Abstract
Competition may lead to changes in a species’ environmental niche in areas of sympatry and shifts in the niche of weaker competitors to occupy areas where stronger ones are rarer. Although mainland Mediterranean (Rhinolophus euryale) and Mehely’s (R. mehelyi) horseshoe bats mitigate competition by habitat partitioning, this may not be true on resource-limited systems such as islands. We hypothesize that Sardinian R. euryale (SAR) have a distinct ecological niche suited to persist in the south of Sardinia where R. mehelyi is rarer. Assuming that SAR originated from other Italian populations (PES) – mostly allopatric with R. mehelyi – once on Sardinia the former may have undergone niche displacement driven by R. mehelyi. Alternatively, its niche could have been inherited from a Maghrebian source population. We: a) generated Maxent Species Distribution Models (SDM) for Sardinian populations; b) calibrated a model with PES occurrences and projected it to Sardinia to see whether PES niche would increase R. euryale’s sympatry with R. mehelyi; and c) tested for niche similarity between R. mehelyi and PES, PES and SAR, and R. mehelyi and SAR. Finally we predicted R. euryale’s range in Northern Africa both in the present and during the Last Glacial Maximum (LGM) by calibrating SDMs respectively with SAR and PES occurrences and projecting them to the Maghreb. R. mehelyi and PES showed niche similarity potentially leading to competition. According to PES’ niche, R. euryale would show a larger sympatry with R. mehelyi on Sardinia than according to SAR niche. Such niches have null similarity. The current and LGM Maghrebian ranges of R. euryale were predicted to be wide according to SAR’s niche, negligible according to PES’ niche. SAR’s niche allows R. euryale to persist where R. mehelyi is rarer and competition probably mild. Possible explanations may be competition-driven niche displacement or Maghrebian origin.
Introduction
Species distribution patterns may potentially result from a range of causes, historical or current, involving abiotic factors as well as biotic interactions [1]. Identifying which factors determine species distribution among the several potential candidates may not be obvious.
A paradigm of ecology is that long-term coexistence is impossible for species sharing an identical ecological niche due to competitive and stochastic factors [2]–[4]. Opposite evolutionary pressures may act on sympatric species in the same guild. Ecomorphological convergence may take place as a result of selective pressures associated with optimal exploitation of the same resources; on the other hand, if such resources are limiting, interspecific competition may occur, leading to niche segregation.
Several types of such mechanisms have been described, including spatial or temporal niche separation [5]–[7] and resource partitioning by morphological divergence [8]–[10]. Interspecific competition may lead to ecological character displacement: differences in morphological and behavioural traits between species are greater where the latter occur in sympatry, smaller or absent in allopatric conditions [2], [11], [12]. Character displacement is often referred to morphological divergence, whose relationship with resource utilization may sometimes be questionable [13], [14]. However, other functionally important traits characterizing a species’ ecological niche may undergo displacement, with crucial consequences for geographical distribution. Yet, the spatial dimension of competition – i.e., the large-scale alteration of species distribution due to biotic interactions – is a poorly explored issue. Interspecific competition might involve changes in the environmental niche of a species where the latter is sympatric to competitors, a process hereafter termed as “niche displacement” [14]. Assessing niche displacement constitutes a key approach to a better understanding of factors influencing species’ geographical range and niche features, offering a major insight into present and future distributional dynamics [14]. Clearer patterns are expected where competition is especially harsh. This is the case with insular environments, where resources are often limiting [15]: thus, islands provide an ideal set to study these processes.
However, caution is needed when interpreting the current characteristics of the ecological niche: rather than resulting from forces acting in situ, they could have been shaped by historical processes occurred ex situ, i.e. in the population’s geographical source, and then retained by their descendents in the newly established population (such as in a process of island colonization from the mainland). Although the rapid change of ecological traits have attracted the attention of scientists for centuries, there is increasing evidence that the tendency for many ecological traits to be retained over time, called niche conservatism [16] is an important, general phenomenon with major evolutionary and ecological consequences – among which, the stability of species assemblages [17].
Bats represent interesting models to test the effect of interactions between species that share habitats and ecomorphological traits due to adaptive convergence, phylogenetic relatedness or crypticism [18]–[20]. Although among bats several examples of niche segregation due to divergence in morphology, sensory ecology, foraging strategies or habitat partitioning are known [21]–[24], no evidence of competition-driven geographical displacement is available.
In this study we focus on two rhinolophid bat species, the Mediterranean (Rhinolophus euryale) and Mehely’s (Rhinolophus mehelyi) horseshoe bats. These largely sympatric Mediterranean bats [25] may be regarded as sibling species as they derive from a close common ancestor and are morphologically very similar [26], [27]. They are thought to have diverged only 3 My ago [26] and only in 1901 were they recognized as separate species by the German zoologist Paul Matschie. R. euryale is widely distributed from sea level to ca. 1,000 m a.s.l. in the south of the continent as well as north-west Africa, and the Near East [28]. Classified globally as near threatened, R. euryale populations are declining in most of the geographical range [28]. R. mehelyi is confined to the Mediterranean where it shows a patchy occurrence from north Africa and southern Europe through Asia Minor, Anatolia, to Transcaucasia, Iran and Afghanistan [29]. The species is classified as vulnerable on a global scale, and is reported to be extinct in north-east Spain, Mallorca [30], Croatia and Israel [31] and close to extinction in France [32] and Romania [33].
These species have been regarded as potential competitors when foraging in sympatry for marked similarities in morphology, echolocation and habitat selection [34]–[36] yet, provided environmental conditions are sufficiently heterogeneous, they may mitigate competition by fine-scale habitat partitioning [35], [36].
The Italian distribution of these bats is puzzling. R. mehelyi is frequent and relatively abundant on Sardinia, whereas in the rest of Italy is almost absent – in fact on the brink of extinction, being restricted to two sites in Sicily where only small colonies occur, and one site on the mainland (Apulia, south-east Italy) where only in 2013 was a single individual observed after 40 years since the latest sighting [37]. R. euryale is widespread in most of the Italian peninsula and also occurs in Sicily. On Sardinia, although both species are present, R. mehelyi occurs in allopatry in most of the island while their sympatry is restricted to a small area. There, the two species show divergence in echolocation call frequency [38]. Specifically, Sardinian R. euryale shows lower frequencies than the peninsular conspecifics, a difference thought to represent an acoustic character displacement pattern driven by the dominant R. mehelyi probably to avoid interspecific frequency overlap and maintain separate communication frequency bandwidths [38].
Islands are ecological systems where spatial and trophic resources are often limited and may lead to increased competition [39], [40]. In this study we used distributional data, maximum entropy models (Maxent) and Niche Analysis to test the main hypothesis that in an insular, food-limited environment (Sardinia), R. euryale may have at least partly accomplished geographical separation from its sibling species thanks to a distinct ecological niche which has allowed it to settle in an area where R. mehelyi is rare and competition probably negligible.
This hypothesis generates two predictions:
Significant overlap will occur between the environmental niches of Sardinian R. mehelyi and allopatric R. euryale populations from the mainland and Sicily (hereafter termed PES), setting the scene for interspecific competition;
although conspecifics, the niche of Sardinian R. euryale (hereafter termed SAR) will diverge from that of PES. This divergence will allow SAR to mitigate interspecific competition with R. mehelyi.
