Abstract
The DNA damage response (DDR) is an essential signaling pathway that detects DNA lesions, which constantly occur upon either endogenous or exogenous assaults, and maintains genetic integrity. An infection by an invading pathogen is one such assault, but how bacteria impact the cellular DDR is poorly documented. Here, we report that infection with Listeria monocytogenes induces host DNA breaks. Strikingly, the signature response to these breaks is only moderately activated. We uncover the role of the listerial toxin listeriolysin O (LLO) in blocking the signaling response to DNA breaks through degradation of the sensor Mre11. Knocking out or inactivating proteins involved in the DDR promotes bacterial replication showing the importance of this mechanism for the control of infection. Together, our data highlight that bacterial dampening of the DDR is critical for a successful listerial infection.
Author Summary
In eukaryotic cells both normal metabolic activities and environmental factors such as UV radiation can cause DNA lesions or mutations. The ability of a cell to restore integrity to its genome is vital, and depends on a signaling cascade called the DNA damage response (DDR) that both senses and responds to the assaults. Bacterial infection is one such assault, but its effect on the DDR of the invaded cell remains elusive. Here we used the bacterial pathogen Listeria monocytogenes to study its effect on host DNA damage and its impact on the DDR. Our results show that although Listeria is able to induce DNA damage, the ensuing response is surprisingly low, demonstrating that this bacterium is able to dampen the DDR. We have also shown that the listerial toxin listeriolysin O (LLO) is responsible for the observed block in the DDR. In fact, we find that LLO induces protein degradation of the main DNA damage sensor, Mre11, thereby blocking downstream signaling. Furthermore, we have studied the impact of mutating the DDR on the infectious process and find that it negatively regulates infection with Listeria. In conclusion, our findings reveal that dampening of the DDR is crucial for a productive infection.
Introduction
In human cells, the genomic integrity is continuously challenged by environmental and endogenous factors, which induce DNA damage. Cells have evolved an impressive array of repair and signaling pathways to restore the structure of DNA which are collectively termed the DNA damage response (DDR). Briefly, eukaryotic double strand DNA breaks are detected by PARP1 and PARP2 proteins that assemble poly (ADP-ribose) chains on histone H1 and H2B to mediate recruitment of the MRE11-RAD50-NBS1 (MRN) complex, which is assembled at the damaged site and is crucial for engaging the downstream response [1], [2]. The MRN complex recruits the Ataxia-telangiectasia mutated (ATM) protein that contributes to the phosphorylation of H2AX on serine 139 (γH2AX) and of the Mediator of DNA-Damage Checkpoint 1 (MDC1). MDC1 bound to γH2AX acts as an interaction platform for other DDR components including the ubiquitin E3 ligase Ring finger protein 8 (RNF8), which ubiquitinates H2A-type histones and associates with the ubiquitin E2 enzyme UBC13 [3]. H2A ubiquitination is essential for recruitment of another E3 ubiquitin ligase RNF168, itself important for p53 binding protein (53BP1) and breast cancer 1 early onset (BRCA1) foci formation [4], [5]. When the DNA lesion is not repaired, the DDR slows down the cell cycle, leading to either cell cycle arrest, senescence or death [1], [6]. Thus, maintenance of genome integrity is critical for cell homeostasis and defects in the DDR lead to severe genetic diseases or premature ageing [7].
Due to its important role in genomic stability, the DDR is a common target of many viruses [8]. Indeed, the DDR benefits the replication of some viruses, in particular a number of DNA viruses, but for other, such as Herpes virus, the DDR is an obstacle to overcome during infection. Some bacterial pathogens have also been shown to induce genomic instability upon infection, but the impact of the DNA-damage response on infection efficiency is largely unknown [9], [10], [11], [12], [13], [14]. It has, however, been hypothesized that the cell cycle arrest provoked by bacteria-induced DNA damage could prolong bacterial colonization in vivo by slowing down the epithelium renewal or exfoliation [14], [15]. More studies are thus needed to understand the action of the DDR components on either bacterial clearance or persistence.
L. monocytogenes is the etiological agent of listeriosis, a food borne disease acquired by ingestion of contaminated food. Listeriosis manifests itself as febrile gastroenteritis, or in severe cases as meningoencephalitis, abortion and septicaemia leading to death in 30% of cases [16]. At the cellular level, L. monocytogenes is a facultative intracellular bacterium that is well equipped to survive in the cytoplasm of the infected cell. Indeed, L. monocytogenes promotes its internalization into host cells via two bacterial proteins InlA and InlB that bind the host receptors E-cadherin and c-Met respectively. Activation of these receptors leads to a series of events involving the recruitment of endocytic effectors, and actin cytoskeletal rearrangements, that induce entry of Listeria into the host cell [17]. Once inside the cell, L. monocytogenes escapes from the endocytic vacuole using the pore-forming toxin listeriolysin O (LLO), and multiplies in the cytoplasm. There, Listeria overexpresses ActA, which allows escape from autophagy and also polymerizes host actin, in order to move and spread to the neighboring cells [18]. Importantly, in addition to mediating vacuole escape, LLO also targets several host functions such as, host gene transcription, mitochondrial dynamics, host protein synthesis or SUMOylation [19], [20]. Thus, L. monocytogenes has evolved many mechanisms to manipulate the host to its advantage.
In this study we investigate the effects of L. monocytogenes infection on the host DNA integrity. We show that infection with L. monocytogenes induces DNA breaks and activates the DDR in host cells in vitro and in vivo. Strikingly, L. monocytogenes is able to dampen activation of the DNA-damage signaling pathway through the bacterial factor LLO. We show that LLO induces the degradation of Mre11, one of the major sensors of DNA damage through a mechanism independent of proteasome degradation. In addition, our results reveal that dampening of the DDR is important for infection as inhibition of proteins implicated in this pathway increases bacterial infection. Thus, our study uncovers a subversion of the DDR during infection by L. monocytogenes and a role for the DDR in blocking bacterial proliferation.
Results
L. monocytogenes induces DNA breaks during infection
To determine whether infection with L. monocytogenes induces DNA damage to the host cell, we performed a “comet assay”, a single cell gel electrophoresis assay that is used to show the occurrence of DNA breaks [21]. In this assay cleaved DNA fragments migrate out of the nucleus under the influence of an electric field, whereas most of the DNA does not, or less. By evaluating the DNA comet tail shape and length (arrows on images in figure 1A), one can assess the amount of DNA damage, which can be quantified and averaged over many cell nuclei. Images of control cells that have undamaged DNA and cells treated with H2O2 to induce DNA breaks are shown in figure 1A. Upon infection for 24 h with L. monocytogenes the length of the DNA tail was significantly longer than that measured in uninfected cells, or cells incubated with the non-invasive and non-pathogenic Listeria innocua (figures 1A). Therefore pathogenic Listeria induces DNA breaks during infection.
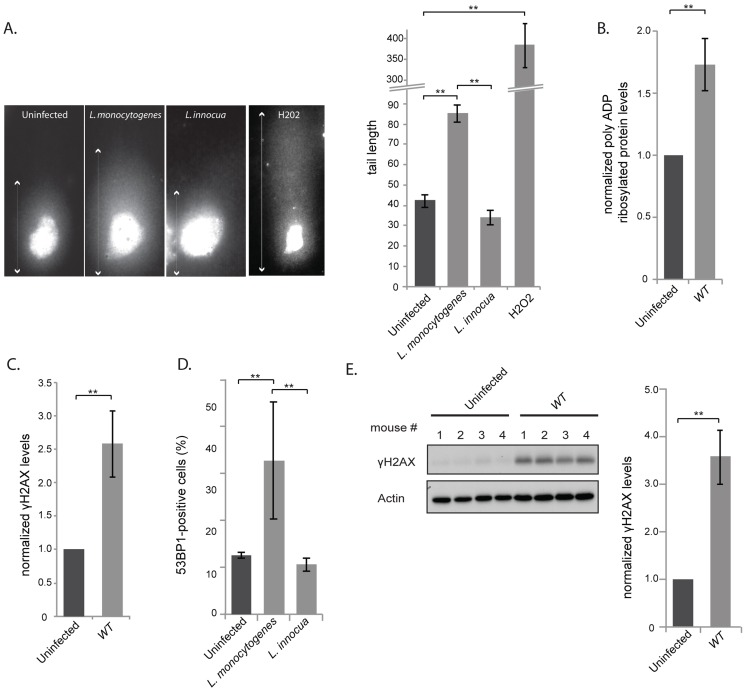
Figure 1. L. monocytogenes induces DNA breaks and mildly activates the DNA damage response.
