Abstract
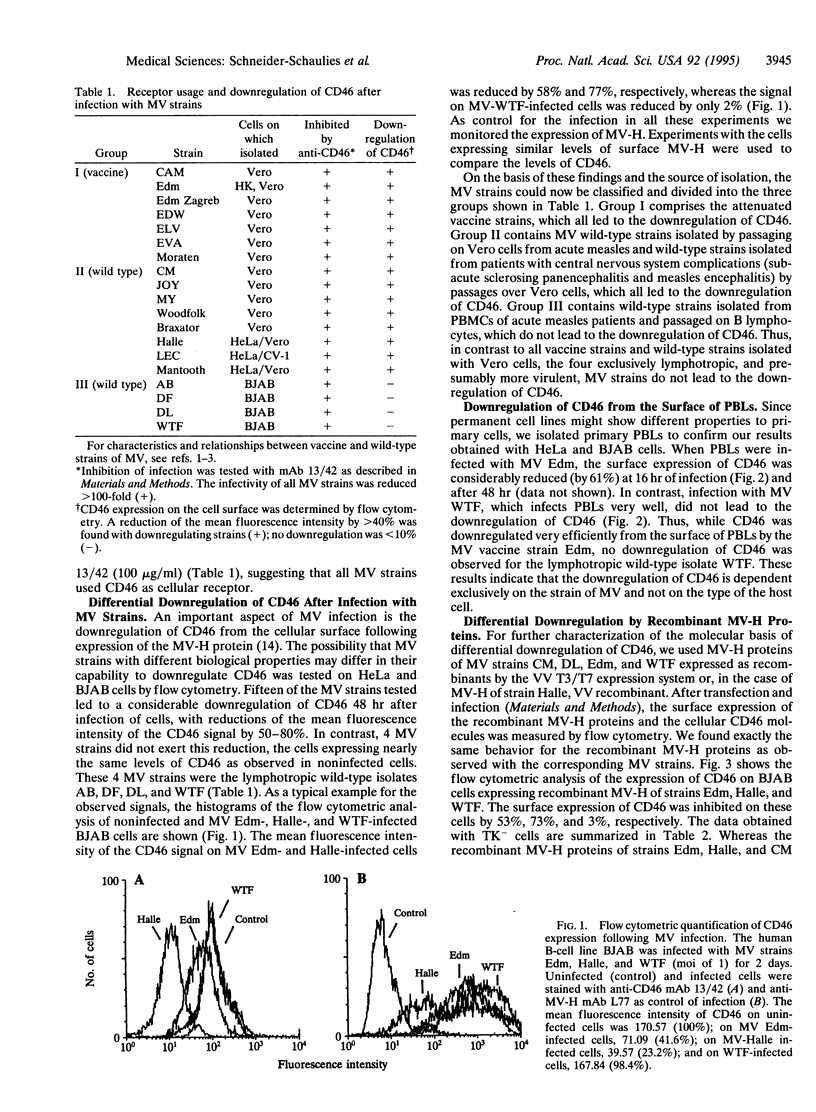
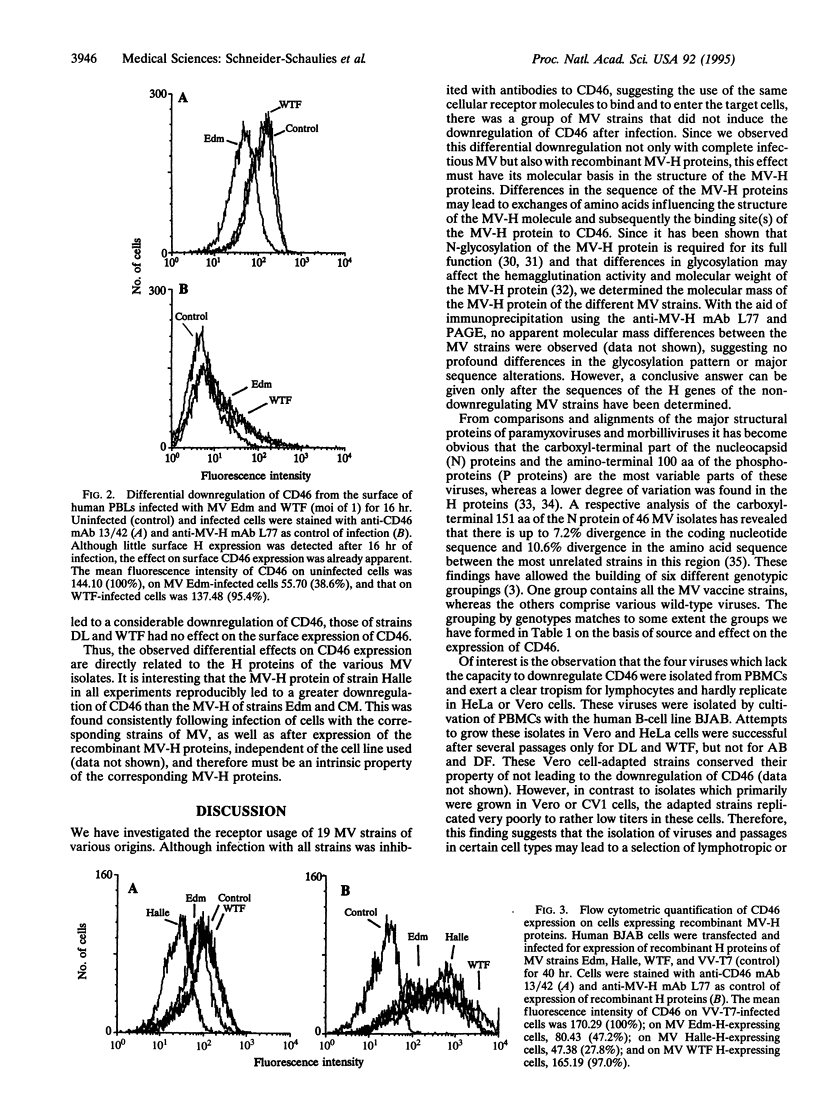
Recently, two cell surface molecules, CD46 and moesin, have been found to be functionally associated with measles virus (MV) infectivity of cells. We investigated the receptor usage of MV wild-type, subacute sclerosing panencephalitis, and vaccine strains and their effect on the down-regulation of CD46 after infection. We found that the infection of human cell lines with all 19 MV strains tested was inhibitable with antibodies against CD46. In contrast, not all strains of MV led to the downregulation of CD46 following infection. The group of CD46 non-downregulating strains comprised four lymphotropic wild-type isolates designated AB, DF, DL, and WTF. Since the downregulation of CD46 is caused by interaction with newly synthesized MV hemagglutinin (MV-H), we tested the capability of recombinant MV-H proteins to downregulate CD46. Recombinant MV-H proteins of MV strains Edmonston, Halle, and CM led to the down-regulation of CD46, whereas those of DL and WTF did not. This observed differential downregulation by different MV strains has profound consequences, since lack of CD46 on the cell surface leads to susceptibility of cells to complement lysis. These results suggest that lymphotropic wild-type strains of MV which do not downregulate CD46 may have an advantage for replication in vivo. The relatively weak immune response against attenuated vaccine strains of MV compared with wild-type strains might be related to this phenomenon.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baczko K., Lampe J., Liebert U. G., Brinckmann U., ter Meulen V., Pardowitz I., Budka H., Cosby S. L., Isserte S., Rima B. K. Clonal expansion of hypermutated measles virus in a SSPE brain. Virology. 1993 Nov;197(1):188–195. doi: 10.1006/viro.1993.1579. [DOI] [PubMed] [Google Scholar]
- Baczko K., Pardowitz I., Rima B. K., ter Meulen V. Constant and variable regions of measles virus proteins encoded by the nucleocapsid and phosphoprotein genes derived from lytic and persistent viruses. Virology. 1992 Sep;190(1):469–474. doi: 10.1016/0042-6822(92)91236-n. [DOI] [PubMed] [Google Scholar]
