Abstract
Robotic surgery is an evolving technology that has been successfully applied to a number of surgical specialties, but its use in liver surgery has so far been limited. In this review article we discuss the challenges of minimally invasive liver surgery, the pros and cons of robotics, the evolution of medical robots, and the potentials in applying this technology to liver surgery. The current data in the literature are also presented.
Keywords: Robotics, hepatectomy, minimally invasive surgery
Overview of minimally invasive liver surgery
Liver resection, once regarded as an operation with prohibitively high mortality and morbidity, has now become a routine operation in expert hands. As laparoscopic techniques for other major abdominal operations such as splenectomy, colectomy, and fundoplication have matured, the interest in applying minimally invasive techniques to liver resection also developed. Technical developments such as more sophisticated energy devices and articulated laparoscopic staplers have enabled surgeons to tackle liver resection laparoscopically.
Some of the major technical challenges in liver surgery include the difficult access to the vena cava and major hepatic veins, precision required for dissection at the hilum, and propensity for the liver to bleed. These are made more difficult with laparoscopy due to the limitations in depth perception, restricted movement by rigid instruments and fixed fulcrum at the ports, unnatural ergonomics, and difficult suturing particularly in presence of hemorrhage. There is a steep learning curve making its practice outside high-volume centers difficult.
As a result, the uptake of minimally invasive hepatectomy has been slow and cautious. But with increasing experience, surgeons have gradually increased the difficulty and complexity of surgery, from staging and deroofing cysts initially, to resecting readily accessible parts of the liver such as the lateral sector and wedge resections from the anteroinferior segments, to major hepatectomies (1). However, certain scenarios are still considered prohibitively challenging, such the presence of extensive adhesions, resection of the caudate or posteriorly placed tumors, and bile duct resection and reconstruction. In 2008, a panel of 45 international experts on laparoscopic liver surgery gathered in Louisville, Kentucky to discuss the state of the art. There was a consensus that the best indications for laparoscopic resection are in patients with solitary lesions, 5 cm or less, located in segments 2 to 6 (2). Of note, the participants of this consensus conference recommended against routine laparoscopic resection of segments 7, 8, 1. This is due to difficulties in visualizing and working in these areas of the liver with straight laparoscopic instruments.
Single incision laparoscopic surgery (SILS) has been touted as the next stage in minimally invasive surgery with enhanced cosmesis and possibly recovery compared to conventional laparoscopic surgery. Small series of single-port laparoscopic hepatectomy have been published showing its feasibility (3,4). However, limited views, clashing of the surgeons’ hands, “sword-fighting” of instruments and inability to triangulate remain significant limitations. Attempts have been made to reduce collision by creating articulated instruments, however they may need to be used cross-handed, an unnatural and un-ergonomical operating position (5).
Pros of robotic surgery
Robotic assistance was developed in part to compensate for some of these limitations. The unfavorable ergonomics of rigid laparoscopic instruments are partially overcome by articulated ones to mimic the dexterity of the human hand. This allows tissue manipulation and suturing in small spaces, at angles not possible with rigid instruments, and facilitates curved transection lines for more complex resections. Tremor is filtered to allow precise suture placement useful for bleeding, and for creating biliary and enteric anastomoses. The surgeon’s motions are scaled so that small, precise movements are effected at the patient’s end. Operating via a console allows the surgeon to work sitting down in a comfortable position, and the 3-dimensional projection of images partially overcomes the lack of depth perception. The surgeon is in control of the camera, which is mounted on a stable platform, avoiding poor camera work due to a tired or inexperienced assistant. Laparoscopic retractors are also controlled by the surgeon and can be locked into position, further avoiding inappropriate or ineffective retraction.
One of the big theoretical advantages of robotic assistance in complex surgery is the shorter learning curve compared with conventional laparoscopy. Port placement is more forgiving as instruments are not completely restricted by a rigid fulcrum. Currently complex laparoscopic liver resections are generally performed by surgeons who are both expert hepatobiliary surgeons and expert laparoscopic surgeons. Open techniques are more readily translated to robotics and thus surgeons who are expert in hepatobiliary but not necessarily advanced laparoscopy may become proficient quickly.
