Abstract
Introduction:
Confirming abstinence during smoking cessation clinical trials is critical for determining treatment effectiveness. Several biological methods exist for verifying abstinence (e.g., exhaled carbon monoxide [CO], cotinine), and while cotinine provides a longer window of detection, it is not easily used in trials involving nicotine replacement therapy. The Society for Research on Nicotine and Tobacco’s Subcommittee on Biochemical Verification cite 8–10 parts per million (ppm) for CO as a viable cutoff to determine abstinence; however, recent literature suggests this cutoff is likely too high and may overestimate the efficacy of treatment.
Methods:
This study examined the relationship between CO and cotinine in a sample of 662 individuals participating in a smoking cessation clinical trial. A receiver operating characteristics curve was calculated to determine the percentage of false positives and false negatives at given CO levels when using cotinine as confirmation of abstinence. Differences were also examined across race and gender.
Results:
A CO cutoff of 3 ppm (97.1% correct classification) most accurately distinguished smokers from nonsmokers. This same cutoff was accurate for both racial and gender groups. The standard cutoffs of 8 ppm (14.0% misclassification of smokers as abstainers) and 10 ppm (20.6% misclassification of smokers as abstainers) produced very high false-negative rates and inaccurately identified a large part of the sample as being abstinent when their cotinine test identified them as still smoking.
Conclusions:
It is recommended that researchers and clinicians adopt a more stringent CO cutoff in the range of 3–4 ppm when complete abstinence from smoking is the goal.
INTRODUCTION
Confirming smoking abstinence during smoking cessation treatment is critical in determining treatment effectiveness. Although self-report has been one primary method of verifying smoking status, self-report may not be accurate in high-demand situations such as requiring smoking abstinence as a condition of hiring (McDaniel & Malone, 2012; Schmidt, Voigt, & Emanuel, 2013; Voigt, 2012), determining smoking status in pregnant women (e.g., Shipton et al., 2009) or adolescents (e.g., Mermelstein et al., 2002), or during smoking cessation treatment (Dolcini, Adler, & Ginsberg, 1996; Patrick et al., 1994; Velicer, Prochaska, Rossi, & Snow, 1992). Several biological methods for verifying abstinence are widely used and include measuring exhaled carbon monoxide (CO) as well as nicotine or cotinine concentrations in plasma, saliva, and urine (see Society for Research on Nicotine and Tobacco [SRNT] Subcommittee on Biochemical Verification, 2002). Although cotinine has a half-life of 10–30hr, allowing detection for several days to a week (Benowitz, 1999; SRNT Subcommittee on Biochemical Verification, 2002), cotinine cannot be easily used in trials that provide nicotine replacement therapy (NRT) as cotinine is a metabolite of nicotine (SRNT Subcommittee on Biochemical Verification, 2002). Conversely, CO provides a good estimate of smoking and is not affected by the use of NRT. Although the short half-life of CO (2–3hr) limits the range to detection to about 24hr (e.g., Perkins, Karelitz, & Jao, 2013), CO remains one of the least expensive and invasive methods for verifying smoking abstinence. CO has comparable detection rates to cotinine for short-term abstinence (Fritz et al., 2010), with cutoffs of 8–10 parts per million (ppm) commonly cited as indicative of recent smoking (SRNT Subcommittee on Biochemical Verification, 2002).
Recent studies have suggested that this standard cutoff for smoking (8–10 ppm) may be too high and thus, may classify light to moderate smokers as abstinent (Cropsey, Eldridge, Weaver, Villalobos, & Stitzer, 2006; Javors, Hatch, & Lamb, 2005; Perkins et al., 2013; Raiff, Faix, Turturici, & Dallery, 2010). This is particularly relevant as many individuals receiving smoking cessation treatment reduce, but do not completely quit smoking during treatment (e.g., Hatsukami et al., 2004). For example, previously heavy smokers may significantly reduce the number of cigarettes smoked per day, but still remain light smokers with a CO below the 8–10 ppm cutoff (Centers for Disease Control and Prevention [CDC], 2011). It is critical to accurately identify individuals who continue to smoke, despite treatment, as this may prompt the use of more intensive and effective treatment strategies to help smokers attain full abstinence.
Several studies have established that cutoffs of 3–5 ppm provide better sensitivity and specificity for differentiating between current smokers and abstainers or nonsmokers (Cropsey et al., 2006; Javors et al., 2005; Perkins et al., 2013; Raiff et al., 2010). In fact, this lowered cutoff appears to be valid when comparing light smokers (<10 cigarettes/day [CPD]) to heavier smokers, as well as Black smokers to White smokers (Cropsey et al., 2006). Further, use of a cutoff of 4 ppm or lower provided optimal detection of 24hr overnight abstinence compared with higher values of 8–10 ppm (Perkins et al., 2013). Exhalation speed also influences CO values, with faster exhalation speeds resulting in lower CO values such that a cutoff of 3 ppm is optimal during fast exhalation or when speed of exhalation is not monitored and a cutoff of 4 ppm is optimal during slow exhalation (Raiff et al., 2010). Despite the consistency of these recent findings, the research community as well as treatment providers appear reluctant to adopt more stringent CO cutoffs. One possible reason for the reluctance to adopt lower CO thresholds is that these lower cutoffs would reduce the reported quit rates of smokers as well as the previously established associations between cessation (as indicated by higher cutoffs of 10 ppm) and known predictors of cessation (Brose, Tombor, Shahab, & West, 2013). However, the associations found when using the lower cutoff of 3 ppm were similar to the 10 ppm cutoff and remained statistically significant in predicting smoking cessation (Brose et al., 2013).