(A) Images on the left show comet assays of HeLa cell infected with the indicated strain or treated with purified listeriolysin O or hydrogen peroxide (0.5 mM), and on the right is a quantification of the images. Each box on the left measures 48,3×90 µm, except for the H2O2 treated condition, where the size is double. The white arrows indicate the measured tail length which is recorded and averaged in the histogram on the right. Each bar in the histogram is an average of at least 30 nuclei from at least 3 independent experiments. (B) and (C) HeLa cells were infected with L. monocytogenes EGD for 24 h. Cell extracts were collected for immunoblot analysis. The polyADP and γH2AX levels are normalized to actin and to the uninfected condition (n≥3). (D) HeLa cells were infected with Listeria for 24 h. 53BP1 foci were visualized by immunofluorescence and quantitated over at least 3 experiments, for a total of more than 500 cells counted. (E) Immunoblot images are shown on the left and quantifications on the right. The left image is shown for 1 experiment on spleen homogenates from C57Bl/6J mice infected with L. monocytogenes EGD for 72 h. The histogram on the right integrates 2 experiments (n = 8 animals per condition). All quantifications in graphs show the mean +/− SEM (** indicates p<0.01).
DNA breaks are detected by the cell, which then initiates the DDR pathway. To determine whether infection with L. monocytogenes activates this pathway, we focused on three well-characterized DDR markers: accumulation of poly-ADP ribosylated proteins, phosphorylation of H2AX and increase in the number of 53BP1 foci [22]–[24]. We first studied the levels of poly-ADP ribosylated proteins by western blotting of HeLa cells extracts infected for 24 h with wild type bacteria. A small but significant accumulation of poly-ADP ribosylated proteins could be detected upon infection. Modified proteins ranged from 150 to 200 kDa in size and the accumulation of these proteins was proportional to the multiplicity of infection (MOI) used in the experiment (figure 1B, S1A).
Next, we examined the level of γH2AX, another marker of DNA double stranded breaks. The levels of γH2AX have been shown to increase upon infection with Shigella flexneri [9], another invasive bacterium that we took as control. Surprisingly, although L. monocytogenes induces DNA breaks upon infection, as shown by the comet assay, the levels of γH2AX showed a significant albeit modest increase in the cells (figure 1C). The increase in γH2AX levels was proportional to the multiplicity of infection used in the experiment (figure S1A), yet lower than that detected upon infection with S. flexneri (figure S1B). Interestingly, accumulation of γH2AX was not observed upon infection with the non pathogenic Listeria innocua, or with Staphylococcus aureus or with Escherichia coli K12 (figure S1B).
The last marker we monitored was 53BP1 foci. Resting cells usually show a small number of 53BP1 foci [25]. We therefore counted the number of cells that contained more than 3 foci per cell (figure S2A) and showed that upon infection, there was a higher number of 53BP1 positive cells when infected with L. monocytogenes compared to uninfected conditions or to cells incubated with L. innocua (figure 1D).
As Listeria was inducing DNA breaks and recruitment of associated markers, we investigated whether these events induced an arrest in the cell cycle as generally observed after double strand breaks [26]. We thus synchronized cells with nocodazole and infected them 4 hours after release from the mitotic block. Although a delay of the cell cycle was observed at 16 h and 20 h of infection, this difference was no longer visible at 24 h (figure S2B), suggesting that Listeria does not have a significant and long lasting effect on the host cell cycle. Taken together all these results show that although Listeria induces a significant level of DNA breaks, as detected by the comet assay, the response to these breaks through the DDR is unexpectedly low.
We further studied whether an increase in γH2AX levels was detectable during an in vivo infection. We infected mice for 72 h and harvested the spleens. Figure 1E shows that γH2AX levels displayed a 3.5 fold increase in spleens of infected mice compared to uninfected mice. The larger increase in γH2AX levels observed in vivo compared to in vitro is probably due to the duration of the infection (72 h vs. 24 h). Therefore we conclude that L. monocytogenes induces an increase in γH2AX levels during infection both in tissue-cultured cells and in mice.
To further assess how L. monocytogenes induces DNA damage, we investigated using the comet assay, the phenotype of a ΔinlB mutant, which lacks InlB the protein crucial for bacterial invasion. Our results show that the size of the comet observed upon infection with a ΔinlB mutant was similar to the comet observed upon infection with wild type Listeria (figure S3A). Similarly, we treated cells with the actin polymerization inhibitor cytochalasin D to block bacterial entry and measured the size of the cellular comets observed. These results also show that bacterial internalization was not necessary for inducing host cell DNA damage (figure S3A). Consistent with these results, extracellular Listeria (either infection with the ΔinlB mutant or pretreatment of cells with cytochalasin D prior to infection with wild type bacteria) showed the same level of γH2AX as observed upon infection with Listeria able to invade host cells (figure 2 and S3B).
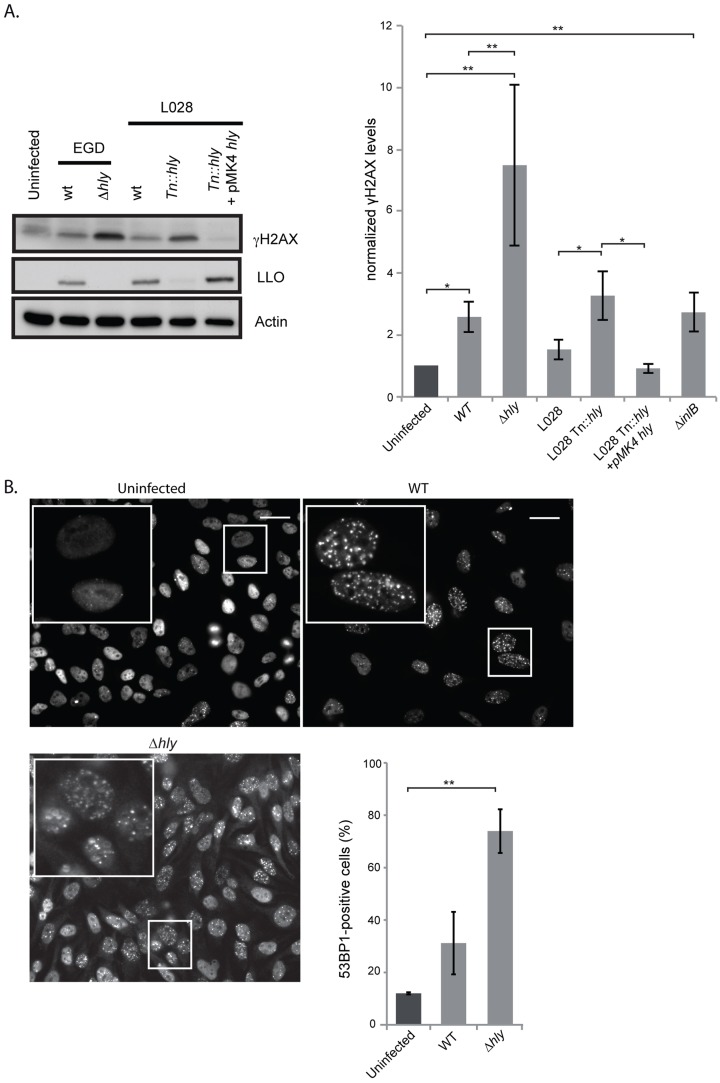
Figure 2. A Δhly mutant induces a higher DNA damage response than wild type Listeria.
(A) HeLa cells were infected for 24 h with the indicated strains of Listeria. Cell extracts were collected for immunoblot analysis. A representative immunoblot (left) and a quantification (right) of at least 3 independent experiments are shown. The γH2AX levels are normalized to actin and to the uninfected condition. (B) Immunofluorescence of 53BP1 in HeLa cells infected with Listeria for 24 h and quantification of 53BP1-positive cells from at least 3 independent experiments, for a total of more than 500 cells counted. All quantifications in graphs show the mean +/− SEM (* indicates p<0.05, ** p<0.01). Size bars represent 30 µm, insert is 2.5 times larger than box in original image.
L. monocytogenes deleted for LLO activates the DDR to higher levels than the wild type parent strain
Our finding that extracellular Listeria induces DNA breaks during infection led us to investigate the role of the secreted toxin listeriolysin O (LLO), a well-known virulence factor [19]. Using the comet assay, we showed that infection with a Δhly mutant, lacking LLO, led to the formation of the same size comets as when infecting with wild type Listeria (figure S3A). Consistent with these results, the purified toxin itself does not provoke DNA breaks (figure S3A). Therefore, the LLO toxin is not important for inducing DNA breaks upon infection with L. monocytogenes.
We next studied the effect of LLO on the DDR by measuring the level of H2AX phosphorylation. Surprisingly, as shown in figure 2A, a Δhly mutant induced a greater increase in the level of γH2AX than the wild type. These results were reproduced in another cell type and with another strain of L. monocytogenes, L028, suggesting that LLO prevents H2AX phosphorylation (figure S4 and 2A). In addition, upon overexpression of LLO in a Δhly mutant background, the level of γH2AX was reduced to the same level as that observed in uninfected cells (figure 2A). These data, along with the comet assay results, thus suggested that LLO dampens the DDR downstream of DNA breaks.
We also measured the number of 53BP1 foci upon infection with a Δhly mutant. As shown in figure 2B similarly to what is observed for γH2AX, infection with a mutant lacking LLO caused an over 2-fold increase in the number of 53BP1 foci compared to wild type bacteria (around 2000 cells were counted per condition). Together these data show that during infection LLO dampens the DNA damage response, which is normally activated upon DNA breaks.
