and suggest that its antiparkinsonian effects may be due to components other than L-Dopa or that its components might have L-Dopa-enhancing effects [25] [26] [27] on one hand, as well as L-Dopa-induced dyskinesias (LID)-preventive effects, on the other.
Intriguingly, PINK1B9 mutations have been linked to both autosomal recessive and sporadic forms of PD and, given the role of PINK1-parkin pathway in regulating mitochondrial function, our findings highlight its role as a potential target for the described actions of Mpe [30] on mitochondria. This interpretation finds further support in the observation of mitochondrial stress-dependent neurodegeneration [7] and dysfunction in PINK1 knock-out mice [31].
A large body of literature documents that mutations of PINK1 gene are associated with mitochondrial dysfunction. In particular, complex I deficiency [24] [32] has been characterized as a mechanism of energy balance failure [33] resulting also in dramatic loss of dopaminergic neurons [34]. Although in the present study we did not attempt any direct measurement of mitochondrial energy impairment, this dysfunction was indirectly determined by assessing the number of damaged, swollen and with clearly fragmented cristae, mitochondria and we found that 0.1% Mpe administration could dramatically recover their morphology to that of WT controls (Fig. 5). This indicates that Mpe may play beneficial actions by interfering with the mechanisms responsible of energy production [24] or linked to maintenance of membrane gradients as well as to protection against the raise of reactive oxygen species within mitochondria [16]. In this regard, it is intriguing to observe that Mp has antioxidant properties [35] and it was suggested that its “rescue” properties may be due to increased complex-I activity and presence of nicotinamide adenine dinucleotide and coenzyme Q-10 [25]. This interpretation is also supported by the observation that also enhancement of nucleotide production, by feeding PINK1 mutant Dm with folic acid, results in rescued loss of mitochondrial mass and function [31]. Thus, on the basis of these reports and of our results it seems possible to speculate that Mpe administration interferes with the pathway regarding the mitochondrial rescue from oxidative stress but not on the complex apoptosis mechanism. In fact, the clock of the end of the life is not modified as also suggested by the results regarding the effect of Mpe on life span according to which the amelioration is slight, albeit significant. In agreement with Poddighe et al. [4], PINK1B9 mutants showed steeper slope life span curves and overall shortened lifespan with respect to WT. Mpe significantly attenuated these conditions only when administered to L+/A+ at 0.1%, but not when administered to adults only (L−/A+) no matter the concentration tested (Fig. S1A), nor when administered with 0.01% L-Dopa. These results can be explained by taking into account that in Drosophila the cluster of neurons is manly conserved from larval to adult stage [28]. Conversely in L+/A+ mutants treated at the highest concentration administered (1–10%) even if not significant, a worsening trend was observed (Fig. S1B). The effects of Mpe on flies’ lifespan resemble those of the Mpe component, nicotine, described in a Drosophila autosomal recessive-juvenile model of parkinsonism [29].
In addition to the observed rescue of damaged mitochondria in PINK1B9 mutants treated with 0.1% Mpe, we observed that this treatment significantly recovered the expression of BRP and the reduction of T-bars density in both PINK1B9 ALs and thoracic ganglia, strengthening the tenet that BRP is crucial for the correct formation of T-bars at active zones [36]. Mutation-induced mitochondrial degeneration may also have led to the observed diminished expression of BRP, known to be critical also for neurotransmitter release [37] [38]. Accordingly, PINK1B9 mutants show degeneration of flight muscle and of dopaminergic neurons accompanied by locomotive defects [23] [39] [40]. Humphrey et al. [40] also showed that climbing deficit is related to dysfunction of dopaminergic cells and we found that PINK1B9 mutants also showed compromised motor capabilities as assessed by climbing behavior (Fig. 2). Hence, Mpe-increased expression of BRP may have increased the ability to release neurotransmitters that would result in improved locomotion, as suggested by Yellman et al. [41]. Therefore, our data suggest that the effects of Mpe treatment on BRP expression, climbing and T-bars in PINK1B9 mutants may represent the convergence toward an unified mechanism grounded on mitochondria functional rescue.