This study was part of a large clinical trial to compare four sessions of counseling to brief physician advice among participants who received 12 weeks of bupropion treatment enrolled in a clinical trial for smoking cessation. The objective of this research was to determine the optimal CO cutoff to use for abstinence with urinary cotinine used as the gold standard for verification of smoking abstinence (SRNT Subcommittee on Biochemical Verification, 2002). Few studies have directly compared CO to urine cotinine in determining CO cutoffs (e.g., Marrone, Paulpillai, Evans, Singleton, & Heishman, 2010) even though CO may be one of the few ways to determine abstinence when less invasive procedures are needed or when NRT is used for smoking cessation treatment. Furthermore, although previous research has demonstrated that the same cutoff can be used for light and heavy smokers, as well as for White and Black smokers (Cropsey et al., 2006), no studies to date have compared male and female smokers to determine if the cutoff remains the same between these groups. Thus, this project sought to verify CO cutoffs in relation to urinary cotinine and extend these findings to male and female smokers.
METHODS
Participants
A total of 673 participants provided informed consent and completed baseline measures upon entry into the study. Eleven participants had one or more key variables missing and were excluded. The final sample of 662 participants were at least 19 years or older (the age of legal adulthood in Alabama), reported smoking at least 5 CPD, were under some form of criminal justice supervision in the community (e.g., probation, parole, drug court, community corrections, and so forth), were not currently incarcerated in prison or jail, living in an environment that allowed smoking, reported wanting to quit smoking, and willing to take pharmacotherapy and receive four sessions of behavioral counseling. Because bupropion was the pharmacotherapy provided in this trial, participants were excluded from the study if they had a demonstrated history of bipolar disorder, seizure disorder, eating disorder, current suicidal ideation, or a suicide attempt in the past six months, current pregnancy or lactating, non-English speaking, cognitive impairment such that they were unable to provide informed consent, or were medically unstable (e.g., had an elevation of liver function tests 3 times upper limit of normal). Participants with stable psychiatric or medical disorders were included in the study. In addition, all participants were confirmed to be smoking by verifying a cotinine level more than 200ng/ml and a CO reading more than 3 ppm. All participants received 12 weeks of bupropion treatment at standard smoking cessation dosage (150-mg bupropion sustained release twice daily) and were instructed to take the medication consistently and attempt to quit in the second week. Participants were randomized to either four 20–30-min sessions of standard behavioral smoking cessation counseling or brief physician advice to quit. For the purposes of this study, all participants who completed informed consent and baseline measures were analyzed even though not all participants (n = 173; 25% excluded) went on to receive the study intervention.
Procedures
All participants were recruited via flyers placed at a community corrections supervision site. Participants were prescreened for study inclusion/exclusion criteria in person or over the phone and scheduled for a baseline study visit onsite at offices located at the community corrections site if initially eligible. At the baseline study visit, participants gave written informed consent, provided urine to test for cotinine and illicit substances, and expired air to measure for CO. For CO testing, participants were instructed to take a deep breath, hold for 20 s, and then exhale slowly. Samples were tested using the Vitalograph Breath CO, which provides a measurement of CO in parts per million. Cotinine was assessed using the qualitative COT One-Step Cotinine Device (urine; Reditest®; Redwood Toxicology Laboratory), which uses a lateral flow chromatographic immunoassay to detect cotinine levels more than 200ng/ml in human urine. This cutoff is sensitive enough to detect daily smoking, but high enough to not detect secondhand or passive smoking (Yeh, Levasseur, & Kaiserman, 2011). Demographic questionnaires assessed race, age, gender, and education level. Smoking history was assessed including age of daily smoking, average CPD, cigarettes smoked that day, time since last cigarette, cigarettes smoked yesterday, difficulty of quitting smoking in the past, type of cigarette typically smoked (e.g., menthol, light, regular), and use of other tobacco products. Participants received medication for 12 weeks and were randomized to receive both counseling and brief physician advice to quit or to receive only brief physician advice to quit. Both groups were scheduled to return for visits at weeks 2, 3, 4, 8, and 12. At each subsequent visit, participants completed smoking measures, exhaled air for CO measurement, and provided urine for cotinine testing. This study was approved by the University of Alabama at Birmingham Institutional Review Board, and this trial was registered with ClinicalTrials.gov (NCT01257490).