We further investigated the effect of LLO on the γH2AX levels in vivo. For this, we performed peritoneal infections of mice with either the wild type or Δhly mutant L. monocytogenes. We chose this artificial infection route as it is well known that a Δhly mutant is strongly attenuated in the intravenous model of infection and rapidly eliminated [27]. After 6 hours of infection the content of the peritoneum was harvested and the level of γH2AX was assessed by western blot. Upon infection with wild type Listeria an increase in the level of γH2AX similar to that occurring in tissue culture was observed, and an even larger increase in the level of γH2AX was detected upon infection with the Δhly mutant, even though the same number of bacteria was present in the peritoneal lavage (figure S5). These in vivo results therefore confirm our in vitro observations, i.e. that LLO prevents phosphorylation of H2AX.
LLO blocks the DDR induced by cytotoxic agents
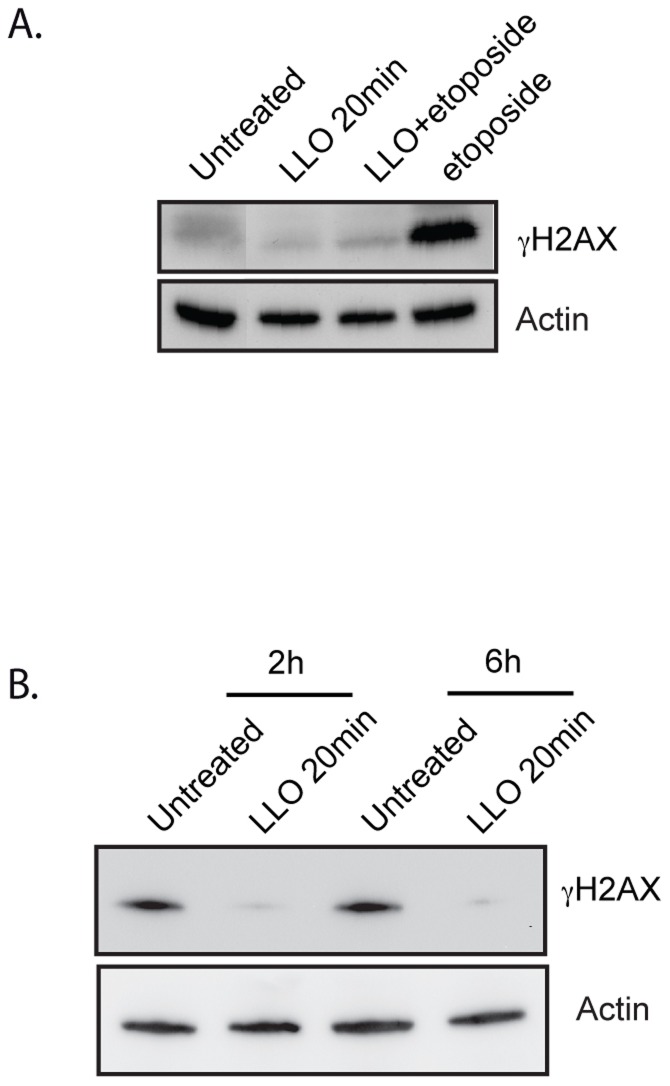
Since our results showed that LLO dampened the DDR induced by infection, we investigated whether LLO could also dampen the DDR induced by known cytotoxic agents such as etoposide or irradiation. To test this hypothesis, we treated cells with purified LLO for 20 min before adding etoposide for 24 h or before X-ray irradiating cells. Our results show that etoposide alone, as expected, induced a large increase in the level of γH2AX, whereas purified LLO on its own did not (figure 3A). Strikingly, upon pre-treatment of cells with LLO, etoposide no longer caused an increase in the level of γH2AX. Similarly, X-ray irradiation, also induces an increase in the level γH2AX within 2 h of the treatment (figure 3B). However, pre-incubation with LLO blocked H2AX phosphorylation induced by X-irradiation (figure 3B). Therefore, the LLO toxin is a potent inhibitor of the DDR.
Figure 3. LLO dampens the DDR.
(A) and (B) Immunoblots of HeLa cells treated with LLO or left untreated. Toxin treatment was performed for 20 minutes and removed prior to treatment with etoposide (A) or prior to X-irradiation (B). Cells were irradiated with 10 Gray and harvested either 2 h or 6 h after irradiation. Images are representative of experiments performed at least 3 times.
LLO induces a proteasome-independent degradation of Mre11
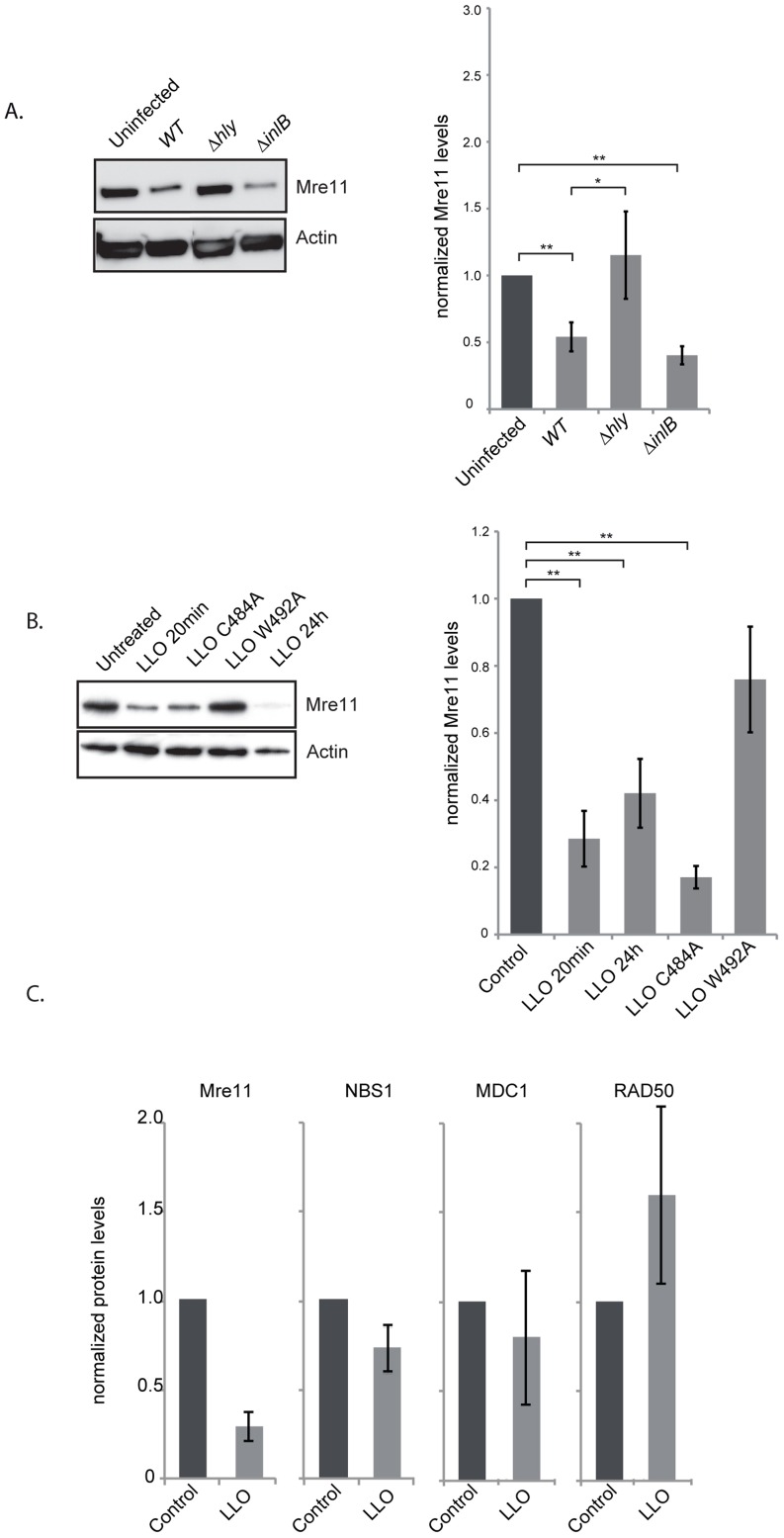
To understand the mechanism by which LLO dampens the DDR we investigated the effect of infection and the effect of the purified LLO toxin on the host proteins, Mre11, RAD50 and NBS1, involved in sensing DNA breaks [6]. Strikingly, after 24 h of infection with wild type L. monocytogenes the level of Mre11 significantly decreased (figure 4A). This decrease is dependent on LLO as a Δhly mutant does not induce such a decrease (figure 4A). These results can also be observed in JEG3 cells, revealing that the effect is not restricted to HeLa cells (figure S4B). Interestingly, a ΔinlB mutant, which is defective in entry into HeLa cells, also induces a decrease in the level of Mre11 similarly to the wild type strain further suggesting that secreted LLO is necessary for this effect (figure 4A). We further investigated whether purified LLO was sufficient to induce Mre11 degradation. As shown in figure 4B incubation of cells with a sub-lytic dose of LLO (3 nM) for 20 minutes was sufficient for decreasing Mre11 levels more than 4 fold. Interestingly, whereas LLO induced a decrease in the levels of Mre11, the levels of RAD50, NBS1, or another DDR protein, MDC1 were unchanged (figure 4C). LLO is a pore-forming toxin. We therefore investigated whether pore formation was necessary for Mre11 degradation by incubating cells with mutant forms of LLO which are affected in their hemolytic activity [28]. A point mutation in LLO at residue 492 (W-A) renders LLO cytolytically inactive, whereas a mutation at residue 484 (C-A) only reduces LLO's activity by 25%. As shown in figure 4B the LLO C484A mutant induces the same decrease in Mre11 levels as wild type LLO, whereas the LLO W492A mutant no longer induces Mre11 degradation. Therefore, pore formation by LLO is required for the impact on Mre11 protein levels. Therefore LLO induces a specific degradation of Mre11, an effect mediated through pore formation at the plasma membrane.