Assuming that SAR has originated from PES, once bats colonized Sardinia the original ecological niche may have undergone a niche displacement process driven by R. mehelyi’s competition and generated the difference forecast by prediction b). However, the origin of R. euryale’s population on Sardinia is unknown. Along with Europe, northern Africa represents an important geographical source for Sardinian bats [41]–[43]. Under a niche conservatism assumption [16], any niche difference spotted in SAR relative to PES might rather represent a legacy of an extra-European source population which colonized Sardinia and founded SAR. It may be hypothesized that once bats colonized the island, they retained their ecological niche by stabilizing selection [44] because it performed well in the southern region of Sardinia where competition with R. mehelyi was limited.
Accordingly, to search for clues on SAR’s origin, we tested whether SAR’s niche would perform better than PES’ niche in the Maghrebian geographical set. This prediction would be consistent with a northern African origin of SAR. We tested this under different temporal scenarios: we trained distribution models with SAR and PES occurrences respectively and projected them to northern Africa in two snapshots – current time and Last Glacial Maximum (LGM, 21,000 years PB). We chose LGM because at that time geographical distances between islands and mainland were reduced by the emergence of land bridges favouring island colonization by bats, including that of Sardinia from northern Africa [42], [45].
Materials and Methods
Study area
For this study we considered the entire Italian territory comprised ca. between latitudes 45° N–36° N and longitudes 6°E–18°E (corresponding to ca. 301.000 km2, elevation range = 0–4810 m a.s.l.).
Presence species data
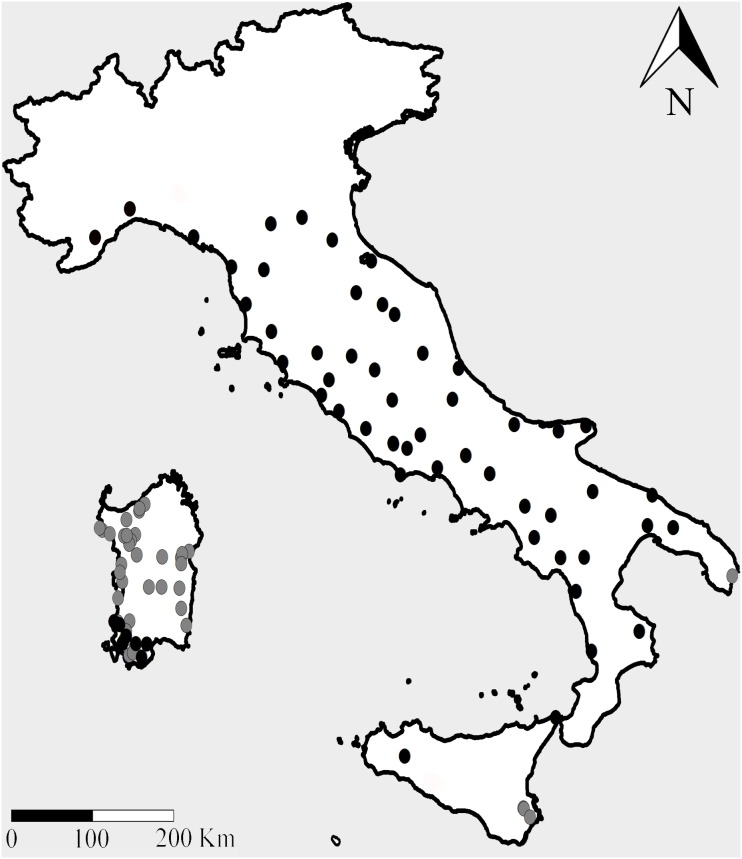
Presence records for R. euryale (n = 210) and R. mehelyi (n = 60) came from authors’ personal databases (Figure 1). Most faunal records were taken by either direct observation or acoustic surveys – activities requiring no specific permission according to Italian laws and regulations. On Sardinia, when roosts were surveyed for the first time the distinction between R. euryale and R. mehelyi was made by temporarily capturing bats under licence from the Italian Ministry of Environment (licence numbers: DPN/2D/2004/7489, DPN-2007-0003938, DPN-2010-0009609). Records were screened in ArcGis (version 9.2) for spatial autocorrelation using average nearest neighbour analyses and Moran’s I measure of spatial autocorrelation to remove spatially correlated data points and guarantee independence. After this selection, 65 and 40 presence data respectively for R. euryale and R. mehelyi were used to generate SDMs.
Figure 1. Presence records for Rhinolophus euryale (n = 65; black symbols) and R. mehelyi (n = 40; grey symbols) considered for this study.
The publicly available map layer was obtained from www.fao.org/geonetwork/srv/en/main.home and the image prepared with the Quantum Gis 2.2.0 Valmiera open source software.
Ecogeographical variables
To predict habitat suitability for the two species, we used a set of 21 Eco-Geographical Variables (EGVs). We included one topographical and 19 bioclimatic variables obtained from WorldClim database (www.worldclim.org/current) [46]. The latter variables are derived from the monthly temperature and rainfall values in order to generate more biologically meaningful factors [47]. Land cover was obtained from Global Land Cover 2000 (http://bioval.jrc.ec.europa.eu/products/glc2000/products.php). All variable formats were raster files (grid) with a resolution of 30 arc second (0.93×0.93 km = 0.86 km2 at the equator) and 1,307,195 grid cells. In order to remove the highly correlated variables for the final distribution models, we calculated a correlation matrix using Pearson’s technique and selected only the variables with r<0.5. We converted the eleven final EGVs used to model habitat suitability of both species in ASCII files.
Maximum entropy approach
We used Maxent – maximum entropy modelling of species geographic distributions [48] – to develop a geographic distribution model for R. euryale and R. mehelyi. Maxent is a machine learning method developed to detect habitat suitability of each grid cell as function of the interaction between EGVs and occurrence data [48]. This approach does not require absence data to model, an especially important feature for nocturnal, elusive animals such as bats. To build the models, we used Maxent ver. 3.3.3 k (http://www.cs.princeton.edu/~schapire/maxent), the presence record for R. euryale and R. mehelyi selected as described above, and the following EGVs: Altitude, Land cover, Mean Diurnal Range, Isothermality, Temperature Seasonality, Temperature Annual Range, Mean Temperature of Wettest Quarter, Mean Temperature of Driest Quarter, Precipitation Seasonality, Precipitation of Wettest Quarter and Precipitation of Coldest Quarter. Further details on EGV are given in Table S1. In the setting panel, we selected the following options: random seed; remove duplicate presence records; write plot data; regularisation multiplier (fixed at 1); 10,000 maximum number of background points; 1000 maximum iterations; and, finally, 20 replicate effects with cross-validate replicated run type. For the latter procedure, 80% of records were randomly extracted for training and 20% for testing the model and the procedure was repeated 20 times. The average final map obtained had a logistic output format with suitability values from 0 (unsuitable habitat) to 1 (suitable habitat). The 10th percentile (the value above which the model classifies correctly 90% of the training locations) was selected as the threshold value for defining the species’ presence. This is a conservative value that is commonly used in species distribution modelling studies especially when considering datasets gathered over a long time by different observers and methods of collection. This threshold was used to reclassify our model into binary presence/absence maps [49].