Data Analyses
In order to achieve independence among observations and assure that each participant was included in the analysis only once, data for assessment points representing a negative cotinine status were selected using the first observation of the negative cotinine observation (e.g., if participant obtained a negative outcome for cotinine on weeks 3 and 4, data for week 3 assessment were chosen). In other words, because there were more observations that indicated a positive cotinine test relative to a negative cotinine test, the first timepoint that indicated a negative cotinine for each person was chosen, if applicable. All other assessment points for those individual cases were then removed from the data set. For individuals who did not achieve a negative cotinine status for the duration of the study, the first positive cotinine value was used.
In order to determine the most appropriate cutoff level of CO indicative of abstinence as verified by urinary cotinine, a receiver operating characteristics (ROC) curve was calculated. In a ROC analysis, the “gold standard” assessment method (in this case, urine cotinine) for the disorder/disease in question dictates “actual” group membership and is the metric for comparison (Streiner & Cairney, 2007). Values of CO are plotted against the classification groups determined by urinary cotinine (negative or positive cotinine) to determine the optimal cutoff for CO measurements. Thus, ROC analyses examine sensitivity and specificity across the entire range of cutoff values, which are plotted against each other on a scatterplot graph to produce the ROC curve.
Calculating the area under the ROC curve (area under the curve [AUC]) provides an index of the overall discrimination performance of the test and can be considered a global estimate of diagnostic accuracy (Streiner & Norman, 2003), which can range from .5 (no discrimination) to 1 (perfect discrimination). Generally, accuracy of tests with AUC values falling between .5 and .7 are considered low, .7 and .9 moderate, and above .9 high (Fischer, Bachmann, & Jaeschke, 2003). The AUC can be interpreted as the percentage of time that a positive case will have a higher score on the test than a negative case (Hanley & McNeil, 1982). Points (representing scores) plotted closer to the upper left corner of the ROC curve are associated with the highest accuracy (i.e., optimal balance between sensitivity and specificity; Tripepi, Jager, Dekker, & Zoccali, 2009).
This study used a ROC approach to determine the optimal cutoff point for CO as a biomarker to identify smoking status. Additionally, to assure equivalent functioning across gender and race, independent ROCs (predicting cotinine status using CO) were calculated and compared for men and women and Black and White smokers. Sensitivity, specificity, positive predictive value, negative predictive value, and efficiency were also calculated for each subgroup. The primary analyses were conducted using the ROC5 program (available at www.stanford.edu/~yesavage/ROC.html), which is based on Kraemer’s (2002) analytic approach. Preliminary and secondary analyses (i.e., data set preparation, regression-based group comparisons, and so forth) were conducted using SPSS version 21.
RESULTS
Sample Characteristics
The sample was comprised of 662 participants (32.6% female and 67.4% male); 34.5% self-identified as White/non-Hispanic, 63.0% as Black, and 2.5% belonged to other racial ethnic categories. The average age was 37.11 (SD = 11.1) years. Approximately, one third of the sample (31.8%) had less than a high school education, 35.6% had a high school diploma or General Educational Development, 21.7% had some college, 3.8% had a Bachelor’s degree, and 1.8% completed some postgraduate work.
The prevalence rate of quitting smoking in the current sample was 5.6% with 625 participants obtaining a positive cotinine outcome and 37 obtaining a negative cotinine outcome. CO values were calculated for participants with a positive (M = 16.5±10.26; range = 0–84) and negative (M = 2.6±3.51; range = 0–18) cotinine status (see Figure 1). Among participants who tested positive for smoking (i.e., positive cotinine), the average number of cigarettes smoked per day was 15.0 (SD = 9.2). The average total score on the Fagerström Test for Cigarette Dependence (Fagerström, 2012) at baseline was 5.3 (SD = 2.0).
Figure 1.