Figure 4. LLO induces a decrease in Mre11 protein levels.
(A) Immunoblots (left) and quantification of Mre11 protein levels in HeLa cells infected for 24 h with the indicated listerial strain. (B) Purified LLO (3 nM), or mutant purified LLO (C484A, or W492A) were used for 20 minutes and cells were harvested immediately (LLO 20 min) or after 24 h following removal of LLO (LLO 24 h). Immunoblots (left) and quantification of Mre11 levels (right) are shown. (C) Protein levels of other DDR proteins were assessed by immunoblots in HeLa cells treated with LLO (3 nM) for 20 minutes. All immunoblot quantifications are an average of at least 3 independent experiments, which are normalized to actin and to the uninfected control. All quantifications in graphs show the mean +/− SEM (* indicates that p<0.05, ** p<0.01).
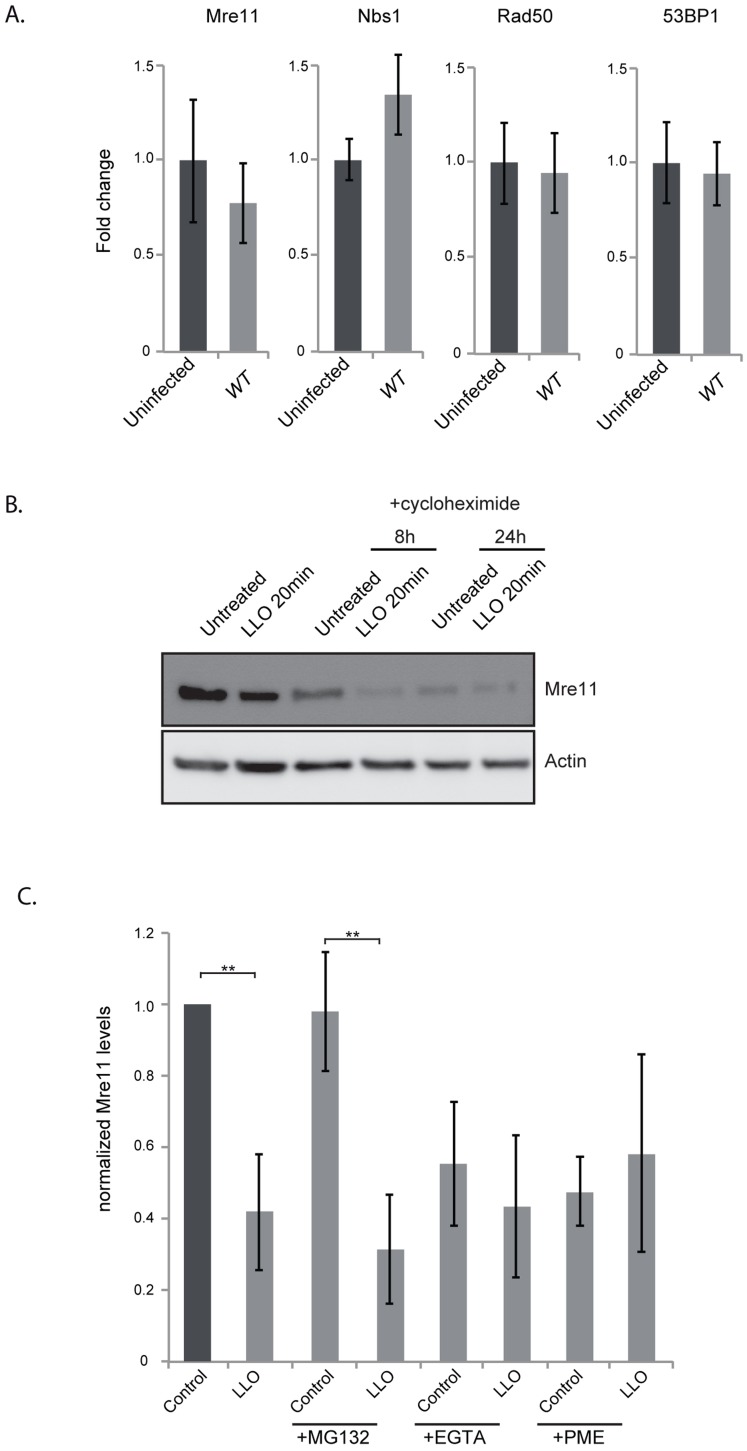
We next aimed at understanding the mechanism underlying the decrease in the protein level of Mre11. By quantitative PCR we determined that infection was not decreasing transcription levels of the Mre11 gene, or any other gene of the DNA sensing complex (figure 5A). To determine whether LLO was inducing a block in protein synthesis, we performed experiments using cycloheximide which blocks translational elongation. Our results show that cycloheximide did not prevent the LLO-induced decrease in Mre11 levels strongly suggesting that LLO was triggering a degradation of Mre11 (figure 5B). We further used chemical inhibitors to uncover the mechanism of Mre11 degradation. MG132 was used to block the proteasome, however this inhibitor did not block LLO-mediated Mre11 degradation (figure 5C). However, we found that using a cell permeable inhibitor of aspartyl-proteases, pepstatin-A-methylester (PME), or incubating cells in EGTA, which quenches extracellular calcium, blocked LLO-mediated Mre11 degradation (figure 5C). Therefore, our data show that LLO induces a proteasome-independent degradation of Mre11, a mechanism that appears to require a calcium dependent aspartyl-protease.
Figure 5. LLO induces degradation of Mre11 in an aspartyl-protease dependent manner.
(A) Quantitative PCR experiments comparing uninfected HeLa cells to cells infected for 24 h with wild type L. monocytogenes. Error bars are standard deviation as calculated by the Qiagen RT2 Profiler PCR analysis program. (B) Immunoblot of HeLa cells treated with cycloheximide for the indicated times. (C) Quantification of immunoblots of cells treated with the indicated inhibitors. For all experiments LLO was used at 3 nM for 20 minutes. All quantifications in graphs show the mean +/− SEM (** indicates p<0.01).
Dampening of the DDR is required for productive infection
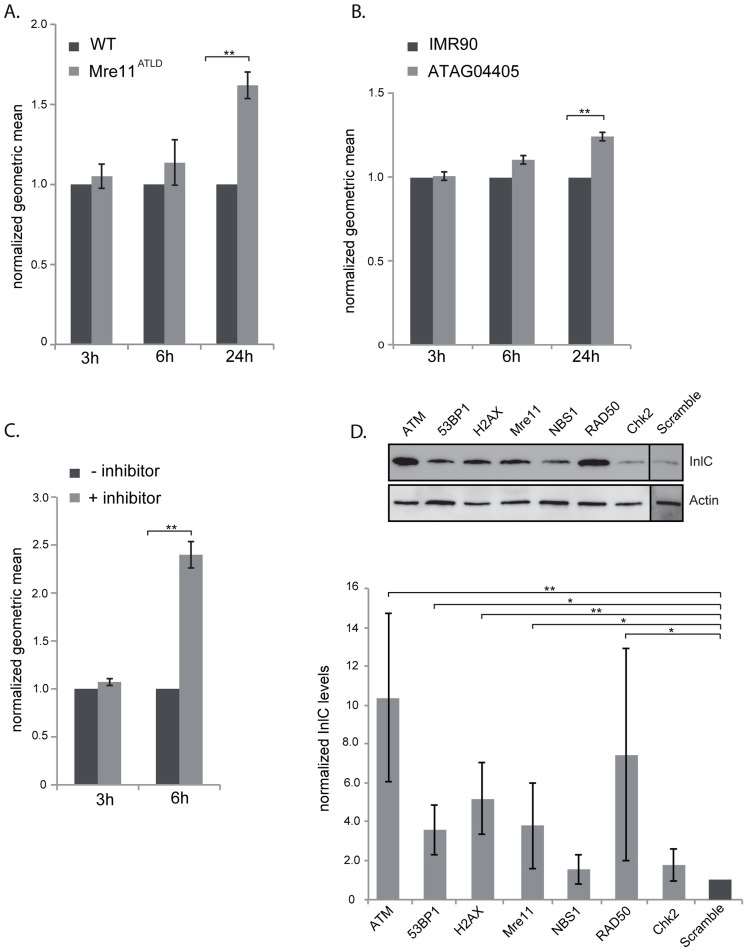
Our data show that L. monocytogenes, through the activity of LLO, dampens the DDR during infection. To determine whether dampening of the DDR was important for infection, we first focused on mre11, whose gene product is degraded during infection. We infected mouse embryonic fibroblasts (MEFs) from Mre11ATLD mice, in which the mre11 gene is truncated [29], with L. monocytogenes expressing GFP and compared, infection of these cells to infection of wild type MEFs by FACS analysis. We monitored the number of bacteria per cell through detection of GFP levels, which are directly proportional to the number of bacteria [30]. At early times of infection, i.e. 3 and 6 hours post infection, there was no difference in the GFP levels detected in wild type MEFs and Mre11ATLD MEFs, indicating that there is no difference in entry and early stages of bacterial replication between the two cell types (figure 6A). However, at 24 hours of infection there was a significantly higher level of GFP detected in Mre11ATLD MEFs compared to wild type MEFs (figure 6A). These results show that an impaired Mre11 function promotes bacterial growth at late infection time points, suggesting that Mre11 dependent DDR controls L. monocytogenes proliferation.