Olfactory dysfunction is a clinical early non-motor symptom of PD [3] and, accordingly, we observed loss of olfaction in PINK1B9 mutant Dm [4]. The physiopathology of olfactory dysfunction is not known. However, many studies have suggested involvement of dopaminergic system [42] [43]. In our investigation we observed improved olfactory responsiveness underlined by both behavioral and electrophysiological experiments.
It is interesting to observe that the shape of EAG responses recorded in the WT revealed a dose-related hyperpolarizing part (Fig. S2). This observation seems in accordance with the stimulating power of 1-hexanol that is reported to involve both the appetitive and the aversive stimuli [19]. The EAG represent the summed activity of all antennal sensory neurons involved in stimulation. This activity can result in the EAG recordings in a depolarization and/or hyperpolarization signal, that is elicited according to the stimulating effect of the odor tested as well as of its concentration. In details, in WT strain, the rapid depolarization is followed by a slow recovery phase at the lower concentration (0.01%) while at the highest concentration (1%), a greater hyperpolarizing phase was recorded. This phase could represent the activation of a pool of receptors that hyperpolarize when stimulated at this high concentration. With regards to this, a similar response was not present in untreated PINK1B9 (Fig. S2). Future electrophysiological analysis of the olfactory response should take into account both shape and amplitude whose variations might be a promising tool to study peripheral olfactory responses. Furthermore, our behavioural results show that PINK1 B9 mutants have a decreased responsiveness to 1-hexanol and water (Fig. 4) that reveals an impairment of also other chemoreceptors such as hygroreceptors [44]. In other words the mutants seem to present a general sensory impairment.
In agreement with study by Katzenschlager and Lees [45], suggesting a possible association between olfaction, increased TH and dopamine in the olfactory bulbs, we observed that PINK1B9 mutation-dependent impairment of olfaction behavior and whole brain TH expression were improved by Mpe treatment (Figs. 4 and 6, respectively). Surprisingly, L-Dopa administration on its own failed to recover TH expression to WT controls levels. However, since our analysis was done in whole brain homogenates, if analysis was restricted to the ALs, the homologous structures of human olfactory bulbs, we cannot exclude the possibility that L-Dopa would have brought different results.
The physiopathology of LID is still largely unknown and LID has consistently been related to excessive DA release [46]. Furthermore, in parkinsonian non-human primates [47], L-Dopa produces LID without enhancing striatal DA release. Interestingly, Katzenschlager et al. [26] observed a reduced severity of dyskinesias after Mp as compared to levodopa/carbidopa combination and an increased DDC expression associated with LID has been reported in rats [48]. This intriguing prospective remains to be fully demonstrated in the Dm mutant model. In conclusion, our study confirms in this translational model the validity of Mucuna pruriens as a valuable approach for PD treatment, discloses mechanistic insights at the basis of its effects and confirms the use of PINK1B9 Dm as a model of PD that fulfills the required face, construct and predictive validity criteria to follow up on these investigations.
Supporting Information
Effects of Mpe administered at different concentration on lifespan. (A): Lifespan, expressed as % survival rates of untreated and Mpe-treated PINK1B9 at the 4 dose-step tested: 0, 0.1, 1 and 10% (w/w) only when adults (L−/A+). (B): lifespan of untreated and Mpe-treated PINK1B9 at the 4 dose-step tested: 0, 0.1, 1 and 10% (w/w) only when adults (L+/A−).
(TIF)
EAGs samples. Dose-response relationships for olfactory stimulations in WT and PINK1B9 adult flies and their differences in signal amplitude and shape.