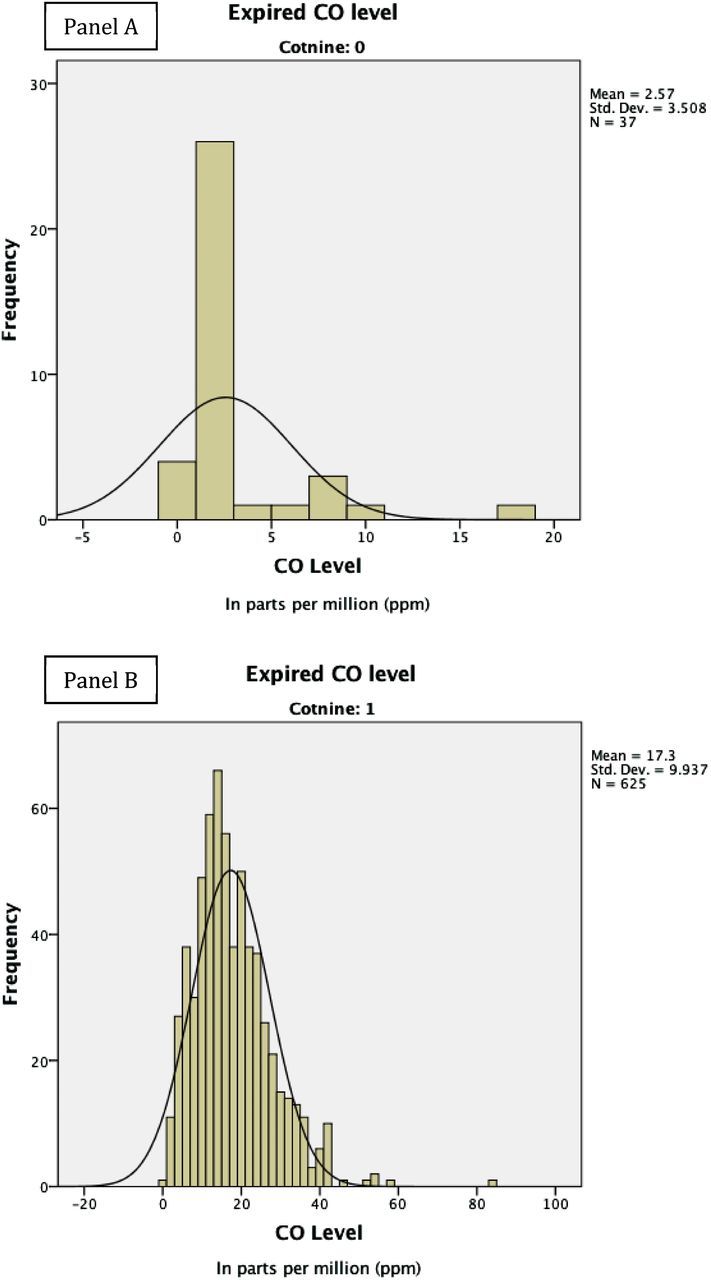
Histograms for carbon monoxide (CO) distribution of cotinine negative (Panel A) and positive (Panel B) smokers (N = 662).
ROC Analysis Predicting Cotinine Status Using CO
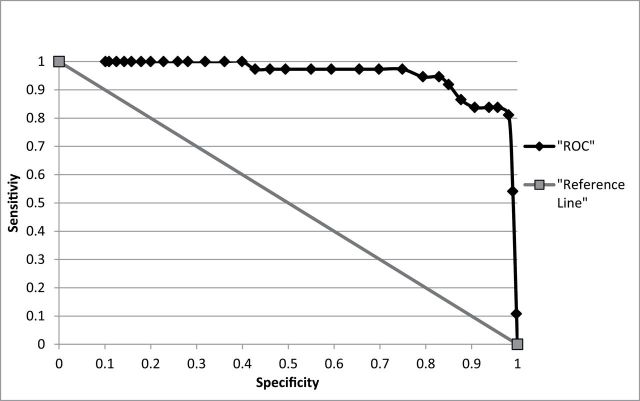
When CO was used to predict cotinine status, the AUC was .956 (95% CI = .922, .991), and the optimal cut-point for CO was found to be 3 ppm (see Figure 2). Table 1 shows sensitivity, specificity, positive predictive value, negative predictive value, and efficiency for different CO levels in the current sample. A CO cutoff of 3 ppm demonstrated the highest value for efficiency, accurately classifying 97.1% of cases (i.e., only misclassifying 2.9%). In order to demonstrate the practical significance of ROC results, Table 1 also includes information concerning the number participants who were misclassified at each CO cutoff. Specifically, the value in the false-negative column represents the number of smokers who would be mislabeled as “abstinent,” while the value in the false-positive column represents the number of abstinent individuals who would be mislabeled as “smokers” (using the corresponding CO level as a cutoff). For example, if the standard cutoff of 8 ppm was used, this would misclassify 94 (14.2%) participants from the sample as abstinent when they were actually smoking, while only misclassifying 3 (0.4%) participants who were abstinent as smoking. If a cutoff of 10 ppm was used, this would misclassify 129 (20.6%) participants as abstinent, while misclassifying 2 (0.3%) participants who were abstinent as smoking.
Figure 2.
Receiver operating characteristics (ROC) curve for smokers versus nonsmokers (N = 662).
Table 1.