We used Jacknife analysis to estimate the actual contribution that each variable provided to the geographic distribution models. During this process, Maxent generated three models: first, each EGV was excluded in turn and a model created with the remaining variables to check which of the latter was most informative. Second, a model was created using individually each EGV to detect which variable had the most information not featuring in the others. Third, a final model was generated based on all variables. Response curves derived from univariate models were plotted to know how each EGV influences the presence probability.
For R. mehelyi we generated a distribution model based on Sardinian occurrences only. For R. euryale, we generated two models: one calibrated with Sardinian occurrences only and projected to Sardinia (SAR), another based on occurrences from both the Italian peninsula and Sicily (PES) which was also projected to Sardinia. We also generated palaeo-distribution models based on bioclimatic variables only. These were trained with PES and SAR occurrences and projected to the Maghreb in the LGM (23,000–18,000 year BP). The two LGM models were based respectively on the Community Climate System Model, CCSM, and the Model for Interdisciplinary Research on Climate, MIROC [46], [50]. Projecting SDMs to regions other than those on which models were calibrated, or to past or future times is a widespread approach to make inferences such as forecasting the spreading of alien organisms, providing palaeo-reconstructions or predicting distributional patterns in future epochs [51]–[53]. In order to project to a new area models calibrated elsewhere, whether in the current epoch or in the LGM, variables in the projection area must meet a condition of environmental similarity to the environmental data used for training the model. Therefore, we preliminarily ascertained that this condition was verified for both current and past projections, which were thus legitimate, by inspecting Multivariate Environmental Similarity Surfaces [54] (data not shown in the results for brevity). All digital information had a resolution of 2.5 arc-minutes (4.6 km).
Model validation
We evaluated model performance with different methods: the receiver operated characteristics (ROC), analyzing the area under curve (AUC) [55]; the true skill statistic (TSS) [56]; and the minimum difference between training and test AUC data (AUCdiff) [57]. Such statistics were averaged across the 20 replicates run on the 80% (training) vs. 20% (testing) dataset split.
AUC established the discrimination ability of the models and may range from 0 (equalling random distribution) to 1 (perfect prediction). AUC values >0.75 correspond to high discrimination performances [58]. TSS compares the number of correct forecasts, minus those attributable to random guessing, to that of a hypothetical set of perfect forecasts. It considers both omission and commission errors, and success as a result of random guessing, and ranges from − 1 to +1, where +1 indicates perfect agreement and values of zero or less correspond to a performance no better than random [56]. By minimizing the difference between training and test AUC data, in fact we reduce the risk that models are over-parameterized in such a way as to be overly specific to the training data [57].
Niche analysis
We performed niche overlap analyses using the analytical framework proposed by [59] and recently adopted in different studies [60], [61]. The procedure follows three steps: data pre-processing, calculation of the niche overlap measure and testing niche similarity. Further details are given in file S1.
To quantify niche overlap, we used the following ordination (for details see [59]) and SDMs methods: Principal Component Analysis calibrated with EGV values associated with the occurrences of the species (PCA-occ); Principal Component Analysis calibrated on the whole environmental space including the presence records where the species occur (PCA-env); Between-group and Within-group analyses (BETWEEN-occ and WITHIN-occ); Within-group calibrated on the whole environmental space (WITHIN-env); Linear Discriminant Analysis (LDA); Multidimensional scaling (MDS); and Maximum Entropy algorithm (MAXENT). For the application of the latter, two tests were carried out (named Maxent 1 and 2) corresponding to the analysis made using in turn one of the two Maxent outputs generated by either population (or species) as the comparison background against which the output of the remainder was contrasted.
Results
Niche differences between R. mehelyi, SAR and PES
Maxent models showed high levels of predictive performance as can be seen from AUC, TSS and AUCdiff values (Table 1).
Table 1. Validation methods applied to Maxent Species Distribution Models for Rhinolophus euryale and R. mehelyi.
| Model | AUC Training | SD | AUC Test | SD | AUCdiff | SD | TSS | SD |
| Current | ||||||||
| SAR | 0.965 | 0.005 | 0.932 | 0.021 | 0.033 | 0.024 | 0.680 | 0.165 |
| PES projected to Sardinia | 0.997 | 0.000 | 0.993 | 0.003 | 0.004 | 0.003 | 0.804 | 0.170 |
| R. mehelyi Sardinia | 0.863 | 0.018 | 0.782 | 0.040 | 0.082 | 0.057 | 0.523 | 0.109 |
| LGM | ||||||||
| SAR CCSM | 0.944 | 0.004 | 0.926 | 0.014 | 0.018 | 0.022 | 0.768 | 0.065 |
| SAR MIROC | 0.987 | 0.006 | 0.917 | 0.016 | 0.070 | 0.005 | 0.754 | 0.045 |
| PES CCSM | 0.884 | 0.002 | 0.854 | 0.005 | 0.030 | 0.015 | 0.844 | 0.022 |
| PES MIROC | 0.885 | 0.003 | 0.877 | 0.002 | 0.008 | 0.022 | 0.799 | 0.056 |
SAR = Sardinian population of R. euryale; PES = Populations of R. euryale of Peninsular Italy and Sicily.
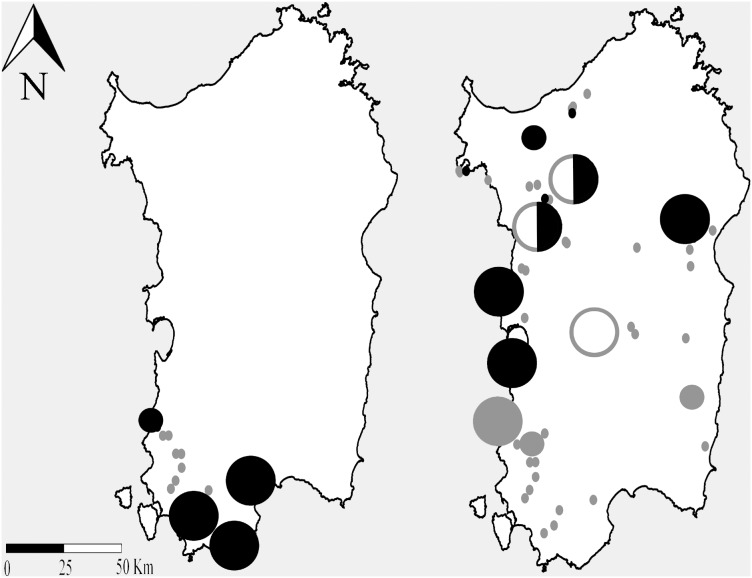
Distributional data showed that the two species are sympatric only in the southern portion of the island, to which R. euryale is confined (Figure 2). This distribution matches the prediction made by Maxent model for SAR (Figure 3). Besides, large colonies of R. mehelyi are found in most of the island (where only this bat, but not R. euryale occurs) except in the restricted area where R. euryale is present: there R. mehelyi only occurs with small numbers (Figure 2).
Figure 2. Distribution and colony size on Sardinia for Rhinolophus euryale (left) and R. mehelyi (right).