An inherent imperfection in surgical training is the need for inexperienced trainees to operate on real patients while overcoming the learning curve of the procedure, thus exposing patients to a degree of risk. Robotic surgery lends itself well to computer based virtual reality training, similar to how pilots train on flight simulators. Such training systems have been developed and validated, such as the dV-Trainer (Mimic Technologies, Inc, Seattle, WA, USA), and the da Vinci Skills Simulator (Intuitive Surgical, Sunnyvale, CA, USA). Studies have found that structured training exercises improved simulator performance, although the translation to actual surgical performance has not been well studied (6,7).
Cons of robotic surgery
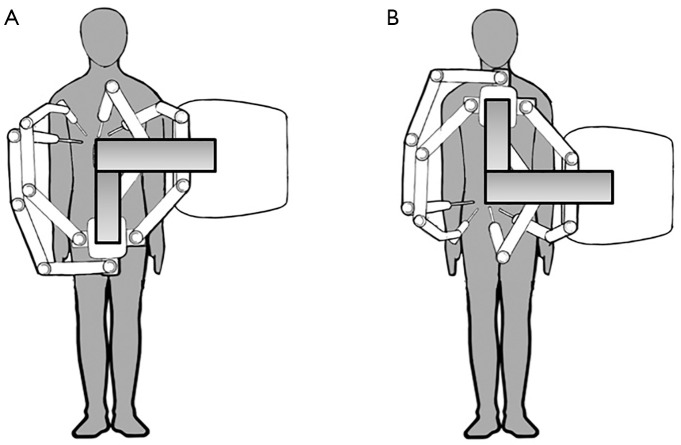
There are a number of disadvantages with robotic surgery. The current generation of robots has a large footprint and bulky arms, in addition to the size of the operating console. Spacious operating rooms are required, and dexterity is limited by collision of robotic arms (Figure 1). A skilled assistant is needed for suction, change of instruments, application of argon plasma, and stapling. There is no tactile feedback so the retraction pressure on the liver may be more difficult to gauge, and suture breakage may be more common, although experienced surgeons adjust to it by visually judging the tension on sutures (8). Changing patient position requires the robot to be undocked and redocked, adding time to the procedure and interrupting the flow of the operation. The separation of surgeon and patient potentially leading to delays in managing intraoperative complications and emergent conversion can be a source of anxiety for the operating team. Studies have generally shown that robotic surgery take longer time than their laparoscopic counterparts, in part due to time setting up and docking the robot, and time spent changing instruments (9-11). However, with increasing experience and proficiency this is likely to reduce.
Figure 1.
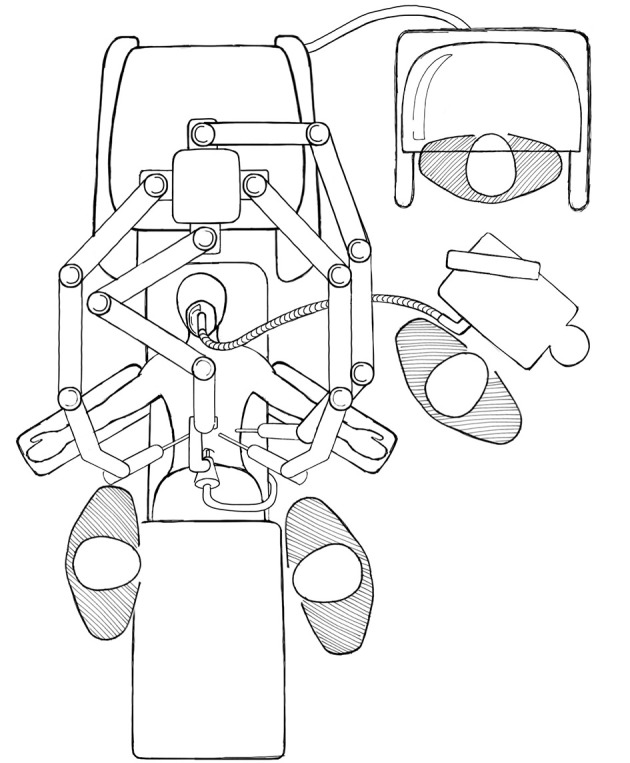
Typical room setup for a robotic hepatectomy.
