The Combined Oxaliplatin Neurotoxicity Prevention Trial (CONcePT) tested intermittent oxaliplatin (IO) administration and the use of concurrent calcium and magnesium salts (Ca/Mg) in patients with colorectal cancer with intent to reduce neurotoxicity and extend treatment duration. An IO dosing schedule had a significant benefit on both time-to-treatment failure and time-to-tumor progression versus continuing dosing. The use of Ca/Mg had no effect on response.
Keywords: bevacizumab, calcium and magnesium salts, colorectal cancer, FOLFOX, intermittent, drug-induced neurotoxicity
Abstract
Background
Oxaliplatin is an integral component of colorectal cancer treatment, but its use is limited by neurotoxicity. The Combined Oxaliplatin Neurotoxicity Prevention Trial (CONcePT) tested intermittent oxaliplatin (IO) administration and the use of concurrent calcium and magnesium salts (Ca/Mg), two modifications intended to reduce neurotoxicity and extend the duration of treatment.
Patients and methods
In this trial involving double randomization, 140 patients were randomized to receive modified FOLFOX7 plus bevacizumab with IO (eight-cycle blocks of oxaliplatin treatment) versus continuous oxaliplatin (CO); and Ca/Mg versus placebo (pre- and postoxaliplatin infusion). The primary end point was time-to-treatment failure (TTF).
Results
One hundred thirty-nine patients were entered and treated up to the point of early study termination due to concerns by the data-monitoring committee (DMC) that Ca/Mg adversely affected tumor response. Tumor response was not a study end point. Given DMC concerns, an additional independent, blinded radiology review of all images showed no adverse effect of treatment schedule or Ca/Mg on response by Response Evaluation Criteria In Solid Tumors. The IO schedule was superior to CO [hazard ratio (HR) = 0.581, P = 0.0026] for both TTF and time-to-tumor progression (TTP) (HR = 0.533, P = 0.047).
Conclusions
An IO dosing schedule had a significant benefit on both TTF and TTP versus CO dosing in this trial despite the very attenuated sample. There was no effect of Ca/Mg on response.
introduction
Oxaliplatin with infusional 5-fluorouracil (5-FU) and leucovorin (LV) (FOLFOX) is an established treatment of metastatic colorectal cancer (CRC) [1–5]. Oxaliplatin-based therapy also shows improved efficacy combined with bevacizumab (BEV) [6–8]; however, neurotoxicity is a dose-limiting adverse event (AE) for oxaliplatin [3, 4, 9, 10] and the maximal benefit of BEV cannot be achieved without treatment to disease progression [7]. This necessitates a strategy to ‘optimize’ use of oxaliplatin. Intermittent oxaliplatin (IO) administration and prophylactic calcium/magnesium salts (Ca/Mg) are two treatment modifications that may reduce neurotoxicity, allowing more extended use of oxaliplatin [11, 12].
We report the findings of Combined Oxaliplatin Neurotoxicity Prevention Trial (CONcePT). The objective was to compare efficacy and safety (including neurotoxicity) of IO ± Ca/Mg versus continuous oxaliplatin (CO) ± Ca/Mg as first-line treatment in patients with advanced CRC receiving modified FOLFOX7 (mFOLFOX) plus BEV. Patients were originally randomized to receive either Ca/Mg or placebo combined with the IO or CO regimen; however, a protocol amendment after 140 patients enrolled allowed Ca/Mg prophylaxis in all patients [13, 14]. An unplanned interim analysis by the data-monitoring committee (DMC) indicated a possible negative effect of Ca/Mg on tumor response (which was not a study end point); the study was terminated, with treatment and follow-up of all patients immediately stopped per DMC mandate. Herein, efficacy and safety data are presented for patients randomized to the original 2 × 2 factorial design.
methods
patient selection
Eligible patients had histologically or cytologically documented inoperable metastatic adenocarcinoma of the colon or rectum. Additional inclusion/exclusion criteria and ethical conduct of this study is detailed in Appendix 1.