Circle sizes are proportional to colony sizes. Black: nursery colonies; white: hibernacula; grey: other day-roost. Mixed-colour (white + black) symbols correspond to sites used by bats year round for both hibernation and reproduction. The publicly available map layer was obtained from www.fao.org/geonetwork/srv/en/main.home and the image prepared with the Quantum Gis 2.2.0 Valmiera open source software.
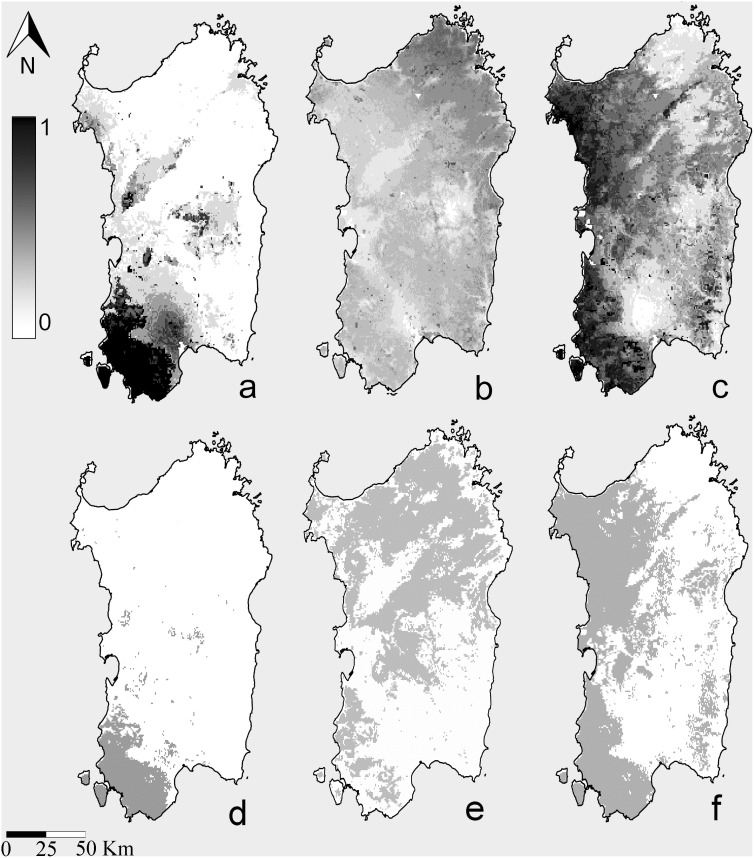
Figure 3. Maxent Species Distribution Models (SDM).
a: SDM for R. euryale on Sardinia calibrated with Sardinian records only; b: SDM for Rhinolophus euryale on Sardinia calibrated with presence records from Italian populations except that of Sardinia and projected to the island; c: SDM for R. mehelyi on Sardinia calibrated with Sardinian records only; c: binary map for R. euryale on Sardinia calibrated with Sardinian records only; d: binary map for Rhinolophus euryale on Sardinia calibrated with presence records from Italian populations except that of Sardinia and projected to the island; e: binary map for R. mehelyi on Sardinia calibrated with Sardinian records only The publicly available map layer was obtained from www.fao.org/geonetwork/srv/en/main.home and the image prepared with the Quantum Gis 2.2.0 Valmiera and Maxent open source software packages.
Maximum entropy models trained with PES presence data and projected to Sardinia show that according to PES ecological requirements, R. euryale would occupy a larger area and, compared to SAR, have a reduced probability of presence in the southern portion of the island (Figure 3). This distributional pattern would determine larger areas of sympatry with R. mehelyi, as also shown by Maxent’s prediction for this species (Figure 3) hence increasing the likelihood of competition. From binary maps it can be derived that the predicted range overlap between R. mehelyi and PES is ca. 60%, while the former overlaps with SAR only by ca. 20% (Figure 3). The competition scenario is also supported by the fact that six out of nine niche analysis methods showed a significant similarity of R. mehelyi with PES (Table 2). Only two methods supported the similarity of PES with R. mehelyi (Table 2).
Table 2. Schoener’s D and Niche similarity significance levels relative to ordination methods and Species Distribution Models used to carry out niche comparison.
| Comparison | Method | Schoener’s D | Niche Similarity | |
| R. mehelyi vs. SAR | SAR → R. mehelyi | R. mehelyi → SAR | ||
| Between group | 0.473 | 0.039+ | 0.019+ | |
| LDA | 0.561 | 0.019+ | 0.019+ | |
| Maxent1 | 0.594 | 0.019+ | 0.019+ | |
| Maxent2 | 0.628 | 0.019+ | 0.019+ | |
| MDS | 0.205 | 0.019+ | 0.950 | |
| PCA environmental | 0.215 | 0.059 | 0.495 | |
| PCA Occurrence | 0.229 | 0.019+ | 0.099 | |
| Within environmental | 0.215 | 0.039+ | 0.455 | |
| Within group | 0.127 | 0.079 | 0.871 | |
| SAR vs. PES | PES → SAR | SAR → PES | ||
| Between group | 0.000 | 2.000 | 2.000 | |
| LDA | 0.000 | 2.000 | 2.000 | |
| Maxent1 | 0.014 | 2.000 | 2.000 | |
| Maxent2 | 0.014 | 0.792 | 0.019− | |
| MDS | 0.000 | 2.000 | 1.584 | |
| PCA environmental | 0.000 | 2.000 | 2.000 | |
| PCA Occurrence | 0.000 | 0.673 | 0.495 | |
| Within environmental | 0.124 | 0.970 | 0.119 | |
| Within group | 0.124 | 0.891 | 0.733 | |
| R. mehelyi vs. PES | PES → R. mehelyi | R. mehelyi → PES | ||
| Between group | 0.409 | 0.554 | 0.019+ | |
| LDA | 0.060 | 0.831 | 0.198 | |
| Maxent1 | 0.190 | 0.039+ | 0.415 | |
| Maxent2 | 0.215 | 0.376 | 0.534 | |
| MDS | 0.103 | 0.594 | 0.019+ | |
| PCA environmental | 0.176 | 0.099 | 0.019+ | |
| PCA Occurrence | 0.170 | 0.178 | 0.019+ | |
| Within environmental | 0.216 | 0.396 | 0.019+ | |
| Within group | 0.243 | 0.039+ | 0.019+ | |
LDA = Linear Discriminant Analysis; Maxent 1 and 2 = Maximum Entropy Algorithm analysis made using in turn one of the two Maxent outputs generated by either population (or species) as the comparison background against which the output of the remainder was contrasted; MDS = Multidimensional scaling; PCA environmental = Principal Component Analysis calibrated on the whole environmental space including the presence records where the species occur; PCA occurrence = Principal Component Analysis calibrated with EGV values associated with the occurrences of the species; Within environmental = Within-group calibrated on the whole environmental space. + = similarity; − = dissimilarity.