ROC Analysis: Sensitivity and Specificity of Expired CO Output (N = 662)
| CO cutoff | Sens | Spec | PPV | NPV | Eff | False − (n)a | False + (n)a |
|---|---|---|---|---|---|---|---|
| 0 | 1 | 0 | .94 | .99 | .944 | 0 | 37 |
| 1 | .998 | .108 | .95 | .8 | .949 | 1 | 33 |
| 2 | .99 | .541 | .97 | .77 | .965 | 6 | 17 |
| 3 | .981 | .811 | .99 | .71 | .971 | 12 | 7 |
| 4 | .957 | .838 | .99 | .53 | .950 | 27 | 6 |
| 5 | .938 | .838 | .99 | .44 | .932 | 39 | 6 |
| 6 | .907 | .838 | .99 | .35 | .903 | 58 | 6 |
| 7 | .877 | .865 | .99 | .29 | .876 | 77 | 5 |
| 8 | .85 | .919 | .99 | .27 | .854 | 94 | 3 |
| 9 | .829 | .946 | 1 | .25 | .836 | 107 | 2 |
| 10 | .794 | .946 | 1 | .21 | .802 | 129 | 2 |
| 11 | .749 | .973 | 1 | .19 | .762 | 157 | 1 |
| 12 | .698 | .973 | 1 | .16 | .713 | 189 | 1 |
| 13 | .655 | .973 | 1 | .14 | .673 | 216 | 1 |
| 14 | .594 | .973 | 1 | .12 | .615 | 254 | 1 |
| 15 | .550 | .973 | 1 | .11 | .573 | 281 | 1 |
Note. Bold and italic values correspond to ideal cut-off value. ROC = receiver operating characteristics; CO cutoff = carbon monoxide (CO) level in ppm used as cutoff to identify smokers (i.e., positive cotinine outcome); sens = sensitivity (i.e., proportion of smokers accurately identified; positive outcome); spec = specificity (i.e., proportion of nonsmokers accurately identified; negative outcome); PPV = positive predictive value (i.e., the percent of participants identified as smokers who are actually smokers); NPV = negative predictive value (i.e., the percent of participants identified as nonsmokers who are actually nonsmokers); eff = efficiency (i.e., weighted average of sensitivity and specificity taking into account disease prevalence); false − = false negatives (i.e., smokers mislabeled as “nonsmokers”); false + = false positives (i.e., nonsmokers mislabeled as “smokers”).
aActual smoking status: 37 nonsmokers (i.e., negative cotinine) and 625 smokers (i.e., positive cotinine).
Subgroup Comparisons
Participants’ data were divided into subgroups based on gender and race in order to examine possible differences on key smoking outcomes and assure equivalent functioning of CO’s predicative ability across these groups (results are reported in Table 2). Significant differences were found between White and Black smokers on expired CO level, with White smokers obtaining significantly higher expired CO levels (CO = 18.95±9.71) than Black smokers (CO = 16.39±9.99; p < .002). Additionally, White smokers reporting smoking significantly more CPD (18.62±7.89 cigarettes) than Black smokers (12.18±7.32 cigarettes; p < .001). No significant differences were found between males and females for expired CO or CPD (see Table 2).
Table 2.
Descriptive Statistics and ROC Outcome for Gender and Race
| Sample | CO level, M (SD) | CPD, M (SD) | AUC (95% CI) | ROC outcome (CO = 3 ppm) | ||||
|---|---|---|---|---|---|---|---|---|
| Cot + | Cot − | Cot + | Cot − | Sens | Spec | Eff | ||
| Total | ||||||||
| N = 662 | 16.46 | 2.57 | 15.01 | 1.64 | .956 | .981 | .811 | .971 |
| P = .944 | (10.26) | (3.51) | (9.22) | (3.95) | (.922–.991) | |||
| Gender | ||||||||
| Male | ||||||||
| n = 446 | 16.69 | 2.23 | 15.06 | 1.90 | .970 | .988 | .864 | .982 |
| P = .951 | (9.36) | (2.58) | (9.65) | (4.87) | (.943–.998) | |||
| Female | ||||||||
| n = 216 | 18.53 | 3.07 | 14.91 | 1.27 | .937 | .965 | .733 | .949 |
| P = .931 | (10.99) | (4.60) | (8.29) | (2.22) | (.869–1.000) | |||
| Race | ||||||||
| Black | ||||||||
| n = 418 | 16.39* | 2.83 | 12.18** | 2.30 | .943 | .99 | .783 | .969 |
| P = .945 | (9.99) | (3.96) | (7.32) | (4.743) | (.887–.999) | |||
| White | ||||||||
| n = 229 | 18.95* | 1.67 | 18.62** | 0.50 | .985 | .98 | .917 | .978 |
| P = .948 | (9.71) | (1.50) | (7.89) | (1.45) | (.985–1.000) | |||
Note. ROC = receiver operating characteristics; CO level = carbon monoxide level in ppm; COT= cotinine; CPD = cigarettes smoked per day; AUC = area under the curve; CI = confidence interval; sens = sensitivity (i.e., proportion of smokers accurately identified; positive outcome); spec = specificity (i.e., proportion of nonsmokers accurately identified; negative outcome); eff = efficiency (i.e., weighted average of sensitivity and specificity taking into account disease prevalence); P = prevalence of smoking within sample.
*Significantly different at p < .002.
**Significantly different at p < .001.
Results of the independent ROC analyses within subgroups identified the optimal cutoff as 3 ppm for men as well as women with similar AUC values produced (.970 vs. .937). Outcome for the separate ROCs performed on Black and White participants’ data mirrored these results also demonstrating the optimal CO cutoff as 3 ppm as well as similar AUC values for Black and White participants (.943 vs. .985).