randomization
This was a 2 × 2 parallel-group, randomized, double-blind (for Ca/Mg), phase III trial comparing CO versus IO treatment schedules ± Ca/Mg for neurotoxicity prophylaxis. Due to revised practice patterns as the trial enrolled, the trial was amended to a two-arm design that eliminated Ca/Mg randomization (supplementary Figure S1, available at Annals of Oncology online). Cohort 1 patients were randomized by the original 2 × 2 factorial design (i.e. IO versus CO × Ca/Mg versus placebo), and patients subsequently recruited to the study (Cohort 2) all received Ca/Mg and were randomized to either CO or IO. In the CO arms, patients received mFOLFOX plus BEV every 2 weeks (see Appendix 1). In the IO arms, patients alternated between eight cycles of the above regimen and eight cycles of the same regimen without oxaliplatin until treatment failure. This design was selected as most patients achieve maximum response within the first eight cycles and before the onset of cumulative neurotoxicity is generally seen. We believed that 5-FU/LV plus BEV would at least hold this response until restarting oxaliplatin. Patients assigned to the Ca/Mg arms received infusional calcium gluconate (1 g) plus magnesium sulfate (1 g) in 100 ml of 5% dextrose or normal saline over 30 min before and after oxaliplatin and LV. Placebo infusions contained 5% dextrose or normal saline. These were assigned in a double-blind fashion with only the local pharmacist knowing the treatment assignment. Patients with tumor regrowth to >50% of baseline at the 6-month computed tomography scan while on IO were instructed to reintroduce oxaliplatin therapy early. Those with <50% regrowth were continued on IO. Treatment was continued until death, disease progression, unacceptable toxicity, patient withdrawal, or treatment delay. Criteria for treatment delays and/or patient withdrawal are detailed in Appendix 1.
assessment of efficacy and safety
The primary end point was time-to-treatment failure (TTF) for oxaliplatin, a composite which assessed time from randomization to discontinuation of oxaliplatin-based treatment for any reason. TTF was measured from randomization to date of treatment discontinuation due to AEs, progression, withdrawal of consent, or death. TTF was chosen as primary end point, since the goal of therapy was to achieve optimal efficacy and tolerability for the longest possible time. Secondary end points included time-to-tumor progression (TTP), incidence of AEs, and quality of life, including oxaliplatin-specific neurologic symptoms (see Appendix 1). Response was not an end point and was not reported by investigators, but scans were carried out every 2 months using modified Response Evaluation Criteria In Solid Tumors version 1 criteria to determine TTP. All responses and response rates reported here were determined retrospectively by the blinded independent radiology review committee (IRRC) when the question of interference was raised by the DMC. Toxicities were graded according to National Cancer Institute Common Terminology for Adverse Events Version 3.0 [15].
statistical methods
Study populations for analysis defined in Appendix 1 include the intent-to-treat, as-treated, and safety populations. However, because of early trial termination, only the as-treated population in cohort 1 was used for primary analysis. Minimal data were obtained on cohort 2 given the short interval between initiation of the amendment and study termination. Statistical power considerations are detailed in Appendix 1. Kaplan–Meier estimates were used to calculate median TTF and TTP. Patients still on study at the time of study termination were censored at that point. Effects of treatment schedule and neuroprophylaxis on TTF and TTP were analyzed using Cox regression modeling; P values were calculated via log-rank test. Effects of treatment schedule and neuroprophylaxis on tumor response were analyzed using exact logistic regression modeling. Analysis of the changes in oxaliplatin-induced peripheral sensory neuropathy (PSN) (Patient Neurotoxicity Questionnaire–Oxaliplatin [PNQ-oxali] data) was carried out on the as-treated population via Fisher's exact test.
results
study conduct and termination
CONcePT was initiated (first subject enrolled) in February 2005. In August 2006, an amendment eliminated placebo groups so all subjects would receive Ca/Mg, and removed the requirement for neurologic/neurosensory assessments. Previously enrolled patients continued treatment as randomized (cohort 1). Subjects enrolled after elimination of the placebo arm were designated as cohort 2.