(TIF)
Acknowledgments
We are indebted to Natural Remedies Ltd., Bangalore, India for generous gift of Mucuna pruriens extract. The Authors would like to thank Prof. Giovanni Biggio (University of Cagliari, Italy) and Dr. Andrea Falqui (Istituto Italiano di Tecnologia, Genoa, Italy) for scientific support and productive discussions, and Dr. Valter Seu, Mrs Alessia Caredda and Dr. Valentina Corda (University of Cagliari) for taking care of flies and technical support. A special thank goes to Prof. A.M. Angioy.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, et al.. (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 24∶ 197–211. [DOI] [PubMed]
- 2.Haehner A, Hummel T, Reichmann H (2011) Olfactory Loss in Parkinson’s Disease. Parkinsons Dis. doi:10.4061/2011/450939. [DOI] [PMC free article] [PubMed]
- 3.Ferraris A, Ialongo T, Passali GC, Pellecchia MT, Brusa L, et al.. (2009) Olfactory dysfunction in Parkinsonism caused by PINK1 mutations. Mov Disord. 24∶ 2350–2357. [DOI] [PubMed]
- 4. Poddighe S, Bhat KM, Setzu MD, Solla P, Angioy AM, et al. (2013) Impaired Sense of Smell in a Drosophila Parkinson’s Model. PLoS ONE. 8: e73156 doi:10.1371/journal.pone.0073156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo M (2010) What have we learned from Drosophila models of Parkinson's disease? Prog Brain Res. 184: 3–16. [DOI] [PubMed] [Google Scholar]
- 6. Celotto AM, Palladino MJ (2005) Drosophila: A Model System To Study Neurodegeneration. Mol Inter. 5: 292–303. [DOI] [PubMed] [Google Scholar]
- 7. Moisoi N, Fedele V, Edwards J, Martins LM (2014) Loss of PINK1 enhances neurodegeneration in a mouse model of Parkinson's disease triggered by mitochondrial stress. Neuropharmacology. 77: 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katzenschlager R, Lees AJ (2002) Treatment of Parkinson's disease: levodopa as the first choice. J Neurol. 249: 19–24. [DOI] [PubMed] [Google Scholar]
- 9. Mercuri NB, Bernardi G (2005) The magic of L-dopa: why is it the gold standard Parkinon’s therapy? Trend Pharmacol Sci. 26: 341–344. [DOI] [PubMed] [Google Scholar]
- 10. Brooks DJ (2008) Optimizing levodopa therapy for Parkinson’s disease with levodopa/carbidopa/entacapone: implications from a clinical and patient perspective. Neuropsychiatr Dis Treat. 4: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morelli M, Carta AR, Jenner P (2009) Adenosine A2A Receptors and Parkinson’s Disease. Handb Exp Pharmacol. 193: 589–615. [DOI] [PubMed] [Google Scholar]
- 12. Fox SH (2013) Non-dopaminergic treatments for motor control in Parkinson's disease. Drugs. 73: 1405–15. [DOI] [PubMed] [Google Scholar]
- 13. Dhanasekaran M, Tharakan B, Manyam BV (2008) Antiparkinson drug Mucuna pruriens shows antioxidant and metal chelating activity. Phytother Res. 22: 6–11. [DOI] [PubMed] [Google Scholar]
- 14. Lieu CA, Kunselman AR, Manyam BV, Venkiteswaran K, Subramanian T (2010) A water extract of Mucuna pruriens provides long-term amelioration of parkinsonism with reduced risk for dyskinesias. Parkinsonism Relat Disord. 16: 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kasture S, Mohan M, Kasture V (2013) Mucuna pruriens seeds in treatment of Parkinson’s disease: Pharmacological review. Orien Pharm Exp Ther. 13: 165–174. [Google Scholar]
- 16. Obata T, Yamanaka Y, Kinemuchi H, Oreland L (2001) Release of dopamine by perfusion with 1-methyl-4-phenylpyridinium ion (MPP(+)) into the striatum is associated with hydroxyl free radical generation. Brain Res. 906: 170–175. [DOI] [PubMed] [Google Scholar]