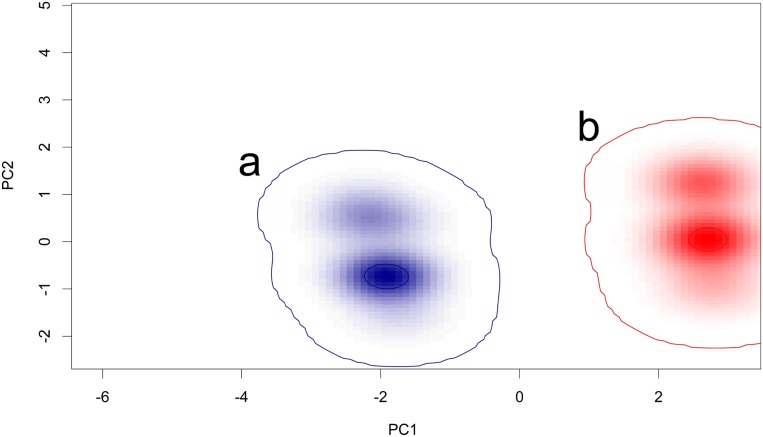
Niche comparison between SAR and PES showed no significant similarity (Table 2; Figure 4). The climatic variables that were most important to explain the potential distribution of SAR and PES were different. SAR is mainly localized in areas characterized by high isothermality values, mean temperature of wettest quarter of ca. 10–11°C, low standard deviation values of temperature seasonality and mean diurnal range between 8.5°C–10.5°C. SAR is also more likely to occur in areas of bare ground and mixed-leaved woodland at lower altitude. Such characteristics are found in SW Sardinia where SAR occurs. Suitability for PES decreases with increasing temperature seasonality. In the areas where the species’ likelihood of occurrence is high (central and northern Sardinia), the mean diurnal temperature range is ca. 8°C and % precipitation seasonality is low.
Figure 4. Graphical representation of the environmental niches for Rhinolophus euryale.
a: Sardinian population; b: other Italian populations. In the example, niche were generated with Principal Component Analysis calibrated on the whole environmental space including the presence records where the species occur.
Niche comparison carried out for R. mehelyi vs. SAR showed a substantial similarity, supported respectively by 7 (R. mehelyi vs. SAR) and 4 (SAR vs. R. mehelyi) methods in either direction (Table 2). As found for PES, R. mehelyi probability of occurrence on Sardinia decreased for increasing values of temperature seasonality (Figure S1) and was also associated to wooded habitats.
Overall, the analysis supports the existence of niche divergence between SAR and PES and shows that this results in a smaller overlap between the ranges of R. mehelyi and R. euryale on the island.
Niche difference as a legacy of biogeographic origin?
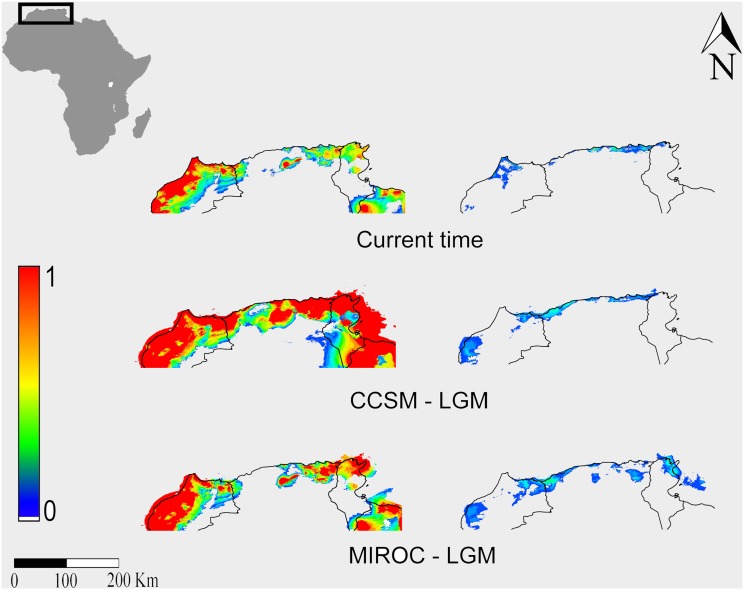
Palaeo-distribution models too showed excellent levels of predictive performance as can be seen from AUC, TSS and AUCdiff values (Table 1). Our reconstruction showed that both in the present time and under the LGM scenario SAR would largely occur in northern Africa whereas PES would be practically absent, in agreement with a Maghrebian origin of SAR (Figure 5).
Figure 5. Maxent SDMs for Rhinolophus euryale calibrated respectively on presence records of bats from Sardinia (left) and from the remaining Italian areas (right) and projected to northern Africa.
LGM = Last Glacial Maximum. CCSM = Community Climate System Model; MIROC = Model for Interdisciplinary Research on Climate. The publicly available map layer was obtained from www.fao.org/geonetwork/srv/en/main.home and the image prepared with the Quantum Gis 2.2.0 Valmiera and Maxent open source software packages.
Discussion
Niche differences between R. mehelyi, SAR and PES
We showed that R. euryale on Sardinia is confined to a small southern portion of the island where it occurs in sympatry with R. mehelyi although in that area the latter is far less numerous than in the north, where R. euryale is absent. The niches of PES and R. mehelyi are similar but PES and SAR niches are not. If SAR had shown a niche identical to that of PES, the geographical distribution of R. euryale and R. mehelyi on the island would be largely sympatric, potentially leading to stronger interspecific competition. We conclude that niche differences between R. mehelyi and SAR minimize sympatry and thus potential competition. This result is consistent with the hypothesis that Sardinian R. euryale experienced a niche displacement process to mitigate competition with the numerically dominant R. mehelyi. Based on our models, the probabilities of occurrence of both R. mehelyi and SAR in the north-east sector of the island are small, whereas PES shows higher values. Why R. euryale has not occupied that region where competition would be low (at least based on PES ecological characteristics) appears less clear and perhaps explained by the biogeographic origin of Sardinian R. euryale (discussed below). Besides, based on the niche analysis results, the competition hypothesis would not be fully supported since despite the separation of SAR and PES niches, the former still partly overlaps with that of R. mehelyi. This may be explained by the fact that species distribution models were built from occurrence records but did not take colony size into account. Survey data instead showed that R. mehelyi occurs with large colonies in the areas of allopatry with SAR, but where the two species are sympatric, it is only present with smaller numbers. In other words, modelling based on presence records probably overestimated niche similarity by disregarding local population size: the difference between the two niches of Sardinian R. mehelyi and R. euryale may thus be even larger than that estimated here.
Whatever the reason for its peculiarity, SAR niche must have allowed R. euryale to establish a viable population in an area where R. mehelyi appears to perform less well and thus be less competitive, as can be inferred from the smaller colony sizes of the latter in the southern area of sympatry. Noticeably, the two species are known to share roosting sites in their Mediterranean regions of sympatry [38], including Sardinia (this study). Bats often form mixed-species groups when they have common thermal preferences, and interspecific associations may result in mutual thermoregulatory ( = energetic) benefits [62]. Based on such considerations, we rule out that the species we considered compete for roosting sites.
Food is much more likely to trigger competition. The diet of both species is well known, and is mostly made of moths both in sympatry and allopatry; the amounts of other prey only show small interspecific differences [35], [63], [64] In most areas of sympatry but not on Sardinia, echolocation call frequencies of R. mehelyi and R. euryale largely overlap each other [35], [38], [65] leading to the detection of similar prey [35], [38]. Although Sardinian R. euryale have lower frequencies than local R. mehelyi [38], the difference is too small to account for niche partitioning. Since echolocation calls also convey individual information among conspecifics [66], [67] this difference is best explained as a way to maintain separate communication bandwidth in the area of sympatry [38].