Results of an unplanned analysis by the independent DMC (June 2007) of all enrolled patients, including cohort 2, indicated an apparently inferior response rate in patients receiving Ca/Mg (based on DMC interpretation of submitted radiology reports for 174 subjects), and prompted the DMC to recommend study termination. In this analysis, cohort 2 patients (all of whom received Ca/Mg) were largely considered to be nonresponders, mainly because of the very short follow-up period and paucity of scan results, which may have led to an underestimation of the response rate for Ca/Mg patients. The DMC-mandated trial closure resulted in all patients immediately stopping protocol-based therapy and affected the feasibility of statistical analyses, including primary efficacy assessment. All patients still on study were censored at the time of study closure.
disposition of patients
One-hundred eighty patients were randomized: 140 to cohort 1 and 40 to cohort 2 (supplementary Figure S1, available at Annals of Oncology online). All but two patients received treatment as randomized (one did not receive treatment; one received the wrong treatment).
IO regimen compliance
Seventy-one patients were treated in the IO arm (range up to 35 cycles) and 22 remained on study beyond cycle 16. Twenty-one of the 22 patients (95%) had oxaliplatin reintroduced per protocol, including one early reintroduction at cycle 13 for regrowth. Eight patients remained on treatment beyond cycle 24 and one of these beyond cycle 32; all of these received IO, in eight-cycle blocks, correctly per protocol.
efficacy
Patients with IO had a significantly longer median TTF than those receiving CO in cohort 1. Overall hazard ratio (HR) for IO versus CO was 0.58 [Cox regression using treatment schedule and neuroprophylaxis; 95% confidence interval (CI) 0.41–0.83; P = 0.0026]. Administration of Ca/Mg or placebo had a statistically nonsignificant effect on TTF (HR = 1.32, 95% CI 0.93–1.86; P = 0.118) and favored Ca/Mg; therefore, four treatment groups were collapsed into two (all IO versus all CO treatment) for further analysis. Median TTF was longer for the combined IO group (Table 1). Kaplan–Meier curves of TTF for IO versus CO schedule (as-treated population, cohort 1) are shown in Figure 1A. There were no imbalances in baseline prognostic factors for TTF (Table 2).
Table 1.
Treatment schedule effect on time-to-treatment failure (TTF) and time-to-tumor progression (TTP) in intent-to-treat patients receiving continuous oxaliplatin (CO) or intermittent oxaliplatin (IO) and placebo or Ca/Mg
| CO (N = 68) | IO (N = 71) | |
|---|---|---|
| TTF | ||
| Patients with an event, n (%) | 67 (98.5) | 65 (91.5) |
| Patients censored, n (%) | 1 (1.5) | 6 (8.5) |
| Median TTF, months (95% CI) | 4.2 (3.7–5.5) | 5.7 (4.7–7.1) |
| HR (95% CI) | 0.58 (0.41–0.83); P = 0.0026 | |
| TTP | ||
| Patients with an event, n (%) | 24 (35.3) | 21 (29.6) |
| Patients censored, n (%) | 44 (64.7) | 50 (70.4) |
| Median PFS, months (95% CI) | 7.4 (6.9–NE) | 12.0 (8.3–NE) |
| HR (95% CI) | 0.53 (0.29–0.99); P = 0.047 | |
CI, confidence interval; HR, hazard ratio; NE, not evaluable; PFS, progression-free survival.
Figure 1.