The other recent advancements in the field that will improve accessibility of robotic surgery for liver resection include the range of new instrumentation that is now available, including robotic suction devices, sealers, and staplers. That has eliminated the routine need for accessory ports and necessity of a skilled bedside assistant. The launch of the Intuitive Xi robot has also allowed ease of multi-field surgery, and provides great ease in repositioning and re-docking (Figure 2). This robot is attached to a mobile boom that allows full 180 change in orientation of instruments without moving the patient, or table, or the robot.
Figure 2.
Flexibility for multi-field robotic surgery for the Intuitive Xi Robot. Without moving the patient, or table, or robotic tower, the working arms can be turned 180 degrees to swap from right upper quadrant work (A) to pelvic work (B). This will allow combined hepatectomy and rectal resections.
Robot malfunction in a variety of general surgical operations has been reported but appears to be relatively uncommon, and rarely lead to significant consequences. Approximately half of documented malfunction cases were attributed to robotic instruments and were resolved by replacing the instruments. Other sources of malfunction included optical systems, robotic arms, and the console. Agcaoglu et al. reported 10 cases of robotic malfunction in 223 cases (4.5%), with no adverse outcomes (12). Buchs et al. reported 18 cases of malfunction in 526 cases (3.4%), with one conversion to laparoscopy due to light source failure (13). Kim et al. reported 43 malfunctions in 1,797 cases of general and urological operations (2.4%), leading to conversion to open in one patient and to laparoscopy in two patients, all due to robotic arm malfunction (14).
One of the major disadvantages of robotic surgery is the high cost. The purchase of a da Vinci robot has been reported to be around US $1.5 million, with annual service cost of around $110,000, plus cost of disposable instruments (15). In a systematic review, Turchetti et al. analyzed 11 studies in the English literature which compared the cost of robotic surgery with the laparoscopic approach for various abdominal operations. The cost of the robotic approach was generally higher due to increased operating time (particularly set-up time) and instruments, while the costs of hospital stay were similar (16). However many studies did not include the purchase and maintenance costs which are significant, particularly in lower volume centers. None of the studies in this review evaluated the potential economic benefits of robotics.
Evolution of robots
Even though robotics in medicine have only recently caught the attention of the public, the technology is not new. One of the first applications of robotics to modern medicine was the Puma 560 in 1985, an industrial robotic arm used by Kwoh et al. to perform stereotactic brain biopsies. In the 1990s, a number of robots were developed, including the PROBOT at the Imperial College of London for transurethral resection of the prostate, the RoboDoc in the USA for femoral coring for hip replacement, and the ARTEMIS in Germany, a precursor to the modern master-slave manipulator system. Subsequently the robots used in modern surgery were developed by two initially competing companies (17,18).
One company was Computer Motion Inc based in California. They were contracted by NASA to develop the AESOP, a voice-activated camera control system that was compatible with standard 5 and 10 mm endoscopes. Subsequently the ZEUS robotic system was developed and became commercially available in 1998. The system consisted of a control console and table-mounted robotic arms incorporating the AESOP camera. In the 1980s, the Stanford Research Institute conducted research funded by the U.S. Army to develop telesurgery in the battlefield. Interest arose to extend its application to civilian surgery, and in 1995, Intuitive Surgical Inc was founded in California to further develop this technology. In 1999, Intuitive Surgical released the da Vinci robot in Europe, and in 2000 FDA approved its use in the USA. The da Vinci robot consists of three parts: a control console, a 3- or 4-armed surgical cart that is docked against the operating table, and a vision system. Central to the technology are a high-definition 3-dimensional viewer, a footswitch to allow the surgeon to swap between camera, retractors, and instrument control, and the Endowrist instruments, articulated instruments that mimic the seven degrees of motion of the human hand (18,19). In 2003, Intuitive Surgical and Computer Motion were merged. The ZEUS model was phased out and continued development was focused on the da Vinci system, now the only commercially available robotic operating system in the world. The second generation da Vinci S was released in 2006, and in 2009, the third generation Si model was released with dual-console capability and improved vision. In 2014, the fourth generation da Vinci Xi robot was approved by the FDA, with a redesigned surgical arm cart, smaller, longer arms, and new camera system to allow more flexibility in cart position and port placement (20).