- 17. Liu Z, Wang X, Yu Y, Li X, Wang T, et al. (2008) A Drosophila model for LRRK2-linked parkinsonism. Proc. Natl Acad. Sci USA. 105: 2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dekker T, Ibba I, Siju KP, Stensmyr MC, Hansson BS (2006) Olfactory shifts parallel superspecialism for toxic fruit in Drosophila melanogaster sibling, D. sechellia. Curr Biol 16: 101–109. [DOI] [PubMed] [Google Scholar]
- 19. Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, et al. (2003) Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in drosophila. J Neurosci. 23: 10495–10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fishilevich E, Vosshall LB (2005) Genetic and Functional Subdivision of the Drosophila Antennal Lobe Curr. Biol (15) 1548–1553. [DOI] [PubMed] [Google Scholar]
- 21. Yadav SK, Prakash J, Chouhan S, Singh SP (2013) Mucuna pruriens seed extract reduces oxidative stress in nigrostriatal tissue and improves neurobehavioral activity in paraquat-induced Parkinsonian mouse model. Neurochem Int. 62: 1039–1047. [DOI] [PubMed] [Google Scholar]
- 22. Yadav SK, Prakash J, Chouhan S, Westfall S, Verma M, et al. (2014) Comparison of the neuroprotective potential of Mucuna pruriens seed extract with estrogen in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mice model. Neurochem Int. 65: 1–13. [DOI] [PubMed] [Google Scholar]
- 23. Park J, Lee SB, Lee S, Kim Y, Song S, et al. (2006) Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 441: 1157–1161. [DOI] [PubMed] [Google Scholar]
- 24.Morais VA, Verstreken P, Roethig A, Smet J, Snellinx A, et al.. (2009) Parkinson's disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol Med. 1∶ 99–111. [DOI] [PMC free article] [PubMed]
- 25. Manyam BV, Dhanasekaran M, Hare TA (2004) Neuroprotective effects of the antiparkinson drug Mucuna pruriens. Phytother Res. 18: 706–712. [DOI] [PubMed] [Google Scholar]
- 26.Katzenschlager R, Evans A, Manson A, Patsalos PN, Ratnaraj N, et al.. (2004) Mucuna pruriens in Parkinson’s disease: a double blind clinical and pharmacological study. J Neurol Neurosurg Psychiatry. 75∶ 1672–1677. [DOI] [PMC free article] [PubMed]
- 27. Lieu CA, Venkiteswaran K, Gilmour TP, Rao AN, Petticoffer AC, et al. (2012) The Antiparkinsonian and Antidyskinetic Mechanisms of Mucuna pruriens in the MPTP-Treated Nonhuman Primate. Evid Based Complement Alternat Med. 2012: 840247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Monastirioti M (1999) Biogenic amine systems in the fruit fly Drosophila melanogaster. Microsc Res Tech. 45: 106–121. [DOI] [PubMed] [Google Scholar]
- 29. Chambers RP, Call GB, Meyer D, Smith J, Techau JA, et al. (2013) Nicotine increases lifespan and rescues olfactory and motor deficits in a Drosophila model of Parkinson's disease. Behav Brain Res. 253: 295–102. [DOI] [PubMed] [Google Scholar]
- 30. Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, et al. (2006) Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 441: 1162–1166. [DOI] [PubMed] [Google Scholar]
- 31. Tufi R, Gandhi S, de Castro IP, Lehmann S, Angelova PR, et al. (2014) Enhancing nucleotide metabolism protects against mitochondrial dysfunction and neurodegeneration in a PINK1 model of Parkinson's disease. Nat Cell Biol 16: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu Z, Yu Y, Li X, Ross CA, Smith WW (2011) Curcumin protects against A53 T alpha-synuclein498 induced toxicity in a PC12 inducible cell model for Parkinsonism. Pharmacol Res. 63: 439–444. [DOI] [PubMed] [Google Scholar]