- Barbanti-Brodano G., Oyanagi S., Katz M., Koprowski H. Presence of 2 different viral agents in brain cells of patients with subacute sclerosing panencephalitis. Proc Soc Exp Biol Med. 1970 May;134(1):230–236. doi: 10.3181/00379727-134-34765. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Rose J. K. Cell fusion by the envelope glycoproteins of persistent measles viruses which caused lethal human brain disease. J Virol. 1993 Mar;67(3):1493–1502. doi: 10.1128/jvi.67.3.1493-1502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunster L. M., Schneider-Schaulies J., Löffler S., Lankes W., Schwartz-Albiez R., Lottspeich F., ter Meulen V. Moesin: a cell membrane protein linked with susceptibility to measles virus infection. Virology. 1994 Jan;198(1):265–274. doi: 10.1006/viro.1994.1029. [DOI] [PubMed] [Google Scholar]
- Dörig R. E., Marcil A., Chopra A., Richardson C. D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell. 1993 Oct 22;75(2):295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- ENDERS J. F., PEEBLES T. C. Propagation in tissue cultures of cytopathogenic agents from patients with measles. Proc Soc Exp Biol Med. 1954 Jun;86(2):277–286. doi: 10.3181/00379727-86-21073. [DOI] [PubMed] [Google Scholar]
- Esolen L. M., Ward B. J., Moench T. R., Griffin D. E. Infection of monocytes during measles. J Infect Dis. 1993 Jul;168(1):47–52. doi: 10.1093/infdis/168.1.47. [DOI] [PubMed] [Google Scholar]
- Forthal D. N., Blanding J., Aarnaes S., Peterson E. M., de la Maza L. M., Tilles J. G. Comparison of different methods and cell lines for isolating measles virus. J Clin Microbiol. 1993 Mar;31(3):695–697. doi: 10.1128/jcm.31.3.695-697.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A., Ayata M., Wang A. H., Wong T. C. Functional analysis of matrix proteins expressed from cloned genes of measles virus variants that cause subacute sclerosing panencephalitis reveals a common defect in nucleocapsid binding. J Virol. 1993 Apr;67(4):1848–1853. doi: 10.1128/jvi.67.4.1848-1853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Hamilton R., Wittig B., Fuccillo D. A., Sever J. L., Vernon M. L. Subacute sclerosing panencephalitis: isolation of suppressed measles virus from lymph node biopsies. Science. 1971 Aug 27;173(3999):840–841. doi: 10.1126/science.173.3999.840. [DOI] [PubMed] [Google Scholar]
- Hu A., Cattaneo R., Schwartz S., Norrby E. Role of N-linked oligosaccharide chains in the processing and antigenicity of measles virus haemagglutinin protein. J Gen Virol. 1994 May;75(Pt 5):1043–1052. doi: 10.1099/0022-1317-75-5-1043. [DOI] [PubMed] [Google Scholar]