Robotic liver surgery
The indications for robotic hepatectomy are similar to those for laparoscopic hepatectomy. Both benign and malignant tumors can be resected robotically. Patients must have the physiological reserve to tolerate general anesthesia and a prolonged pneumoperitoneum. General contraindications to laparoscopy such as uncorrected coagulopathy should be observed.
Laparoscopic hepatectomy for lesions in the superoposterior segments such as segment VII and VIII are particularly challenging due to their positions and the curved transection lines. As a result, laparoscopically lesions in these segments may be more commonly resected via a right hepatectomy, sacrificing a substantial volume of normal liver (21). Robotic hepatectomy helps overcome this problem and some authors have reported success (22). Thus the greatest theoretical advantage of robotic hepatectomy may lie in sectoral, segmental, or subsegmental resections in difficult-to-reach positions, where patients may be spared the large incisions and extensive mobilization required in an open approach. On the other hand, major hepatectomies for malignant conditions where large incisions are required for specimen extraction may be better served by a traditional open approach. Difficult hepatic resections such as those for hilar cholangiocarcinoma requiring caudate lobectomy and bile duct anastomoses are generally not performed laparoscopically but the use of a robot may allow these to be approached in a minimally invasive manner.
Image guided surgery is a developing field where pre-operative imaging is used to aid intraoperative maneuvers. There is considerable experience in applying this technology to neurosurgery and orthopedic surgery, but there is increasing interest in hepatobiliary surgery (23). Computer models built on CT or MRI are registered onto the real-life organs by matching landmarks, which then allows intra-operative navigation to be guided. The need for a computer console in robotic surgery makes it ideal for integration of image-guidance as an adjunct to intraoperative ultrasound, creating an augmented reality where images are superimposed onto the field of view which may help surgeons anticipate vascular structures and obtain adequate margins. This is particularly suited to accurate probe placement for ablation of small, difficult to localize tumors. Image-guidance technology in hepatobiliary surgery is still in its infancy with a number of technical challenges such as deformation correction, and further work is needed before augmented reality can be realized.
Robotic assistance can potentially overcome some of the limitations of SILS, for example by swapping the hand controls to eliminate cross-handed operating. Early experiences with robotic single-port hepatectomy have been reported (24), but the technology will likely have to be modified to adapt to the unique challenges of SILS, particularly the propensity for the robotic arms to clash with each other.
In theory, robotic surgery is an ideal platform for telesurgery. Indeed that was one of the driving forces behind the development of the master-slave robotic system. However, the latency between the surgeon’s movement and the observed effect due to transmission of data to and back from the patient is a significant limitation. Marescaux et al. reported the first transatlantic robot-assisted telesurgery in 2001, where a robotic cholecystectomy was performed by surgeons in New York, USA, and the patient in Strasbourg, France (25). The authors reported a total time delay of 155 ms; however this was performed on a dedicated high-speed terrestrial optical fibre network. Current satellite-based networks and public-internet based connections are inadequate for the widespread application of telesurgery over long distances, particularly for complex procedures with small margins of error (26).
Current data on robotic liver resection
Early experiences with using a robot in cholecystectomy were reported by Gagner et al. and Himpens et al. (27,28). Chan et al. reported their experience with 55 robotic HPB procedures, including 27 hepatectomies, 12 pancreatectomies (including 8 Whipple’s), and 16 biliary operations. Their experience with robotic liver resections for HCC was subsequently also published (29).
The largest series of robotic hepatectomy to date was a single-surgeon series published by Giulianotti et al. from the University of Illinois, with 70 patients (60% malignant, 40% benign). Major hepatectomy was performed in 27 patients, including 20 right hepatectomy, 5 left hepatectomy, and 2 right trisectionectomy. Of note, lesions in segments VII and VIII were only attempted if a right hepatectomy was performed. Three patients had a bile duct resection with biliary reconstruction, which is considered by most surgeons as a contraindication to laparoscopic hepatectomy because of the added complexity of a bile duct anastomosis. The median operative time was 270 min; for major resection it was 313 min, minor resection 198 min, and for biliary reconstruction 579 min. Major morbidity occurred in four patients, and there were no mortalities. Median surgical margin was 18 mm. No survival or oncological outcomes were reported (30).