Figure 6. The DNA damage response is negative regulator of infection.
(A) WT MEFs or Mre11ATLD MEFs, or (B) WT human fibroblasts (IMR90) and fibroblasts bearing a mutation in ATM (ATAG04405) were infected with L. monocytogenes expressing GFP for the indicated times. FACS analysis was performed on infected cells and the geometric mean of 10,000 cells was measured using the FlowJo software. Results are normalized to uninfected cells and averaged over at least 3 independent experiments. (C) HeLa cells were infected with L. monocytogenes expressing GFP for the indicated times. The ATM inhibitor was added (+ inhibitor) 1 h after the start of infection. FACS analysis was performed on infected cells and the geometric mean of 10,000 cells was measured using the FlowJo software. Results are normalized to uninfected cells and averaged over at least 3 independent experiments. (D) HeLa cells were transfected with the indicated siRNA and infected with L. monocytogenes. Cells were collected after 24 h of infection and immunobloted for the Listerial protein InlC (top). Quantifications (bottom) are normalized to actin and to the scramble sample over at least 3 independent experiments. All quantifications in graphs show the mean +/− SEM (* indicates that p<0.05, ** p<0.01).
Mre11, which is part of the MRN complex, is important for recruiting ATM, a kinase that contributes to the phosphorylation of H2AX [1]. We thus also tested the role of ATM in the infectious process by two different approaches. We first infected wild type human fibroblasts (IMR90) and fibroblasts isolated from ataxia-telangiectasia syndrome patients which have a mutation in the ATM gene. Similarly to the results obtained in Mre11ATLD MEFs, we observed at 24 hours of infection a higher GFP signal in ATM deleted fibroblasts compared to the wild-type cells (figure 6B). The difference in the GFP signal was only visible at 24 hours of infection and not at 3 or 6 hours (figure 6B). We further tested the role of ATM by infecting cells treated with a pharmacological inhibitor of ATM (KU60019). In order to keep the incubation time with the inhibitor low, we only monitored infection at 3 and 6 hours of infection. Our FACS analysis showed that at 3 hours of infection the ATM inhibitor had no effect on the GFP levels recovered from cells. However, at 6 hours of infection, the mean fluorescence detected in cells treated with the ATM inhibitor was more than twice that of untreated cells (figure 6C). These data therefore confirm the results obtained with human fibroblasts. To further demonstrate the inhibitory role of ATM on infection we performed another assay in which cells are transfected with siRNA against ATM and infected with L. monocytogenes. Cells are then recovered and monitored by western blot for the levels of InlC, a listerial protein overexpressed when bacteria are intracellular which provides a direct read out of bacterial proliferation [31]. As shown in figure 6D, the level of InlC is over 3 fold higher in cells knocked down for ATM compared to the control siRNA treated cells. These results further demonstrate the inhibitory role of ATM during a Listeria infection.
To extend our analysis to other DDR proteins, we treated HeLa cells with siRNAs against 53BP1, H2AX, Mre11, NBS1, RAD50 and CHK2. As shown in figure 6D, the level of InlC is significantly higher in cells knocked down for 53BP1, H2AX, Mre11, and RAD50 compared to control siRNA treated cells. Intracellular bacteria and InlC can also be visualized by immunofluorescence microscopy. This technique also revealed higher levels of infection in 53BP1, H2AX and ATM knocked down cells (figure S6). Taken together, these data establish that inhibition of the DDR promotes L. monocytogenes infection.
Discussion
In this study, we report that the bacterium L. monocytogenes provokes DNA breaks in its host while at the same time controlling the ensuing host DDR. Indeed, we found that although wild type bacteria modestly induce the DDR, a Δhly mutant lacking LLO did so to a significantly higher level. We further reveal that LLO dampens the DDR, through degradation of the host protein Mre11 (figure 7). Interestingly and consistent with these observations, we find that deletion or inhibition of proteins implicated in the DDR lead to higher levels of infection demonstrating for the first time that the DDR can be a negative regulator of a bacterial infection.
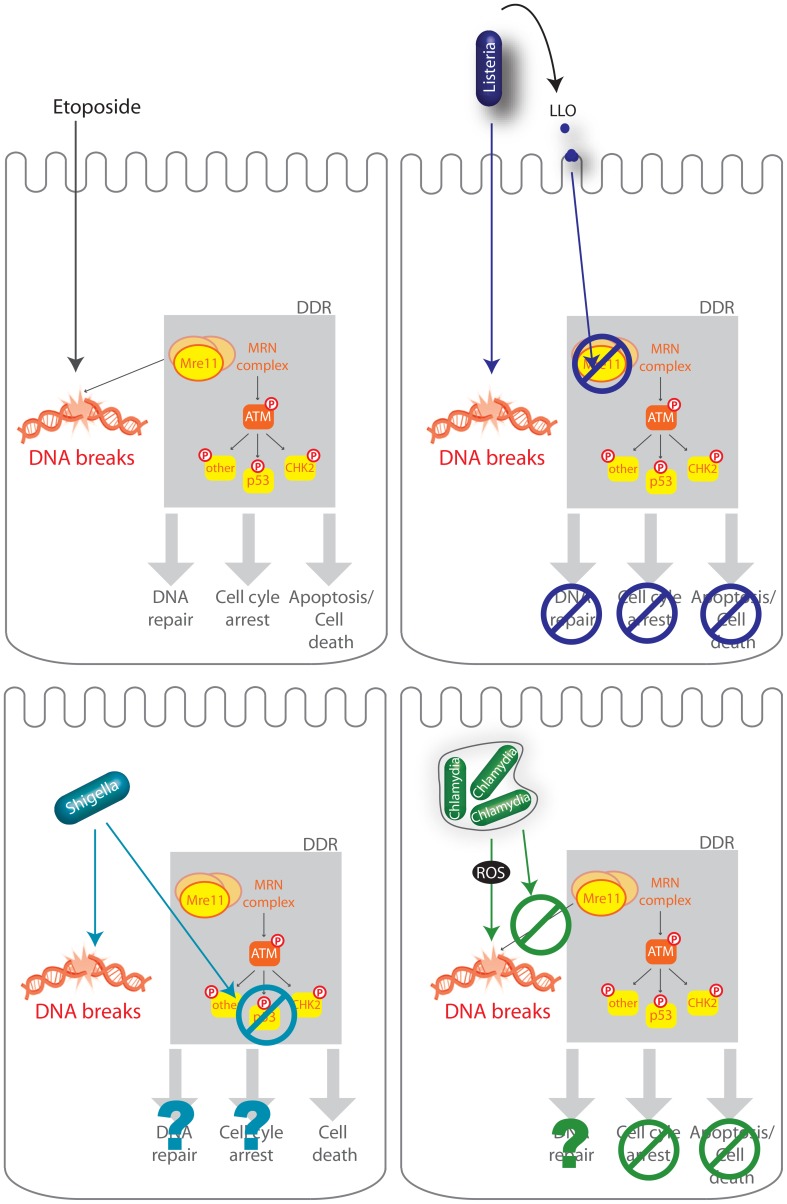
Figure 7. Schematic representation of several infectious processes affecting the DDR.
The DDR regulates DNA repair, cell-cycle checkpoints in order to halt cellular proliferation, or, if the damage is too important, cell death. Etoposide is a cytotoxic agent which causes DNA strand breaks. DNA lesions are recognized by the MRN complex (MRE11, RAD50, and NBS1) and leads to the phosphorylation of ATM and further activation of the DDR including phosphorylation of p53, CHK2, and other substrates. L. monocytogenes (Listeria), S. flexneri (Shigella), C. trachomatis (Chlamydia) all induce DNA breaks during infection but impair the DDR using different mechanisms. L. monocytogenes LLO, through pore formation induces degradation of Mre11, an associated block in the DDR, no cell cycle arrest, and no cell death (Stavru et al., 2011). S. flexneri infection leads to a decrease in the levels of p53 and cell death (Bergonioux et al., 2012), whereas C. trachomatis blocks recruitment of the MRN complex and blocks cell cycle arrest (Chumduri et al., 2013).