Foraging habitat use in these rhinolophids shows no interspecific differences in allopatric populations [35] but does differ in sympatry, where R. mehelyi performs better in less structurally complex habitats than in more closed vegetation [35], [36] due to its lower flight manoeuvrability and agility [35], [36], [68]. Over the millennia, hundreds of human generations have shaped Sardinian landscapes and microclimates through deforestation, stock breeding and fires [69] so that much of the land is covered with Mediterranean scrubland and open forest, where R. mehelyi is probably more competitive than R. euryale. Reduced habitat heterogeneity such as that found on Sardinia as well as the limitedness of food, typical of insular systems [70] can be important factors increasing competition between the two rhinolophids.
Niche difference as a legacy of biogeographic origin?
In principle, our findings are in agreement with a niche displacement process: in fact, one possible scenario is that Sardinia was colonized by R. euryale from mainland Europe and that the newly established population shifted its ecological niche to counter competition pressures from heterospecific bats (R. mehelyi). However, questions arise on the geographical source of Sardinian R. euryale and its ecological consequences as an alternative explanation for SAR’s niche distinctness. R. euryale might have colonized Sardinia from the Maghreb and the peculiar ecological niche of Sardinian bats could thus be a legacy of the African source population rather than the outcome of a niche displacement process. This niche may have been subjected to stabilizing selection [44] and conserved as it must have performed well to allow co-existence with insular R. mehelyi. By projecting respectively SAR and PES niches to the Maghreb both in the current time and in the LGM we found a striking difference in the probability of occurrence, much higher for SAR’s projection. This result is in agreement with a possible SAR’s Maghrebian origin.
Stretches of sea have been found to represent barriers to the movement of bats [71], [72] although their permeability differs across species. The capacity of different species to overcome such barriers is not related to wing morphology and flight performances [73]. Colonization events would seem as difficult from mainland Italy as they would be from northern Africa. Sardinia lies ca. 200 km off the coasts of both regions, so both routes appear equally likely to explain the origin of Sardinian R. euryale. Colonization of Sardinia by bats was only possible across the sea since the end of the Messinian Event, ending 5.33 million years ago [43], [74]. Thus, if SAR had an African origin, its establishment would either date back to the Messinian Event or, if more recent, must have implied crossing the sea. The latter option is possible: there is evidence that after the Messinian Event Sardinia was subject to repeated bat colonization waves at different times (including recent ones) from Europe and northern Africa, as for the Maghrebian bat Myotis punicus [42], long-eared bats [41] and pipistrelles [43]. Glacial episodes that repeatedly occurred in the Pleistocene lowered sea levels and led to the emersion of land bridges [45], interrupting the isolation of Sardinia from the mainland during early and mid-Pleistocene, and favoured island colonization most probably via a stepping stone geographic system. This would be in agreement with the wide northern African distribution we obtained for R. euryale during the LGM by projecting SAR’s niche to that region. Caution is needed when considering our LGM models for the Sahara area, where some overpredictions occurred. These were most likely due to the lack of solid information on the region’s climate at that age [75] inevitably affecting the reliability of climatic variables.
Although these findings do not prove the biogeographical origin of SAR, we hope they will stimulate molecular studies investigating the phylogeography of R. euryale in the Mediterranean Basin for a final answer on the identity of SAR’s geological source and a full reconstruction of this population’s history.
Supporting Information
EGV response curves of Maxent SDMs for selected ecogeographic variables. a: Temperature seasonality for R. mehelyi; b: Temperature seasonality for PES; c: Isothermality for SAR.
(TIF)
List of ecogeographical variables used for this study, their type and measurement unit.
(DOC)
Description of Niche Analysis.
(DOC)
Acknowledgments
We thank Ivy Di Salvo and Mara Calvini for providing information on rhinolophid occurrence respectively in Sicily and Liguria. We are grateful to two anonymous reviewers for their valuable comments on a first version of this manuscript.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. The dataset used for this study includes geographical locations for many important roosts of threatened bat species whose uncontrolled disclosure might affect species conservation. Data are therefore available from the National Database of the Italian Chiroptera Research Group for researchers who meet the criteria for access to confidential data (http://www.pipistrelli.net/drupal/contact).
Funding Statement
DR and LB were funded by Italian Ministry of the Environment and the Protection of Land and Sea, CIG nr. 464598541B (www.minambiente.it). HR was funded by the Programme Investigador FCT, IF/00497/2013 (www.fct.pt). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cox CB, Moore PD (2010) Biogeography: An Ecological and Evolutionary Approach, 8th edn. New York: John Wiley & Sons. 440 p. [Google Scholar]
- 2. Brown W, Wilson E (1956) Character displacement. Systematic Zoology 5: 49–64. [Google Scholar]
- 3. Hutchinson GE (1959) Homage to Santa Rosalia or why are there so many kinds of animals. The American Naturalist 93: 145–159. [Google Scholar]
- 4. Hardin G (1960) The competitive exclusion principle. Science 131: 1292–1297. [DOI] [PubMed] [Google Scholar]
- 5. Kronfeld-Schor N, Dayan T (1999) The dietary basis for temporal partitioning: food habits of coexisting Acomys species. Oecologia 121: 123–8. [DOI] [PubMed] [Google Scholar]
- 6. Albrecht M, Gotelli NJ (2001) Spatial and temporal niche partitioning in grassland ants. Oecologia 126: 134–141. [DOI] [PubMed] [Google Scholar]
- 8. Spencer LM (1995) Morphological correlates of dietary resource partitioning in the African Bovidae. Journal of Mammalogy 76: 448–471. [Google Scholar]
- 9. Castillo-Rivera M, Kobelkowsky A, Zamayoa V (1996) Food resource partitioning and trophic morphology of Brevoortia gunteri and B. patronus . Journal of Fish Biology 49: 1102–1111. [Google Scholar]
- 10. Albertson RC (2008) Morphological divergence predicts habitat partitioning in a Lake Malawi cichlid species complex. Copeia 2008: 689–698. [Google Scholar]
- 11. Grant P (1972) Convergent and divergent character displacement. Biological Journal of the Linnean Society of London 4: 39–68. [Google Scholar]