Lai et al. from Hong Kong reported their experience of 42 patients with HCC and non-cirrhotic liver or Child-Pugh class A cirrhosis. The type of surgical operation included wedge resection in 10 patients, segmentectomy in 7, bisegmentectomy in 4, left lateral sectionectomy in 12, right hepatectomy in 7, and left hepatectomy in 3. Mean operating time was 229 min and median blood loss was 413 mL. Three patients developed complications, and there were no perioperative deaths. Mean hospital stay was 6.2 days. R0 resection was achieved in 40 patients (93%). Follow-up was relatively short at a median of 14 months. Six patients recurred within the liver and the 2-year overall survival was 94% (10).
The hepatopancreatobiliary group at Memorial Sloan Kettering Cancer Center has performed over 70 robotic hepatectomies (Kingham P and Fong Y, 2014, unpublished data). Twenty-three percent of patients have had previous abdominal surgery, including 5 re-operative hepatectomies. Median operating time was 164 minutes, estimated blood loss 100 mL, and four patients required conversion to open (6.1%). There were no mortalities and no re-operations for complications. The major conclusion derived from this series is: lesions in segment 1, 7, and 8 can be performed safely. Unlike the prior series where investigators saw the goal of robotic hepatectomy as trying to perform major hepatectomies, these investigators saw the robot as a means to accomplish resection of ill places minor resections. For major resections, it is unlikely that robotic resection will change much the usual outcomes of hospital stay or complications, since the extent of the hepatic resection and not the incision will be the greatest determinant of outcome. For minor resections of ill placed tumors, the incision usually dominates the clinical outcome. These are likely to be those resections where robotic surgery is likely to be proven superior. These are also those cases where expert opinion has recommended against laparoscopic surgery (2). Positioning of patient and the robot has now been improved to facilitate safe robotic resection of tumors in segments 7 and 8 (Figure 3).
Figure 3.
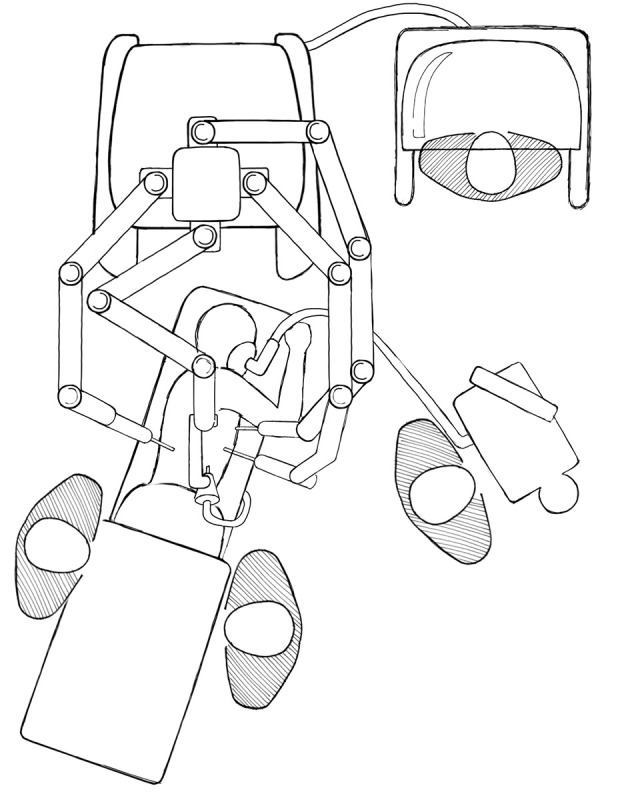
Positioning for robotic hepatectomy for lesions in segment 7 or 8.