- 33. Knott A, Bossy-Wetzel E (2009) Impairing the Mitochondrial Fission and Fusion Balance: A New Mechanism of Neurodegeneration. Ann N Y Acad Sci. 1147: 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coulom H, Birman S (2004) Chronic exposure to rotenone models sporadic Parkinson's disease in Drosophila melanogaster. J Neurosci. 24: 10993–10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dhanasekaran M, Tharakan B, Manyam BV (2008) Antiparkinson drug–Mucuna pruriens shows antioxidant and metal chelating activity Phytother Res. 22: 6–11. [DOI] [PubMed] [Google Scholar]
- 36.Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, et al.. (2006) Bruchpilot, a Protein. [DOI] [PubMed]
- 37. Takao-Rikitsu E, Mochida S, Inoue E, Deguchi-Tawarada M, Inoue M, et al. (2004) Physical and functional interaction of the active zone proteins, CAST, RIM1, and Bassoon, in neurotransmitter release. J Cell Biol 164: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, et al. (2006) Bruchpilot promotes active zone assembly, Ca2+ channel custering, and vesicle release. Science. 312: 1051–1054. [DOI] [PubMed] [Google Scholar]
- 39. Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, et al. (2006) Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA. 103: 10793–10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Humphrey DM, Parsons RB, Ludlow ZN, Riemensperger T, Esposito G, et al. (2012) Alternative oxidase rescues mitochondria-mediated dopaminergic cell loss in Drosophila. Hum Mol Genet. 21: 2698–2712. [DOI] [PubMed] [Google Scholar]
- 41. Yellman C, Tao H, He B, Hirsh J (1997) Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc Natl Acad Sci USA. 94: 4131–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang J, You H, Liu JF, Ni DF, Zhang ZX, et al. (2011) Association of olfactory bulb volume a olfactory sulcus depth with olfactory function in patients with Parkinson disease. Am J Neuroradiol 32: 677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sharot T, Shiner T, Brown AC, Fan J, Dolan RJ (2009) Dopamine enhances expectation of pleasure in humans. Curr Biol. 19: 2077–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sayeed O, Benzer S (1996) Neurobiology Behavioral genetics of thermosensation and hygrosensation in Drosophila Proc. Natl. Acad. Sci. USA 93: 6079–6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Katzenschlager R, Lees AJ (2004) Olfaction and Parkinson's syndromes: its role in differential diagnosis. Curr Opin Neurol. 17: 417–423. [DOI] [PubMed] [Google Scholar]
- 46.Carta M, Bezard E (2011) Contribution of pre-synaptic mechanisms to L-DOPA-induced. [DOI] [PubMed]
- 47.Porras G, De Deurwaerdere P, Li Q, Marti M, Morgenstern R, et al.. (2014) L-dopa-induced dyskinesia: beyond an excessive dopamine tone in the striatum. Sci Rep. 16;4∶ 3730. [DOI] [PMC free article] [PubMed]
- 48. Gil S, Park C, Lee J, Koh H (2010) The roles of striatal serotonin and 455 L -amino-acid decarboxylase on L-DOPA-induced Dyskinesia in a Hemiparkinsonian rat model. Cell Mol Neurobiol. 30: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of Mpe administered at different concentration on lifespan. (A): Lifespan, expressed as % survival rates of untreated and Mpe-treated PINK1B9 at the 4 dose-step tested: 0, 0.1, 1 and 10% (w/w) only when adults (L−/A+). (B): lifespan of untreated and Mpe-treated PINK1B9 at the 4 dose-step tested: 0, 0.1, 1 and 10% (w/w) only when adults (L+/A−).
(TIF)
EAGs samples. Dose-response relationships for olfactory stimulations in WT and PINK1B9 adult flies and their differences in signal amplitude and shape.
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.