Several bacteria have been reported to induce host DNA damage, a phenotype which had not yet been studied during a Listeria infection. Extracellular pathogenic bacteria such as Pseudomonas aeruginosa, Neisseria gonorrhoeae, E. coli, or Helicobacter pylori and intracellular bacteria such as S. flexneri and Chlamydia trichomatis are able to induce host DNA double-strand breaks (DBS) [9], [10], [12]–[14]. Very recently a report has shown that Listeria monocytogenes delays the host cell cycle by increasing the levels of host DNA strand breaks [32]. However, in most cases, the bacterial factor(s) responsible for inducing these DNA breaks has/have not been identified. To our knowledge, only two bacterial virulence factors have been shown to directly induce DNA breaks: Cytolethal distending toxin (CTD) produced by several Gram negative pathogens such as E. coli, and Typhoid toxin (TT) that is expressed by the intracellular S. typhi [15], [33], [11]. Both types of toxin are related and share a common subunit, CdtB, responsible for DNA breaks. However, no homologues of these virulence factors are present in L. monocytogenes. It has recently been reported that C. trachomatis could induce DSB through generation of reactive oxygen species (ROS) [10]. We explored this possibility during infection with L. monocytogenes using the DCFDA reporter, but failed to observe ROS production under our conditions during infection. Thus, ROS production does not appear to contribute to DNA breaks induced by Listeria. A very recent report using multiple bacterial, fungal and plant pathogen species which induce DSBs in plant DNA illustrates the complexity of identifying the cause of pathogen-induced DNA damage [34] and that other factors besides ROS are involved. In our case, we still observe the same increase in γH2AX levels during infection with L. monocytogenes deleted for inlB and impaired in cell invasion, and therefore this suggests that a secreted virulence factor or a surface component is necessary to induce the observed DNA breaks.
Along with the genotoxic stress induced by S. flexneri and C. trachomatis, both bacteria were also shown to impair the DDR (figure 4). Shigella flexneri infection leads to DNA breaks, γH2AX accumulation along with a degradation of the p53 protein, which blocks the p53-dependent DNA repair response [9]. Similarly, C. trachomatis was very recently shown to induce extensive double strand breaks during infection with no associated cell cycle arrest [10]. Indeed, C. trachomatis blocked the formation of 53BP1 foci and phosphorylation of Mre11 through an unknown mechanism. Remarkably, in our report we find that L. monocytogenes, via LLO, also prevents excessive DNA damage signaling, similarly to S. flexneri and C. trachomatis, but through a different mechanism. Interestingly, we show in this report that the DDR is a negative regulator of a listerial infection. Although the inhibitory effect of the DDR on infection had been suggested for S. flexneri and C. trachomatis, this had not been shown experimentally. Our results therefore indicate that dampening of the DDR might be a common and beneficial feature of multiple intracellular pathogen infections.
It is clear that viruses, being obligate intracellular pathogens have an advantage to block the host DDR in order to inhibit cell-cycle checkpoints and p53-mediated apoptosis and keep the cell alive. However, inhibition of the DDR by a bacterial pathogen such as L. monocytogenes, which can survive very well without the host cell, is less intuitive. Nevertheless, It is well known that the DDR induces activation of NF-κB [35], [36]. Furthermore, a very recent report shows that DNA damage induces an immune response [37]. In addition, it has been suggested that the Mre11 protein could be a sensor of exogenous DNA leading to activation of type I interferon [38]. Therefore, our data along with a growing body of evidence suggests that the DDR could have an important role in inducing inflammation during infection.
To our knowledge, this is the first report to show that a bacterium induces Mre11 degradation. Mre11 degradation has only been shown to occur during infection with a virus. Indeed, infection with adenovirus has been shown to result in both reorganization and degradation of members of the Mre11–Rad50–NBS1 complex [39]. Furthermore, and similarly to our findings, inhibition of the DDR is essential for adenovirus infection as in the absence of this block, viral replication is inhibited [39]. Therefore, the DDR is a negative regulator of adenovirus infection. Likewise, in the case of Listeria, we show that downregulation of Mre11 or ATM or H2AX favors infection. This is the first report showing that the DDR has an impact on bacterial infection.
Finally we show here that LLO induces a decrease in the levels of Mre11 in a pore dependent manner. Indeed, point mutants of LLO which are reduced in their hemolytic activity lead to a lesser effect on Mre11 and γH2AX compared to native LLO. LLO is a pore forming toxin which was originally shown to be essential for Listeria escape from the internalization vacuole [40]. Since then, LLO has been shown to have many different extracellular activities on the host cell [19]. Remarkably, the pore formation of LLO has been shown to lead to degradation of at least 2 other proteins during infection, the catalytic subunit of the human telomerase complex (hTERT), and the human E2 SUMO enzyme Ubc9 [20], [41]. Although the mechanism by which hTERT levels were decreased remains to be determined, the degradation of Ubc9 was shown to be partially dependent on an aspartyl-protease. Similarly, we find here that Mre11 is degraded, in a manner that appears to be dependent on an aspartyl-protease. Therefore, the downstream events of LLO pore formation leading to cellular protein degradation are beginning to emerge, however, the specificity of the targeted protein and the molecular mechanism by which degradation occurs remain elusive. Surprisingly, we find that Mre11 levels remain low 24 h after removal of LLO, even though pores have been shown to be repaired very rapidly [42]. The mechanism by which the long lasting effect on Mre11 is achieved will be of particular interest in future studies, and have a wide ranging impact on understanding the long term effects of bacterially induced DNA damage.
Materials and Methods
Ethics statement
The mouse strained used was C56BL/6N. All the procedures were performed in accordance with protocols approved by the Animal Experimentation Ethics Committee of the Institut Pasteur (permit #03-49), in application of the guidelines of the European Commission for the handling of laboratory animals, Directive 2010/63/EU (http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm). Protocols were approved by the veterinary staff of the Institut Pasteur animal facility and were performed in compliance with the NIH Animal Welfare Insurance #A5476-01 issued on 31/07/2012.
Cell lines, bacterial strains
HeLa (CCL-2) and Jeg-3 (HTB-36) cell lines from American Type Culture Collection (ATCC) were grown in Minimum Essential Medium (MEM GlutaMax; Invitrogen) supplemented with 10% Foetal Bovine Serum (FBS) at 37°C in a 10% CO2 atmosphere. LoVo (CCL-229) cells from ATCC were cultured in Ham's F12K, 2 mM glutamine (Invitogen) with 10% FBS. MEF and human fibroblasts were grown in Dubelcco's Modified Eagle Medium (DMEM GlutaMax; Invitrogen) supplemented with 10% FBS. Purified LLO was obtained as described previously [43] and was used at 3 nM. When needed cells were treated with etoposide (Sigma) at 40 µM or hydrogen peroxide at 0.5 mM.
Escherichia coli K12, E. coli K12+invasin from yersinia pseudotuberculosis (BUG2940) and Salmonella typhimurium sri11 (BUG3044) were grown in Luria-Bertani (LB) medium (BD Difco) at 37°C. Listerias strains and Staphylococcus aureus (RN6390) were grown in brain heart infusion (BHI) medium (BD Difco) whereas Shigella flexneri M90T (BUG2505) were cultured in tryptic soy broth (TSB) medium (BD Difco) at 37°C. The Listeria strains used in this study were L. innocua (BUG499), L. monocytogenes EGD (BUG600), L. monocytogenes EGD-GFP (BUG2539), L. monocytogenes EGDΔhly (BUG2132), L. monocytogenes EGDΔinlB (BUG1047), L. monocytogenes L028 (BOF 343), L. monocytogenes L028 Tn::hly (BOF 415), and L. monocytogenes L028 Tn::hly (BOF 415) overexpressing LLO (BUG 210).
Cell treatments
For synchronization, HeLa cells were blocked in mitosis with 300 nM nocodazole (Sigma) for 22 h. Cells were infected 4 h after release in nocodazole free growth medium. Etoposide treatment of cells was performed for 24 h using a concentration of 40 µM. Cells were exposed to 10 Gray X-ray irradiation using an Xstrahl RS320 irradiator. Cycloheximide treatment was performed using a concentration of 100 µM.
MG132 treatment was performed for 5 h prior to infection and was used at a concentration of 10 µM. Pepstatin-A-methylester (PME) was used for 1 h prior to infection at a concentration of 200 µM. 20 mM EGTA was added to cells upon infection.
Cytochalasin D treatments were performed for 15 minutes prior to infection at a concentration of 5 µg/ml.
Bacterial infections
For in vitro infection: Bacteria were cultured overnight and then sub-cultured 1∶10 in BHI medium for 2 h at 37°C. Bacteria were then washed three times in medium without serum. Prior to infection, HeLa cells were incubated in medium without serum. After addition of bacteria, cells were centrifuged at 1,000× g for 1 min and incubated at 37°C for 1 hour. When not specified, infection was achieved with a multiplicity of infection (MOI) of 50. Infected cells were then grown in growth medium supplemented with 20 µg/ml gentamicin.
In vivo infections were performed by intra venous injection of 105 bacteria per animal. Experiments were performed according to the Institut Pasteur guidelines for animal experimentation. Spleens were collected and homogenized. CFUs were evaluated from homogenates and one volume of homogenate was diluted into 4 volumes of Laemli buffer sonicated and loaded on SDS–polyacrylamide gels for immunoblotting experiments.