- 12. Goldberg E, Lande R (2006) Ecological and reproductive character displacement of an environmental gradient. Evolution 60: 1344–1357. [PubMed] [Google Scholar]
- 13. Dayan T, Simberloff D (2005) Ecological and community-wide character displacement: the next generation. Ecology Letters 8: 875–894. [Google Scholar]
- 14. Peers MJL, Thornton DH, Murray DL (2013) Evidence for large-scale effects of competition: niche displacement in Canada lynx and bobcat. Proceedings of the Royal Society B 280: 20132495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lomolino MV (2005) Body size evolution in insular vertebrates: generality of the island rule. Journal of Biogeography 32: 1683–1699. [Google Scholar]
- 16. Wiens JJ, Ackerly DD, Allen AP, Anacker BL, Buckley LB, et al. (2010) Niche conservatism as an emerging principle in ecology and conservation biology. Ecology Letters 13: 1310–1324. [DOI] [PubMed] [Google Scholar]
- 17. Vamosi SM, Heard SB, Vamosi JC, Webb CO (2009) Emerging patterns in the comparative analysis of phylogenetic community structure. Molecular Ecology 18: 572–592. [DOI] [PubMed] [Google Scholar]
- 18. Stadelmann B, Herrera LG, Arroyo-Cabrales J, Flores-Martínez JJ, May BP, et al. (2004) Molecular systematics of the fishing bat Myotis (Pizonyx) vivesi . Journal of Mammalogy 85: 133–139. [Google Scholar]
- 19. Stadelmann B, Lin LK, Kunz TH, Ruedi M (2007) Molecular phylogeny of New World Myotis (Chiroptera, Vespertilionidae) inferred from mitochondrial and nuclear DNA genes. Molecular Phylogenetics and Evolution 43: 32–48. [DOI] [PubMed] [Google Scholar]
- 20. Jones G, Holderied MW (2007) Bat echolocation calls: adaptation and convergent evolution. Proceedings of the Royal Society of London B 274: 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arlettaz R (1999) Habitat selection as a major resource partitioning mechanism between the two sympatric sibling bat species Myotis myotis and Myotis blythii . Journal of Animal Ecology 68: 460–471. [Google Scholar]
- 22. Kingston T, Jones G, Zubaid A, Kunz TH (2000) Resource partitioning in rhinolophoid bats revisited. Oecologia 124: 332–342. [DOI] [PubMed] [Google Scholar]
- 23. Siemers BM, Schnitzler HU (2004) Echolocation signals reflect niche differentiation in five sympatric congeneric bat species. Nature 429: 657–661. [DOI] [PubMed] [Google Scholar]
- 24. Siemers BM, Swift SM (2006) Differences in sensory ecology contribute to resource partitioning in the bats Myotis bechsteinii and Myotis nattereri (Chiroptera: Vespertilionidae). Behavioral Ecology and Sociobiology 59: 373–380. [Google Scholar]
- 25.Mitchell-Jones AJ, Amori G, Bogdanowicz W, Krytufek B, Reijnder PJH, et al.. (1999) The Atlas of European Mammals. T. and A.D. Poyser, London. 250 p. [Google Scholar]
- 26.Guillén A, Francis CM, Ricklefs RE (2003) Phylogeny and biogeography of the horseshoe bats. In: Csorba G, Ujhelyi P, Shropshire TN, editors. Horseshoe Bats of the World. pp. 7–24.
- 27. Zhou ZM, Guillent-Servent A, Lim BK, Eger JL, Wang YX, et al. (2009) A new species from southwestern China in the Afro-Paleartic lineage of the horseshoe bats (Rhinolophus). Journal of Mammalogy 90: 57–73. [Google Scholar]
- 28.Hutson AM, Spitzenberger F, Juste J, Aulagnier S, Alcaldé JT, et al. (2008a) Rhinolophus euryale In: IUCN 2013. IUCN Red List of Threatened Species. Version 2013.1. Available: www.iucnredlist.org. Accessed 2013 November 14.
- 29.Hutson AM, Spitzenberger F, Juste J, Aulagnier S, Alcaldé JT, et al. (2008b) Rhinolophus mehelyi In: IUCN 2013. IUCN Red List of Threatened Species. Version 2013.1. Available: www.iucnredlist.org. Accessed 2013 November 14.
- 30. Flaquer C, Puig X, Fàbregas E, Guixé D, Torre I, et al. (2010) Revisión y aportación de datos sobre quirópteros de Catalunya: Propuesta de Lista Roja. Galemys 22: 29–61. [Google Scholar]
- 31. Puechmaille SJ, Teeling EC (2014) Non-invasive genetics can help find rare species: a case study with Rhinolophus mehelyi and R. euryale (Rhinolophidae: Chiroptera) in Western Europe. Mammalia . Mammalia 78: 251–255. [Google Scholar]
- 32. Rombaut D, Haquart A (2002) Les Chiroptères de la Directive Habitats: le Rhinolophe de Mehely Rhinolophus mehelyi Matschle, 1901. Arvicola 14: 18–20. [Google Scholar]
- 33. Dragu A, Borissov I (2011) Low genetic variability of Rhinolophus mehelyi (Mehely’s horseshoe bat) in Romania. Acta Theriologica 56: 383–387. [Google Scholar]
- 34. Russo D, Jones G, Migliozzi A (2002) Habitat selection by the Mediterranean horseshoe bat, Rhinolophus euryale (Chiroptera: Rhinolophidae) in a rural area of southern Italy and implications for conservation. Biological Conservation 107: 71–81. [Google Scholar]
- 35. Salsamendi E, Garin I, Arostegui I, Goiti U, Aihartza J (2012) What mechanism of niche segregation allows the coexistence of sympatric sibling rhinolophid bats? Frontiers in Zoology 9: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Russo D, Almenar D, Aihartza J, Goiti U, Salsamendi E, et al. (2005) Habitat selection in sympatric Rhinolophus mehelyi and R. euryale (Chiroptera: Rhinolophidae). Journal of Zoology 266: 327–332. [Google Scholar]
- 37. Dondini G, Tomassini A, Inguscio S, Rossi E (2014) Rediscovery of Mehely’s horseshoe bat (Rhinolophus mehelyi) in peninsular Italy. Hystrix 25: 59–60. [Google Scholar]
- 38. Russo D, Mucedda M, Bello M, Biscardi S, Pidinchedda E, et al. (2007) Divergent echolocation call frequencies in insular rhinolophids (Chiroptera): a case of character displacement? Journal of Biogeography 34: 2129–2138. [Google Scholar]
- 39. Krzanowski A (1967) The magnitude of islands and the size of bats (Chiroptera). Acta Zoologica Cracoviensia 12: 281–346. [Google Scholar]
- 40.McNab BK (2009) Physiological adaptation of bats and birds to island life. In: Fleming TH, Racey PA, editors. Island Bats. Evolution, Ecology and Conservation. pp. 153–175.
- 41.Kiefer A ( 2007) Phylogeny of Western-Palaearctic long-eared bats (Mammalia, Chiroptera, Plecotus): a molecular perspective. Ph.D. Thesis, Gutenberg Univeristy, Mainz.
- 42. Biollaz F, Bruyndonckx N, Beuneux G, Mucedda M, Goudet J, et al. (2010) Genetic isolation of insular populations of the Maghrebian bat, Myotis punicus, in the Mediterranean Basin. Journal of Biogeography 37: 1557–1569. [Google Scholar]
- 43. Veith M, Mucedda M, Kiefer A, Pidinchedda E (2011) On the presence of pipistrelle bats (Pipistrellus and Hypsugo; Chiroptera: Vespertilionidae) in Sardinia. Acta Chiropterologica 13: 89–99. [Google Scholar]
- 44. Russo D, Teixeira S, Cistrone L, Jesus J, Teixeira D, et al. (2009) Social calls are subject to stabilizing selection in insular bats. Journal of Biogeography 36: 2212–2221. [Google Scholar]
- 45. Rohling EJ, Fenton M, Jorissen FJ, Bertrand P, Ganssen G, et al. (1998) Magnitudes of sea-level low stands of the past 500,000 years. Nature 394: 162–165. [Google Scholar]
- 46. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- 47.Busby JR (1991) BIOCLIM: A bioclimatic analysis and predictive system. In: Margules CR, Austin MP, editors. Nature Conservation: Cost Effective Biological Surveys and Data Analysis. pp. 64–68.