Few studies have compared robotic to laparoscopic liver resections. Berber et al. found non-different operating time, blood loss, and resection margin (31). Ji et al. found that robotic resections may have longer operating times than laparoscopic or open resections but comparable blood loss and complications (9). Lai et al. found a similar association for patients undergoing minor hepatectomy (<3 segments) only (10). The largest matched comparison between laparoscopic and robotic hepatectomy was published by Tsung et al. and the University of Pittsburgh group (11). In this retrospective study, 57 patients undergoing robotic hepatectomy were matched with 114 patients undergoing laparoscopic hepatectomy on background liver disease, extent of resection, diagnosis, ASA class, age, BMI, and gender. They found that operating times were significantly longer in the robotic group for both major and minor hepatectomies. There were no significant differences in complication rates, length of stay, mortality, and negative margin rates. There was a trend towards less blood loss in the robotic major hepatectomies compared with laparoscopic major hepatectomies, which the authors attributed to superior inflow and outflow control, as well as magnified optics allowing better identification of vessels during parenchymal transection. Interestingly for the minor resections, the robotic approach was associated with a significantly higher blood loss than laparoscopic approach. The authors also noted that conversion to open rates were comparable, and that patients in the robotic group were more likely to have their surgery performed completely laparoscopically, without hand-assistance or a hybrid laparoscopic-open approach (93% vs. 49% for the laparoscopic group) (11).
Conclusions
Current data show that with good patient selection and meticulous technique, robotic hepatectomy is a safe and effective operation that is likely to stay. The goal of robotic assistance is to mimic the techniques of open surgery delivered through a minimally invasive approach. The theoretical advantages of robotic surgery are exciting but the evolution of the technology has been a slow process. In a review article in 2004, Lanfranco et al. outlined the pros and cons of robotic surgery at its relative infancy (18). Ten years later we find ourselves still facing similar limitations. Future directions may include reducing the size of the robot, modifying the arm mechanism to reduce clashing, multi-purpose instruments to reduce the need for frequent instrument exchanges and for an experienced assistant, development of hepatics to allow tactile feedback, and integration of image guidance. There is still skepticism outside the circle of robotic HPB enthusiasts regarding the wide applicability of this technology. For many centers the high cost will be a major deterrent. Despite all its promises, until the benefits are more clearly defined, robotic liver surgery will likely be practiced by a select group of surgeons at high-volume centers.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Cherqui D, Husson E, Hammoud R, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg 2000;232:753-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [DOI] [PubMed] [Google Scholar]
- 3.Shetty GS, You YK, Choi HJ, et al. Extending the limitations of liver surgery: outcomes of initial human experience in a high-volume center performing single-port laparoscopic liver resection for hepatocellular carcinoma. Surg Endosc 2012;26:1602-8. [DOI] [PubMed] [Google Scholar]
- 4.Aikawa M, Miyazawa M, Okamoto K, et al. Single-port laparoscopic hepatectomy: technique, safety, and feasibility in a clinical case series. Surg Endosc 2012;26:1696-701. [DOI] [PubMed] [Google Scholar]
- 5.Romanelli JR, Earle DB. Single-port laparoscopic surgery: an overview. Surg Endosc 2009;23:1419-27. [DOI] [PubMed] [Google Scholar]
- 6.Hung AJ, Patil MB, Zehnder P, et al. Concurrent and predictive validation of a novel robotic surgery simulator: a prospective, randomized study. J Urol 2012;187:630-7. [DOI] [PubMed] [Google Scholar]
- 7.Korets R, Mues AC, Graversen JA, et al. Validating the use of the Mimic dV-trainer for robotic surgery skill acquisition among urology residents. Urology 2011;78:1326-30. [DOI] [PubMed] [Google Scholar]