Concerning peritoneum infection, mice were infected with 1.107 CFU for 6 h. The peritoneum content was washed with PBS to harvest bacteria and cells. A part of this content was treated or not with 0.1% triton X-100 and was plated on BHI agar plates to quantify the number of bacteria by counting CFU. Remaining content was centrifuged and cell pellet was lyzed in 2× laemmli buffer.
Comet assay
HeLa cells were grown adherent to tissue culture plates and infected or treated as detailed in figure. Cells were then treated as recommended in the comet SCGE kit (enzo #ADI-900-166). Briefly, cells were scraped, washed in cold PBS, mixed with LMAgarose and spread on Trevigen comet slides. Agarose embedded cells are then lysed and run on a horizontal electrophoresis apparatus on 32 V for 20 minutes. Slides are then dried and stained with DAPI. Image acquisition of comets was done using a 63× objective on an inverted fluorescence microscope. Image analysis was performed using the comet assay plug-in for Image J.
Flow cytometry, immunofluorescence and immunoblot
Bacterial infection was assessed by flow cytometry using the BD Cytofix/cytoperm kit (BD Biosciences). Briefly, cells were trypsinized and fixed for 20 minute with fixation and permeabilization solution. Cells were then harvested in PBS for analysis. For cell cycle distribution, cells were scrapped, resuspended in PBS and fixed in 70% ethanol for at least 1 h at −20°C. Cells were washed and re-suspended in PBS containing 15 mg/ml of propidium iodide and 100 mg/ml of RNase A. Flow cytometry acquisitions were performed on a FACScalibur flow cytometer (Becton Dickinson). Data were analysed using FlowJo software.
For measuring infection, Listeria monocytogenes expressing GFP were used as described in [31]. Infected cells were trypsinized and analyzed on a FACScalibure. Data was analyzed using the FlowJo software.
To quantify positive cells for 53BP1 or γH2AX by immunofluorescence, infected cells were washed and fixed in 4% paraformaldehyde. After permeabilization, 53BP1 or γH2AX proteins were stained with an anti-53BP1 antibody (Novus biologicals) or anti-γH2AX (#9718, Cell Signaling), and a 546 nM goat anti-rabbit antibody (Invitrogen). References for the antibodies used in our studies are the following: NBS1 (Novus biologicals NB100-143), MDC1 (Novus biologicals NB100-395), RAD50 (Novus biologicals NB100-147).
For Western blot analysis, cells were lysed with 2× Laemmli loading buffer (124 mM Tris-HCl [pH 6.8], 4% SDS, 20% glycerol, 0.02% bromophenol blue, 0.03% dithiothreitol [DTT]), sonicated for 2 s, and then boiled for 5 min. After running on gels, proteins were transferred on a nitrocellulose membrane (GE Healthcare) that was then blocked in 10% milk. The primary antibodies were anti-actin (A5441; Sigma), anti-phospho H3 (ser10) (05-817; Upstate, Lake Placid, NY), anti-Poly (ADP-ribose) (MAB3192 Millipore), anti-γH2AX (#9718, Cell Signaling), Mre11 (#4895, Cell Signaling). Rabbit polyclonal antibodies against InlC and LLO (R176) were raised by immunizing rabbits with purified recombinant LLO and InlC proteins. The sera were then purified against InlC and LLO to obtain the purified antibodies (43). Quantification of immunoblots was performed using a G:Box Syngene and the associated software.
Quantitative PCR
mRNA was extracted from uninfected cells and cells infected with L. monocytogenes using the Qiagen RNeasy kit. cDNA was then synthesized using the Qiagen RT2 first strand kit. Real-time PCR was performed using Qiagen RT2 PCR arrays (DNA damage array PAHS-029Z) on a Biorad CFX384. Data was analysed using the Qiagen RT2 Profiler PCR array data analysis.
siRNA
HeLa cells were reverse transfected with commercial siRNA for ATM (L-003201, Thermo scientific), CHK2 (L-003256, Thermo scientific), 53BP1 (s14313, Ambion), H2AX (s226270, Ambion). Briefly, 25 nM siRNA is mixed with 4 µl Lipofectamine RNAiMAX (Invitrogen) in a 400 µl volume. After 15 minutes of incubation 5.5×104 cells were added per well of a 6 well plate. Infections were done 48 h after siRNA treatment for an additional 24 h.
Statistical analyses
When not specified, results are expressed as the means of three independent experiments. The error bars represent the standard errors of the mean (SEM). The analyses were performed with Student's t test, and the statistical significance was established at P values of <0.05 or <0.001 (indicated by one or two asterisks respectively).
Supporting Information
Levels of poly-ADP ribosylated proteins and γH2AX as a function of multiplicity of infection and bacterial strains. (A) HeLa cells are infected with L. monocytogenes using the indicated multiplicity of infection (MOI) and harvested for immunobloting after 24 h of infection. (B) HeLa cells are infected with the indicated bacterial strains and harvested for immunobloting after 24 h of infection.
(PDF)
Effect of infection with L. monocytogenes on 53BP1 foci and the host cell cycle. (A) HeLa cells are infected with L. monocytogenes for 24 h. Immunofluorescence using an antibody specific to 53BP1 is shown. Arrows show cells with more than 3 foci of 53BP1 which are quantified in figure 1. Scale bar is 30 µm, insert is 2.5 times larger than box in original image. (B) Cell cycle quantification of HeLa cells infected (+) or not (−) with L. monocytogenes for the indicated times. In the table the numbers used to draw the graph are shown +/− SEM. (* p<0.05).
(PDF)
L. monocytogenes mediates its effect on the DDR from the outside of the cell. (A) Quantification of comet assays performed on HeLa cells infected with the indicated strain for 24 h. Cytochalasin D treatment was performed for 15 minutes prior to infection. Each bar in the histogram is an average of at least 30 nuclei from at least 3 independent experiments. Quantifications show the mean +/− SEM (** indicates p<0.01). (B) Immunofluorescence of γH2AX. Each box is 100 µm in length. (C) Immunoblot image representative of 3 independent experiments.
(JPG)
γH2AX and Mre11 levels in JEG3 cells. (A) and (B) JEG3 cells were infected with the indicated strain of Listeria for 24 h. Cell extracts were harvested for immunoblotting. Quantifications are normalized to actin and to the uninfected sample. All quantifications in graphs show the mean +/− SEM (** p<0.01).
(PDF)
γH2AX in vivo. Immunoblot of peritoneal content from C57Bl/6J mice infected with Listeria for 6 hours. n = 6 mice per condition. Quantifications in graphs show the mean +/− SEM (* p<0.05, ** p<0.01). Recovered colonie forming units for each conditions were as follows: WT, 46+/−29 and Δhly, 45+/−19.
(PDF)
Infection levels in siRNA treated cells. HeLa cells are transfected with siRNA (indicated above each image) and infected with L. monocytogenes for 24 h. Immunofluorescence is performed with anti-InlC antibody (green), which measures the level of infection and phalloidin (red), which stains for actin and shows the cell cytoskeleton as well as bacterial induced actin polymerization.
(JPG)
Acknowledgments
We would like to acknowledge Titia de Lange (Rockefeller University) in whose lab the first observations were made. We thank John Petrini (Memorial Sloan-Kettering Cancer Center) for cell lines.