DISCUSSION
The findings from this study confirmed recent findings (Cropsey et al., 2006; Perkins et al., 2013; Raiff et al., 2010) suggesting the need to adopt a lower CO cutoff (i.e., 3 ppm) than what is typically used in clinical trials to verify smoking abstinence. This lower cutoff was found to be true both in Black and White smokers as well as women and men. This is a significant issue as continuing to use higher CO cutoffs results in misclassifying light or intermittent smokers as abstinent, inflating quit rates in treatment studies. For example, using our own data in which cotinine-verified abstinence represents the true abstinence status of participant, using a CO of 8 ppm inflates quit rates from 5.6% to 14.4%, while using a 10 ppm cutoff inflates the quit rate to 19.2%, almost four times higher than cotinine-verified abstinence. The dramatically different abstinence rates between a low cutoff such as 3 ppm and a high cutoff of 10 ppm in a clinical trial such as this study demonstrates the inherent problem with using such a high cutoff and calls into question the true abstinence levels of smokers in trials that utilize higher cutoff points.
Importantly, this study also demonstrated that a cutoff of 3 ppm is optimal across a range of demographic groups, including Black and White smokers, as well as both men and women. This is important as these populations have established differences in smoking characteristics that theoretically could cause differences in CO levels. For example, Blacks tend to smoke fewer CPD and use menthol cigarettes (e.g., Cropsey et al., 2009; Jones, Apelberg, Tellez-Plaza, Samet, & Navas-Acien, 2013), both of which could possibly impact CO readings. Women also tend to smoke fewer cigarettes relative to men (CDC, 2011). However, this study confirms the same CO cutoff of 3 ppm is effective at determining abstinence across these subgroups.
This study does have some limitations. In particular, determining optimal cutoffs through ROC analysis is dependent upon the prevalence of the condition (i.e., reference criterion) in the population under investigation (e.g., prevalence of smokers; Kraemer, 1988; Kraemer & Kupfer, 2006). Thus, if applied within a population with a smoking prevalence rate that differed from that of the current sample, a CO cutoff of 3 ppm may not be as accurate. However, a CO cutoff of 3 ppm would likely be accurate in clinical trials recruiting all smokers given the inherency of high smoking prevalence rates to study inclusion. Additionally, outcomes produced within a defined sample do not necessarily generalize to populations that differ in respect to important variables that affect the construct of interest (i.e., CO levels; Linden, 2006). Although the construct of interest in this investigation is less susceptible to the potential for bias than those using more subjective assessment strategies (e.g., self-report), exposure to environmental sources of CO can alter CO levels making application of findings under different conditions potentially inappropriate (Murphy et al., 1987). Variables that have the potential to alter CO levels include inhalation of secondhand smoke and air pollution (Jaakkola & Jaakkola, 1997; Leaderer, 1990); however, empirical investigations to this point have yet to accrue sufficient evidence to conclude that these environmental variables significantly impact CO levels (Joseph et al., 2005). In addition, the cotinine assay used in this study was not particularly sensitive (200ng/ml) but was chosen to avoid misclassifying individuals who may be passively exposed to cigarette smoke as current smokers. Finally, although our previous study (Cropsey et al., 2006) confirmed a CO cutoff of 3 ppm for lighter (<10 CPD) and heavier smokers, we were unable to perform this same analysis in this sample due to the relatively few light smokers who quit smoking in this study.
Our current findings would likely generalize to other outpatient clinical populations of smokers even though this study specifically recruited individuals in the criminal justice system supervised in the community. Although individuals under criminal justice supervision in the community have reporting requirements for urine drug monitoring and may have additional court appearances, they otherwise work and live in the community with similar restrictions to their smoking as encountered by the average citizen. These results, however, may not generalize to incarcerated samples of smokers with higher rates of environmental tobacco smoke exposure (Callinan, Clarke, Doherty, & Kelleher, 2010; Hammond & Emmons, 2004), although a previous study with incarcerated women found the same 3 ppm cutoff value (Cropsey et al., 2006).
Strengths of this study include an adequate sample to compare racial and gender groups on optimal CO cutoffs. This is important as few studies have examined optimal CO cutoff in different subpopulations of smokers (Cropsey et al., 2006). In addition, this study is one of the first to compare CO to cotinine in urine. This is significant as cotinine is a primary method for biochemical verification of smoking abstinence, particularly in situations where a longer timeframe for determining abstinence is warranted, as may be the case in determining cessation in a clinical trial. In most clinical trials, frequency of follow-up is rarely more than once per week, and cotinine can be detected for up to 1 week in heavy smokers (SRNT Subcommittee on Biochemical Verification, 2002). Thus, although using a lower CO cutoff may inadvertently classify someone who has a slip as a current smoker, cotinine would also likely provide a similar result. Therefore, using a lower cutoff such as 3 ppm may be optimal during clinical trials in which abstinence is the goal and where “slips” are counted as smoking. However, lower cutoffs may not be desirable in situations in which smoking reduction is the goal or where a “slip” is acceptable. Overall, our results highlight the significance of using a more stringent CO cutoff in the range of 3–4 ppm for determining abstinence when complete abstinence is the goal. Using a high cutoff potentially quadruples quit rates, dramatically overestimating the effectiveness of our current cessation treatments.