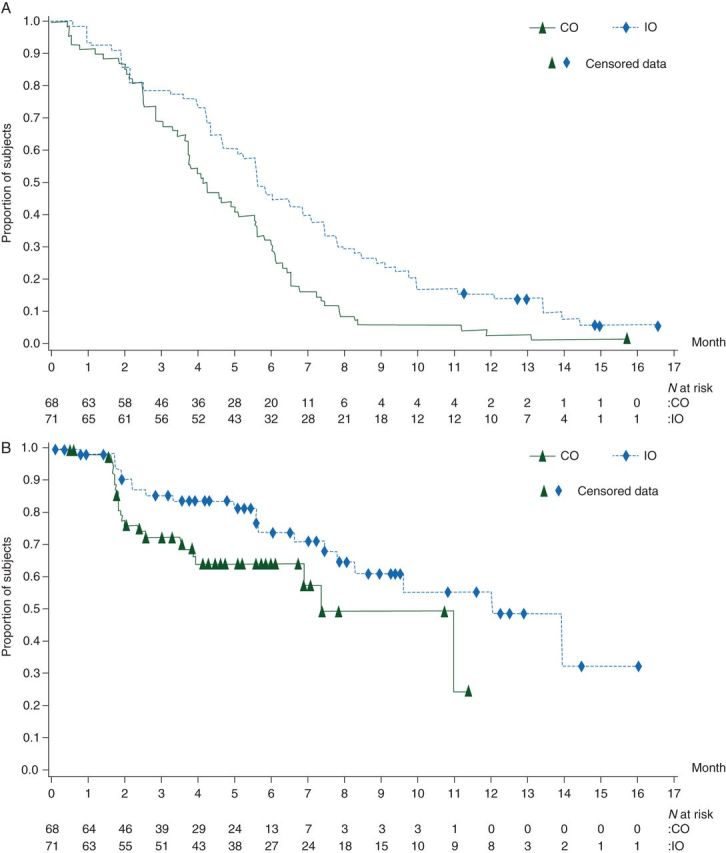
Kaplan–Meier curves for (A) time-to-treatment failure and (B) time-to-tumor progression in the as-treated population (cohort 1) for intermittent oxaliplatin (IO) versus continuous oxaliplatin (CO) treatment.
Table 2.
Patient demographics, as-treated population (cohort 1) at baseline
| No. of patients |
||||
|---|---|---|---|---|
| CO (n = 68) |
IO (n = 71) |
|||
| Placebo (n = 33) | Ca/Mg (n = 35) | Placebo (n = 36) | Ca/Mg (n = 35) | |
| Mean age, years (SD) | 61 (12) | 63 (12) | 62 (12) | 67 (12) |
| Age distribution (years) | ||||
| <65 | 19 | 20 | 21 | 11 |
| ≥65 | 14 | 15 | 15 | 24 |
| Male/female | 16/17 | 24/11 | 18/18 | 18/17 |
| ECOG PS | ||||
| 0 | 19a | 17a | 22 | 16 |
| 1 | 13a | 17a | 14 | 19 |
| Stageb | ||||
| 0–III | 12 | 12 | 10 | 14 |
| IV | 19 | 22 | 25 | 21 |
| Unknown | 2 | 1 | 1 | 0 |
| Site of disease | ||||
| Colon | 25 | 27 | 31 | 28 |
| Rectum | 5 | 6 | 2 | 6 |
| Colorectalc/unknown | 3 | 1 | 3 | 1 |
| Prior treatment | ||||
| Radiotherapy | 4 | 5 | 3 | 4 |
| Surgery | 17 | 18 | 19 | 15 |
| Adjuvant therapy | 9 | 6 | 8 | 7 |
aOne patient not assessed for ECOG PS.
bDisease stage at initial diagnosis.
cNot specified.
Ca/Mg, calcium/magnesium salts; CO, continuous oxaliplatin; ECOG PS, Eastern Cooperative Oncology Group performance status; IO, intermittent oxaliplatin; SD, standard deviation.
Secondary efficacy variables, including TTP, were analyzed using the as-treated population of cohort 1; due to the large number of censored patients, time-to-event results should be interpreted with caution. Patients receiving IO had significantly longer median TTP than those receiving CO (HR = 0.53, 95% CI 0.29–0.99; P = 0.047); the difference remained significant when stratified (by Ca/Mg; P = 0.029). Administration of Ca/Mg or placebo had no significant effect on TTP (HR = 0.99, 95% CI 0.55–1.79; P = 0.973); therefore, four groups were collapsed into two (IO versus CO) for further analysis, and median TTP was longer for the combined IO treatment group (Table 1). Kaplan–Meier curves of TTP for IO versus CO schedule (as-treated population, cohort 1) are shown in Figure 1B. Except for prior chemotherapy, which showed a positive effect in delaying TTP, no baseline prognostic factor showed a significant effect on TTP.