Funding Statement
Work in the Cossart laboratory is funded by the Pasteur Institute, Inserm (U604), INRA (USC2020), the Fondation Louis Jeantet, the ERC (Advanced Grant #233348 MODELIST), the Fondation Le Roch Les Mousquetaires, as well as the ANRs (ERANET “LISTRESS”, and EPILIS). This study has received funding from the French Government's Investissement d'Avenir program, Laboratoire d'Excellence “Integrative Biology of Emerging Infectious Diseases” (grant n°ANR-10-LABX-62-IBEID). LD is funded by the European Research Council under the ERC starting grant agreement # 310917, the Institute Pasteur, the CNRS, the Fondation pour la Recherche Médicale, as well as the Ville de Paris. PC is an international senior research scholar of the Howard Hughes Medical institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ciccia A, Elledge SJ (2010) The DNA damage response: making it safe to play with knives. Mol Cell 40: 179–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schreiber V, Dantzer F, Ame JC, de Murcia G (2006) Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol 7: 517–528. [DOI] [PubMed] [Google Scholar]
- 3. Kolas NK, Chapman JR, Nakada S, Ylanko J, Chahwan R, et al. (2007) Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science 318: 1637–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bekker-Jensen S, Mailand N (2011) The ubiquitin- and SUMO-dependent signaling response to DNA double-strand breaks. FEBS Lett 585: 2914–2919. [DOI] [PubMed] [Google Scholar]
- 5. Zimmermann M, de Lange T (2013) 53BP1: pro choice in DNA repair. Trends Cell Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stracker TH, Petrini JH (2011) The MRE11 complex: starting from the ends. Nat Rev Mol Cell Biol 12: 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jackson SP, Bartek J (2009) The DNA-damage response in human biology and disease. Nature 461: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lilley CE, Schwartz RA, Weitzman MD (2007) Using or abusing: viruses and the cellular DNA damage response. Trends Microbiol 15: 119–126. [DOI] [PubMed] [Google Scholar]
- 9. Bergounioux J, Elisee R, Prunier AL, Donnadieu F, Sperandio B, et al. (2012) Calpain activation by the Shigella flexneri effector VirA regulates key steps in the formation and life of the bacterium's epithelial niche. Cell Host Microbe 11: 240–252. [DOI] [PubMed] [Google Scholar]
- 10. Chumduri C, Gurumurthy RK, Zadora PK, Mi Y, Meyer TF (2013) Chlamydia infection promotes host DNA damage and proliferation but impairs the DNA damage response. Cell Host Microbe 13: 746–758. [DOI] [PubMed] [Google Scholar]
- 11. Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, et al. (2010) Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci U S A 107: 11537–11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elsen S, Collin-Faure V, Gidrol X, Lemercier C (2013) The opportunistic pathogen Pseudomonas aeruginosa activates the DNA double-strand break signaling and repair pathway in infected cells. Cell Mol Life Sci 70: 4385–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toller IM, Neelsen KJ, Steger M, Hartung ML, Hottiger MO, et al. (2011) Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc Natl Acad Sci U S A 108: 14944–14949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vielfort K, Soderholm N, Weyler L, Vare D, Lofmark S, et al. (2013) Neisseria gonorrhoeae infection causes DNA damage and affects the expression of p21, p27 and p53 in non-tumor epithelial cells. J Cell Sci 126: 339–347. [DOI] [PubMed] [Google Scholar]
- 15. Nougayrede JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, et al. (2006) Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313: 848–851. [DOI] [PubMed] [Google Scholar]
- 16. Allerberger F, Wagner M (2010) Listeriosis: a resurgent foodborne infection. Clin Microbiol Infect 16: 16–23. [DOI] [PubMed] [Google Scholar]
- 17. Pizarro-Cerda J, Kuhbacher A, Cossart P (2012) Entry of Listeria monocytogenes in mammalian epithelial cells: an updated view. Cold Spring Harb Perspect Med 2: pii: a010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cossart P (2011) Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. Proc Natl Acad Sci U S A 108: 19484–19491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamon MA, Ribet D, Stavru F, Cossart P (2012) Listeriolysin O: the swiss army knife of Listeria. Trends Microbiology 20: 360–368. [DOI] [PubMed] [Google Scholar]
- 20. Samba-Louaka A, Stavru F, Cossart P (2012) Role for telomerase in Listeria monocytogenes infection. Infect Immun 80: 4257–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olive PL, Banath JP (2006) The comet assay: a method to measure DNA damage in individual cells. Nat Protoc 1: 23–29. [DOI] [PubMed] [Google Scholar]
- 22. Anderson L, Henderson C, Adachi Y (2001) Phosphorylation and rapid relocalization of 53BP1 to nuclear foci upon DNA damage. Mol Cell Biol 21: 1719–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Edwards MJ, Taylor AM (1980) Unusual levels of (ADP-ribose)n and DNA synthesis in ataxia telangiectasia cells following gamma-ray irradiation. Nature 287: 745–747. [DOI] [PubMed] [Google Scholar]
- 24. Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273: 5858–5868. [DOI] [PubMed] [Google Scholar]
- 25. Lukas C, Savic V, Bekker-Jensen S, Doil C, Neumann B, et al. (2011) 53BP1 nuclear bodies form around DNA lesions generated by mitotic transmission of chromosomes under replication stress. Nat Cell Biol 13: 243–253. [DOI] [PubMed] [Google Scholar]
- 26. Zhou BB, Elledge SJ (2000) The DNA damage response: putting checkpoints in perspective. Nature 408: 433–439. [DOI] [PubMed] [Google Scholar]
- 27. Gaillard JL, Berche P, Sansonetti P (1986) Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun 52: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Michel E, Reich KA, Favier R, Berche P, Cossart P (1990) Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol Microbiol 4: 2167–2178. [DOI] [PubMed] [Google Scholar]
- 29. Theunissen JW, Kaplan MI, Hunt PA, Williams BR, Ferguson DO, et al. (2003) Checkpoint failure and chromosomal instability without lymphomagenesis in Mre11(ATLD1/ATLD1) mice. Mol Cell 12: 1511–1523. [DOI] [PubMed] [Google Scholar]
- 30. Balestrino D, Hamon MA, Dortet L, Nahori MA, Pizarro-Cerda J, et al. (2010) Single-cell techniques using chromosomally tagged fluorescent bacteria to study Listeria monocytogenes infection processes. Appl Environ Microbiol 76: 3625–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuhbacher A, Gouin E, Cossart P, Pizarro-Cerda J (2013) Imaging InlC Secretion to Investigate Cellular Infection by the Bacterial Pathogen Listeria monocytogenes. J Vis Exp 19: e51043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leitao E, Costa AC, Brito C, Costa L, Pombinho R, et al. (2014) Listeria monocytogenes induces host DNA damage and delays the host cell cycle to promote infection. Cell Cycle 13: 928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lara-Tejero M, Galan JE (2000) A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290: 354–357. [DOI] [PubMed] [Google Scholar]
- 34. Song J, Bent AF (2014) Microbial pathogens trigger host DNA double-strand breaks whose abundance is reduced by plant defense responses. PLoS Pathog 10: e1004030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Janssens S, Tschopp J (2006) Signals from within: the DNA-damage-induced NF-kappaB response. Cell Death Differ 13: 773–784. [DOI] [PubMed] [Google Scholar]
- 36. Miyamoto S (2011) Nuclear initiated NF-kappaB signaling: NEMO and ATM take center stage. Cell Res 21: 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ermolaeva MA, Segref A, Dakhovnik A, Ou HL, Schneider JI, et al. (2013) DNA damage in germ cells induces an innate immune response that triggers systemic stress resistance. Nature 501: 416–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kondo T, Kobayashi J, Saitoh T, Maruyama K, Ishii KJ, et al. (2013) DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc Natl Acad Sci U S A 110: 2969–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stracker TH, Carson CT, Weitzman MD (2002) Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair complex. Nature 418: 348–352. [DOI] [PubMed] [Google Scholar]
- 40. Gaillard JL, Berche P, Mounier J, Richard S, Sansonetti P (1987) In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun 55: 2822–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ribet D, Hamon M, Gouin E, Nahori MA, Impens F, et al. (2010) Listeria monocytogenes impairs SUMOylation for efficient infection. Nature 464: 1192–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bischofberger M, Gonzalez MR, van der Goot FG (2009) Membrane injury by pore-forming proteins. Curr Opin Cell Biol 21: 589–595. [DOI] [PubMed] [Google Scholar]
- 43. Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA (2002) The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J Cell Biol 156: 1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Levels of poly-ADP ribosylated proteins and γH2AX as a function of multiplicity of infection and bacterial strains. (A) HeLa cells are infected with L. monocytogenes using the indicated multiplicity of infection (MOI) and harvested for immunobloting after 24 h of infection. (B) HeLa cells are infected with the indicated bacterial strains and harvested for immunobloting after 24 h of infection.
(PDF)
Effect of infection with L. monocytogenes on 53BP1 foci and the host cell cycle. (A) HeLa cells are infected with L. monocytogenes for 24 h. Immunofluorescence using an antibody specific to 53BP1 is shown. Arrows show cells with more than 3 foci of 53BP1 which are quantified in figure 1. Scale bar is 30 µm, insert is 2.5 times larger than box in original image. (B) Cell cycle quantification of HeLa cells infected (+) or not (−) with L. monocytogenes for the indicated times. In the table the numbers used to draw the graph are shown +/− SEM. (* p<0.05).
(PDF)
L. monocytogenes mediates its effect on the DDR from the outside of the cell. (A) Quantification of comet assays performed on HeLa cells infected with the indicated strain for 24 h. Cytochalasin D treatment was performed for 15 minutes prior to infection. Each bar in the histogram is an average of at least 30 nuclei from at least 3 independent experiments. Quantifications show the mean +/− SEM (** indicates p<0.01). (B) Immunofluorescence of γH2AX. Each box is 100 µm in length. (C) Immunoblot image representative of 3 independent experiments.
(JPG)
γH2AX and Mre11 levels in JEG3 cells. (A) and (B) JEG3 cells were infected with the indicated strain of Listeria for 24 h. Cell extracts were harvested for immunoblotting. Quantifications are normalized to actin and to the uninfected sample. All quantifications in graphs show the mean +/− SEM (** p<0.01).
(PDF)
γH2AX in vivo. Immunoblot of peritoneal content from C57Bl/6J mice infected with Listeria for 6 hours. n = 6 mice per condition. Quantifications in graphs show the mean +/− SEM (* p<0.05, ** p<0.01). Recovered colonie forming units for each conditions were as follows: WT, 46+/−29 and Δhly, 45+/−19.
(PDF)
Infection levels in siRNA treated cells. HeLa cells are transfected with siRNA (indicated above each image) and infected with L. monocytogenes for 24 h. Immunofluorescence is performed with anti-InlC antibody (green), which measures the level of infection and phalloidin (red), which stains for actin and shows the cell cytoskeleton as well as bacterial induced actin polymerization.
(JPG)