FUNDING
This research was supported by the National Cancer Institute at the National Institutes of Health (R01CA141663 to KLC).
DECLARATION OF INTERESTS
None declared.
ACKNOWLEDGMENTS
The content is solely the responsibility of the authors and does not represent the official views of the National Cancer Institute or the National Institutes of Health.
REFERENCES
- Benowitz N. L. (1999). Biomarkers of environmental tobacco smoke exposure. Environmental Health Perspectives, 107, 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose L. S., Tombor I., Shahab L., West R. (2013). The effect of reducing the threshold for carbon monoxide validation of smoking abstinence—evidence from the English Stop Smoking Services. Addictive Behaviors, 38, 2528–2531. 10.1016/j.addbeh.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Callinan J. E., Clarke A., Doherty K., Kelleher C. (2010). Legislative smoking bans for reducing secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database of Systematic Reviews, 4, CD005992. 10.1002/14651858.CD005992.pub2 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2011). Vital signs: Current cigarette smoking among adults aged ≥ 18 years—United States, 2005–2010. Morbidity and Mortality Weekly Report, 60, 1207–1212. Retrieved from www.cdc.gov/mmwr/preview/mmwrhtml/mm6035a5.htm?s_cid=%20mm6035a5.htm_w [PubMed] [Google Scholar]
- Cropsey K. L., Eldridge G. D., Weaver M. F., Villalobos G. C., Stitzer M. L. (2006). Expired carbon monoxide levels in self-reported smokers and nonsmokers in prison. Nicotine & Tobacco Research, 8, 653–659. 10.1080/14622200600789684 [DOI] [PubMed] [Google Scholar]
- Cropsey K. L., Weaver M. F., Eldridge G. D., Villalobos G. C., Best A. M., Stitzer M. L. (2009). Differential success rates in racial groups: Results of a clinical trial of smoking cessation among female prisoners. Nicotine & Tobacco Research, 11, 690–697. 10.1093/ntr/ntp051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcini M. M., Adler N. E., Ginsberg D. (1996). Factors influencing agreement between self-reports and biological measures of smoking among adolescents. Journal of Research on Adolescence, 6, 515–542. [Google Scholar]
- Fagerström K. (2012). Determinants of tobacco use and renaming the FTND to the Fagerström Test for Cigarette Dependence. Nicotine & Tobacco Research, 14, 75–78. 10.1093/ntr/ntr137 [DOI] [PubMed] [Google Scholar]
- Fischer J. E., Bachmann L. M., Jaeschke R. (2003). A readers’ guide to the interpretation of diagnostic test properties: Clinical example of sepsis. Intensive Care Medicine, 29, 1043–1051. 10.1007/s00134-003-1761-8 [DOI] [PubMed] [Google Scholar]
- Fritz M., Wallner R., Grohs U., Kemmler G., Saria A., Zernig G. (2010). Comparable sensitivities of urine cotinine and breath carbon monoxide at follow-up time points of three months or more in a smoking cessation trial. Pharmacology, 85, 234–240. 10.1159/000280435 [DOI] [PubMed] [Google Scholar]
- Hammond S. K., Emmons K. M. (2004). Inmate exposure to secondhand smoke in correctional facilities and the impact of smoking restrictions. Journal of Exposure Science and Environmental Epidemiology, 15, 205–211. [DOI] [PubMed] [Google Scholar]
- Hanley J. A., McNeil B. J. (1982). The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology, 143, 29–36. [DOI] [PubMed] [Google Scholar]
- Hatsukami D. K., Rennard S., Patel M. K., Kotlyar M., Malcolm R., Nides M. A., … Jamerson B. D. (2004). Effects of sustained-release bupropion among persons interested in reducing but not quitting smoking. The American Journal of Medicine, 116, 151–157. 10.1016/j.amjmed.2003.07.018 [DOI] [PubMed] [Google Scholar]
- Jaakkola M. S., Jaakkola J. J. (1997). Assessment of exposure to environmental tobacco smoke. European Respiratory Journal, 10, 2384–2397. 10.1183/09031936.97.10102384 [DOI] [PubMed] [Google Scholar]
- Javors M. A., Hatch J. P., Lamb R. J. (2005). Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction, 100, 159–167. 10.1111/j.1360-0443.2004.00957.x [DOI] [PubMed] [Google Scholar]
- Jones M. R., Apelberg B. J., Tellez-Plaza M., Samet J. M., Navas-Acien A. (2013). Menthol cigarettes, race/ethnicity, and biomarkers of tobacco use in U.S. adults: The 1999–2010 National Health and Nutrition Examination Survey (NHANES). Cancer Epidemiology, Biomarkers, & Prevention, 22, 224–232. 10.1158/1055–9965.EPI-12–0912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A. M., Hecht S. S., Murphy S. E., Carmella S. G., Le C. T., Zhang Y., … Hatsukami D. K. (2005). Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiology Biomarkers & Prevention, 14, 2963–2968. 10.1158/1055–9965.EPI-04-0768 [DOI] [PubMed] [Google Scholar]