- 8.Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. [DOI] [PubMed] [Google Scholar]
- 9.Ji WB, Wang HG, Zhao ZM, et al. Robotic-assisted laparoscopic anatomic hepatectomy in China: initial experience. Ann Surg 2011;253:342-8. [DOI] [PubMed] [Google Scholar]
- 10.Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic liver resection for hepatocellular carcinoma: short-term outcome. Am J Surg 2013;205:697-702. [DOI] [PubMed] [Google Scholar]
- 11.Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 2014;259:549-55. [DOI] [PubMed] [Google Scholar]
- 12.Agcaoglu O, Aliyev S, Taskin HE, et al. Malfunction and failure of robotic systems during general surgical procedures. Surg Endosc 2012;26:3580-3. [DOI] [PubMed] [Google Scholar]
- 13.Buchs NC, Pugin F, Volontl F, et al. Reliability of robotic system during general surgical procedures in a university hospital. Am J Surg 2014;207:84-8. [DOI] [PubMed] [Google Scholar]
- 14.Kim WT, Ham WS, Jeong W, et al. Failure and malfunction of da Vinci Surgical systems during various robotic surgeries: experience from six departments at a single institute. Urology 2009;74:1234-7. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg PL, Merguerian PA, Bihrle W, 3rd, et al. A da Vinci robot system can make sense for a mature laparoscopic prostatectomy program. JSLS 2008;12:9-12. [PMC free article] [PubMed] [Google Scholar]
- 16.Turchetti G, Palla I, Pierotti F, et al. Economic evaluation of da Vinci-assisted robotic surgery: a systematic review. Surg Endosc 2012;26:598-606. [DOI] [PubMed] [Google Scholar]
- 17.Satava RM. Surgical robotics: the early chronicles: a personal historical perspective. Surg Laparosc Endosc Percutan Tech 2002;12:6-16. [DOI] [PubMed] [Google Scholar]
- 18.Lanfranco AR, Castellanos AE, Desai JP, et al. Robotic surgery: a current perspective. Ann Surg 2004;239:14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yates DR, Vaessen C, Roupret M. From Leonardo to da Vinci: the history of robot-assisted surgery in urology. BJU Int 2011;108:1708-13; discussion 1714. [DOI] [PubMed]
- 20.Intuitive Surgical. Da Vinci Surgical Si System. Available online: http://www.intuitivesurgical.com/products/davinci_surgical_system/davinci_surgical_system_si/
- 21.Cho JY, Han HS, Yoon YS, et al. Feasibility of laparoscopic liver resection for tumors located in the posterosuperior segments of the liver, with a special reference to overcoming current limitations on tumor location. Surgery 2008;144:32-8. [DOI] [PubMed] [Google Scholar]
- 22.Casciola L, Patriti A, Ceccarelli G, et al. Robot-assisted parenchymal-sparing liver surgery including lesions located in the posterosuperior segments. Surg Endosc 2011;25:3815-24. [DOI] [PubMed] [Google Scholar]
- 23.Kingham TP, Scherer MA, Neese BW, et al. Image-guided liver surgery: intraoperative projection of computed tomography images utilizing tracked ultrasound. HPB (Oxford) 2012;14:594-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kandil E, Noureldine SI, Saggi B, et al. Robotic liver resection: initial experience with three-arm robotic and single-port robotic technique. JSLS 2013;17:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marescaux J, Leroy J, Gagner M, et al. Transatlantic robot-assisted telesurgery. Nature 2001;413:379-80. [DOI] [PubMed] [Google Scholar]
- 26.Xu S, Perez M, Yang K, et al. Determination of the latency effects on surgical performance and the acceptable latency levels in telesurgery using the dV-Trainer(®) simulator. Surg Endosc 2014;28:2569-76. [DOI] [PubMed] [Google Scholar]
- 27.Gagner M, Begin E, Hurteau R, et al. Robotic interactive laparoscopic cholecystectomy. Lancet 1994;343:596-7. [PubMed] [Google Scholar]
- 28.Himpens J, Leman G, Cadiere GB. Telesurgical laparoscopic cholecystectomy. Surg Endosc 1998;12:1091. [DOI] [PubMed] [Google Scholar]
- 29.Chan OC, Tang CN, Lai EC, et al. Robotic hepatobiliary and pancreatic surgery: a cohort study. J Hepatobiliary Pancreat Sci 2011;18:471-80. [DOI] [PubMed] [Google Scholar]
- 30.Giulianotti PC, Coratti A, Sbrana F, et al. Robotic liver surgery: results for 70 resections. Surgery 2011;149:29-39. [DOI] [PubMed] [Google Scholar]
- 31.Berber E, Akyildiz HY, Aucejo F, et al. Robotic versus laparoscopic resection of liver tumours. HPB (Oxford) 2010;12:583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]