- Kobune F., Sakata H., Sugiura A. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J Virol. 1990 Feb;64(2):700–705. doi: 10.1128/jvi.64.2.700-705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebert U. G., Flanagan S. G., Löffler S., Baczko K., ter Meulen V., Rima B. K. Antigenic determinants of measles virus hemagglutinin associated with neurovirulence. J Virol. 1994 Mar;68(3):1486–1493. doi: 10.1128/jvi.68.3.1486-1493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszewski M. K., Post T. W., Atkinson J. P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- Loveland B. E., Johnstone R. W., Russell S. M., Thorley B. R., McKenzie I. F. Different membrane cofactor protein (CD46) isoforms protect transfected cells against antibody and complement mediated lysis. Transpl Immunol. 1993;1(2):101–108. doi: 10.1016/0966-3274(93)90002-p. [DOI] [PubMed] [Google Scholar]
- Maisner A., Schneider-Schaulies J., Liszewski M. K., Atkinson J. P., Herrler G. Binding of measles virus to membrane cofactor protein (CD46): importance of disulfide bonds and N-glycans for the receptor function. J Virol. 1994 Oct;68(10):6299–6304. doi: 10.1128/jvi.68.10.6299-6304.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvoisin E., Wild F. The role of N-glycosylation in cell fusion induced by a vaccinia recombinant virus expressing both measles virus glycoproteins. Virology. 1994 Apr;200(1):11–20. doi: 10.1006/viro.1994.1157. [DOI] [PubMed] [Google Scholar]
- Manchester M., Liszewski M. K., Atkinson J. P., Oldstone M. B. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2161–2165. doi: 10.1073/pnas.91.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naniche D., Varior-Krishnan G., Cervoni F., Wild T. F., Rossi B., Rabourdin-Combe C., Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993 Oct;67(10):6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naniche D., Wild T. F., Rabourdin-Combe C., Gerlier D. Measles virus haemagglutinin induces down-regulation of gp57/67, a molecule involved in virus binding. J Gen Virol. 1993 Jun;74(Pt 6):1073–1079. doi: 10.1099/0022-1317-74-6-1073. [DOI] [PubMed] [Google Scholar]
- Okuno Y., Ueda S., Kurimura T., Suzuki N., Yamanishi K. Studies on further attenuated liver measles vaccine. VII. Development and evaluation of CAM-70 measles virus vaccine. Biken J. 1971 Sep;14(3):253–258. [PubMed] [Google Scholar]