- Kraemer H. C. (1988). Assessment of 2 x 2 associations: Generalizations of signal-detection methodology. American Statistician, 42, 37–49. [Google Scholar]
- Kraemer H. C. (2002. ). Evaluating medical tests: Objective and quantitative guidelines. Newbury Park, CA: Sage Publications. [Google Scholar]
- Kraemer H. C., Kupfer D. J. (2006). Size of treatment effects and their importance to clinical research and practice. Biological Psychiatry, 59, 990–996. 10.1016/j.biopsych.2005.09.014 [DOI] [PubMed] [Google Scholar]
- Leaderer B. P. (1990). Assessing exposures to environmental tobacco smoke. Risk Analysis, 10, 19–26. 10.1111/j.1539–6924.1990.tb01016.x [DOI] [PubMed] [Google Scholar]
- Linden A. (2006). Measuring diagnostic and predictive accuracy in disease management: An introduction to receiver operating characteristic (ROC) analysis. Journal of Evaluation of Clinical Practice, 12, 132–139. 10.1111/j.1365-2753.2005.00598.x [DOI] [PubMed] [Google Scholar]
- Marrone G. F., Paulpillai M., Evans R. J., Singleton E. G., Heishman S. J. (2010). Breath carbon monoxide and semiquantitative saliva cotinine as biomarkers for smoking. Human Psychopharmacology, 25, 80–83. 10.1002/hup.1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel P. A., Malone R. E. (2012). Businesses voluntary pro-health tobacco policies: A review and research agenda. Tobacco Control, 21, 66–72. 10.1136/tobaccocontrol-2011–050201 [DOI] [PubMed] [Google Scholar]
- Mermelstein R., Colby S. M., Patten C., Prokhorov A., Brown R., Myers M., … McDonald P. (2002). Methodological issues in measuring treatment outcome in adolescent smoking cessation studies. Nicotine & Tobacco Research, 4, 395–403. 10.1080/1462220021000018470 [DOI] [PubMed] [Google Scholar]
- Murphy J. M., Berwick D. M., Weinstein M. C., Borus J. F., Budman S. H., Klerman G. L. (1987). Performance of screening and diagnostic tests: Application of receiver operating characteristics analysis. Archives of General Psychiatry, 44, 550–555. 10.1001/archpsyc.1987.01800180068011 [DOI] [PubMed] [Google Scholar]
- Patrick D. L., Cheadle A., Thompson D. C., Diehr P., Koepsell T., Kinne S. (1994). The validity of self-reported smoking: A review and meta-analysis. American Journal of Public Health, 84, 1086–1093. 10.2105/AJPH.84.7.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins K. A., Karelitz J. L., Jao N. C. (2013). Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nicotine & Tobacco Research, 15, 978–982. 10.1093/ntr/nts205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiff B. R., Faix C., Turturici M., Dallery J. (2010). Breath carbon monoxide output is affected by speed of emptying the lungs: Implications for laboratory and smoking cessation research. Nicotine & Tobacco Research, 12, 834–838. 10.1093/ntr/ntq090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H., Voigt K., Emanuel E. (2013). The ethics of not hiring smokers. New England Journal of Medicine, 368, 1369–1371. 10.1056/NEJMp1301951 [DOI] [PubMed] [Google Scholar]
- Shipton D., Tappin D. M., Vadiveloo T., Crossley J. A., Aitken D. A., Chalmers J. (2009). Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: A retrospective, cross sectional study. British Medical Journal, 339, b4347. 10.1136/bmj.b4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRNT Committee on Biochemical Verification. (2002). Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research, 4, 149–159. [DOI] [PubMed] [Google Scholar]
- Streiner D. L., Cairney J. (2007). What’s under the ROC? An introduction to receiver operating characteristics curves. Canadian Journal of Psychiatry, 52, 121–128. [DOI] [PubMed] [Google Scholar]
- Streiner D., Norman G. (2003). Health measurement scales. A practical guide to their development and use (3rd ed). New York, NY: Oxford University Press. [Google Scholar]
- Tripepi G., Jager K. J., Dekker F. W., Zoccali C. (2009). Diagnostic methods 2: Receiver operating characteristic (ROC) curves. Kidney International, 76, 252–256. 10.1038/ki.2009.171 19455194 [Google Scholar]
- Velicer W. F., Prochaska J. O., Rossi J. S., Snow M. (1992). Assessing outcome in smoking cessation studies. Psychological Bulletin, 111, 23–41. 10.1037/0033-2909.111.1.23 [DOI] [PubMed] [Google Scholar]
- Voigt K. (2012). Nonsmoker and “nonnicotine” hiring policies: The implications of employment restrictions for tobacco control. American Journal of Public Health, 102, 2013–2018. 10.2105/AJPH.2012.300745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E., Levasseur G., Kaiserman M. J. (2011). Evaluation of urinary cotinine immunoassay test strips used to assess smoking status. Nicotine & Tobacco Research, 13, 1045–1051. 10.1093/ntr/ntr127 [DOI] [PubMed] [Google Scholar]