- 48. Phillips SJ, Anderson RP, Schapire R (2006) Maximum entropy modeling of species geographic distributions. Ecological Modelling 190: 231–259. [Google Scholar]
- 49. Bosso L, Rebelo H, Garonna AP, Russo D (2013) Modelling geographic distribution and detecting conservation gaps in Italy for the threatened beetle Rosalia alpina. . Journal for Nature Conservation 21: 72–80. [Google Scholar]
- 50. Waltari E, Hijmans RJ, Peterson AT, Nyari AS, Perkins SL, et al. (2007) Locating Pleistocene refugia: comparing phylogeographic and ecological niche model predictions. PLoS ONE 2: e563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Medley KA (2010) Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models. Global Ecology and Biogeography 19: 122–133. [Google Scholar]
- 52. Rödder D, Lawing AM, Flecks M, Ahmadzadeh F, Dambach J, et al. (2013) Evaluating the significance of paleophylogeographic species distribution models in reconstructing Quaternary range-shifts of Nearctic chelonians. PLoS ONE 8: e72855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Keith DA, Elith J, Simpson CC (2014) Predicting distribution changes of a mire ecosystem under future climates. Diversity and Distributions 20: 440–454. [Google Scholar]
- 54. Elith J, Kearney M, Phillips SJ (2010) The art of modeling range-shifting species. Methods in Ecology and Evolution 1: 330–342. [Google Scholar]
- 55. Fielding AH, Bell JF (1997) A review of methods for the assessment of prediction errors in conservation presence/absence models. Environmental Conservation 24: 38–49. [Google Scholar]
- 56. Allouche O, Tsoar A, Kadmon R (2006) Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). Journal of Applied Ecology 43: 1223–1232. [Google Scholar]
- 57. Warren DL, Seifert SN (2011) Environmental niche modeling in Maxent: the importance of model complexity and the performance of model selection criteria. Ecological Application 21: 335–342. [DOI] [PubMed] [Google Scholar]
- 58. Elith J, Graham CH, Anderson RP, Dudìk M, Ferrier S, et al. (2006) Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29: 129–151. [Google Scholar]
- 59. Broennimann O, Fitzpatrick MC, Pearman PB, Petitpierre B, Pellissier L, et al. (2012) Measuring ecological niche overlap from occurrence and spatial environmental data. Global Ecology and Biogeography 21: 481–497. [Google Scholar]
- 60. Theodoridis S, Randin C, Broennimann O, Patsiou T, Conti E (2013) Divergent and narrower climatic niches characterize polyploid species of European primroses in Primula sect. Aleuritia . Journal of Biogeography 40: 1278–1289. [Google Scholar]
- 61. Di Febbraro M, Lurz PWW, Maiorano L, Girardello M, Bertolino S (2013) The use of climatic niches in screening procedures for introduced species to evaluate risk of spread: the case of the American Eastern grey squirrel. PLoS ONE 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bogdanowicz W (1983) Community structure and interspecific interactions in bats hibernating in Poznan. Acta Theriologica 28: 357–370. [Google Scholar]
- 63. Goiti U, Aihartza JR, Garin I (2004) Diet and prey selection in the Mediterranean horseshoe bat Rhinolophus euryale (Chiroptera, Rhinolophidae) during the pre-breeding season. Mammalia 68: 397–402. [Google Scholar]
- 64. Salsamendi E, Garin I, Almenar D, Goiti U, Napal M, et al. (2008) Diet and prey selection in Mehely’s horseshoe bat Rhinolophus mehelyi (Chiroptera, Rhinolophidae) in the south-western Iberian Peninsula. Acta Chiropterologica 10: 279–286. [Google Scholar]
- 65. Russo D, Jones G, Mucedda M (2001) Influence of age, sex and body size on echolocation calls of Mediterranean (Rhinolophus euryale) and Mehely’s (Rhinolophus mehelyi) horseshoe bats (Chiroptera: Rhinolophidae). Mammalia 65: 429–436. [Google Scholar]
- 66. Jones G, Siemers BM (2011) The communicative potential of bat echolocation pulses. Journal of Comparative Physiology A 197: 447–457. [DOI] [PubMed] [Google Scholar]
- 67. Schuchmann M, Siemers BM (2010) Variability in echolocation call intensity in a community of horseshoe bats: a role for resource partitioning or communication? PLoS ONE 5: e12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dietz C, Dietz I, Siemers BM (2006) Wing measurement variations in the five European horseshoe bat species (Chiroptera: Rhinolophidae). Journal of Mammalogy 87: 1241–1251. [Google Scholar]
- 69.Weiss S, Ferrand N (2007) Phylogeography of Southern European Refugia. Springer, Dordrecht. 377 p. [Google Scholar]
- 70. Krzanowski A (1967) The magnitude of islands and the size of bats (Chiroptera). Acta Zoologica Cracoviensia 12: 281–346. [Google Scholar]
- 71. Castella V, Ruedi M, Excoffier L, Ibanez C, Arlettaz R, et al. (2000) Is the Gibraltar Strait a barrier to gene flow for the bat Myotis myotis (Chiroptera: Vespertilionidae)? Molecular Ecology 9: 1761–1772. [DOI] [PubMed] [Google Scholar]
- 72. Ruedi M, Walter S, Fischer MC, Scaravelli D, Excoffier L, et al. (2008) Italy as a major ice age refuge area for the bat Myotis myotis (Chiroptera: Vespertilionidae) in Europe. Molecular Ecology 17: 1801–1814. [DOI] [PubMed] [Google Scholar]
- 73. García-Mudarra JL, Ibanez C, Juste J (2009) The Straits of Gibraltar: barrier or bridge to Ibero-Moroccan bat diversity? Biological Journal of the Linnean Society 96: 434–450. [Google Scholar]
- 74. Krijgsman W, Hilgen FJ, Raffi I, Sierro FJ, Wilson DS (1999) Chronology, causes and progression of the Messinian salinity crisis. Nature 400: 652–655. [Google Scholar]
- 75. Brito JC, Godinho R, Martínez-Freirería F, Pleguezuelos JM, Rebelo H, et al. (2014) Unravelling biodiversity, evolution and threats to conservation in the Sahara-Sahel. Biological Reviews 89: 215–231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
EGV response curves of Maxent SDMs for selected ecogeographic variables. a: Temperature seasonality for R. mehelyi; b: Temperature seasonality for PES; c: Isothermality for SAR.
(TIF)
List of ecogeographical variables used for this study, their type and measurement unit.
(DOC)
Description of Niche Analysis.
(DOC)
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. The dataset used for this study includes geographical locations for many important roosts of threatened bat species whose uncontrolled disclosure might affect species conservation. Data are therefore available from the National Database of the Italian Chiroptera Research Group for researchers who meet the criteria for access to confidential data (http://www.pipistrelli.net/drupal/contact).