- Rota J. S., Hummel K. B., Rota P. A., Bellini W. J. Genetic variability of the glycoprotein genes of current wild-type measles isolates. Virology. 1992 May;188(1):135–142. doi: 10.1016/0042-6822(92)90742-8. [DOI] [PubMed] [Google Scholar]
- Rota P. A., Bloom A. E., Vanchiere J. A., Bellini W. J. Evolution of the nucleoprotein and matrix genes of wild-type strains of measles virus isolated from recent epidemics. Virology. 1994 Feb;198(2):724–730. doi: 10.1006/viro.1994.1086. [DOI] [PubMed] [Google Scholar]
- Saito H., Sato H., Abe M., Harata S., Amano K., Suto T., Morita M. Cloning and characterization of the cDNA encoding the HA protein of a hemagglutination-defective measles virus strain. Virus Genes. 1994 Mar;8(2):107–113. doi: 10.1007/BF01703609. [DOI] [PubMed] [Google Scholar]
- Schneider-Schaulies J., Schneider-Schaulies S., Ter Meulen V. Differential induction of cytokines by primary and persistent measles virus infections in human glial cells. Virology. 1993 Jul;195(1):219–228. doi: 10.1006/viro.1993.1363. [DOI] [PubMed] [Google Scholar]
- Taylor M. J., Godfrey E., Baczko K., ter Meulen V., Wild T. F., Rima B. K. Identification of several different lineages of measles virus. J Gen Virol. 1991 Jan;72(Pt 1):83–88. doi: 10.1099/0022-1317-72-1-83. [DOI] [PubMed] [Google Scholar]
- Thormar H., Mehta P. D., Brown H. R. Comparison of wild-type and subacute sclerosing panencephalitis strains of measles virus. Neurovirulence in ferrets and biological properties in cell cultures. J Exp Med. 1978 Sep 1;148(3):674–691. doi: 10.1084/jem.148.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Rustigian R., Stallcup K. C., Byers K. B., Winston S. H., Fields B. N. Measles virus-specified polypeptide synthesis in two persistently infected HeLa cell lines. J Virol. 1979 Sep;31(3):677–684. doi: 10.1128/jvi.31.3.677-684.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa Y., Yamanouchi K. Effect of papaverine treatment on replication of measles virus in human neural and nonneural cells. J Virol. 1984 May;50(2):489–496. doi: 10.1128/jvi.50.2.489-496.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Meulen V., Müller D., Käckell Y., Katz M., Meyermann R. Isolation of infectious measles virus in measles encephalitis. Lancet. 1972 Dec 2;2(7788):1172–1175. doi: 10.1016/s0140-6736(72)92595-0. [DOI] [PubMed] [Google Scholar]