In view of DMC response rate analysis and the trial's early termination, additional analyses were conducted. An IRRC, blinded to all treatment assignments, reviewed the actual images of radiologic studies for randomized patients and scored response based on best response (single scan), subsequently confirmed response (second scan), and sequentially confirmed response (at least two subsequent scans). The latter was the most stringent. No patient achieved complete response (CR). Overall, sequentially confirmed response rates (CR + partial response/N) for assessable patients in the CO arm were 41% compared with 52% for the IO arm (n = 59), with 35% stable disease in each arm. There was no effect of treatment schedule or Ca/Mg for confirmed or subsequently confirmed response (on the next scan). The response rate comparison of IO relative to CO, based on independent review, favored the Ca/Mg groups (data not shown). There was also no effect of treatment schedule or neuroprophylaxis treatment for subsequently confirmed response or unconfirmed response.
adverse events
Overall, treatment duration was longer in cohort 1 subjects receiving IO versus CO; mean treatment duration was 7.4 and 5.5 months for IO placeco and IO Ca/mg respectively compared with 4.7 and 4.6 months for CO pla and CO ca/mg. The most common AE overall was PSN (76.0%), followed by fatigue (70.9%), nausea (63.7%), diarrhea (53.6%), constipation (39.7%), and vomiting (31.3%); other AEs occurred in <30% of subjects. Grade 3 or 4 AEs reported by >2% and >3% of subjects for hematologic and nonhematologic AEs, respectively, in any treatment group are shown in Table 3 for the safety population (n = 179). The most common grade 3 or 4 AE was PSN, which was less frequently observed overall with IO versus CO (Table 3). Incidence of grade 3 or 4 neutropenia was 17.3% in the overall study population. The most common AE leading to treatment discontinuation in cohort 1 was PSN, which occurred in fewer patients in the IO groups (supplementary Table S1, available at Annals of Oncology online). Results for PNQ-oxali are presented in Appendix 2. Overall, neurotoxicity scoring with PNQ-oxali was affected favorably by IO treatment for both chronic and acute PSN, and was affected favorably by Ca/Mg for chronic PSN. The difference between IO and CO was significant in favor of IO for acute PSN (P = 0.037), but not for chronic PSN.
Table 3.
Grade 3 or 4 adverse events (AEs) reported by more than 2% of patients (hematologic AEs) or more than 3% of patients (nonhematologic AEs) in any treatment group, safety population (n = 179), all cohorts
| Grade 3 or 4 AE, n (%) | CO (N = 88) |
IO (N = 91) |
Total (N = 179) | ||
|---|---|---|---|---|---|
| Placebo (N = 33) | Ca/Mg (N = 55) | Placebo (N = 36) | Ca/Mg (N = 55) | ||
| Peripheral sensory neuropathy | 8 (24.2) | 10 (18.2) | 4 (11.1) | 5 (9.1) | 27 (15.1) |
| Peripheral motor neuropathy | 2 (6.1) | 0 | 0 | 0 | 2 (1.1) |
| Neutropenia | 6 (18.2) | 11 (20.0) | 6 (16.7) | 8 (14.5) | 31 (17.3) |
| Thrombocytopenia | 2 (6.1) | 2 (3.6) | 0 | 1 (1.8) | 5 (2.8) |
| Leukopenia | 1 (3.0) | 1 (1.8) | 0 | 0 | 2 (1.1) |
| Lymphopenia | 0 | 1 (1.8) | 1 (2.8) | 0 | 2 (1.1) |
| Fatigue | 2 (6.1) | 2 (3.6) | 1 (2.8) | 5 (9.1) | 10 (5.6) |
| Diarrhea | 1 (3.0) | 2 (3.6) | 3 (8.3) | 2 (3.6) | 8 (4.5) |
| Dehydration | 2 (6.1) | 2 (3.6) | 0 | 4 (7.3) | 8 (4.5) |
| Hypertension | 1 (3.0) | 4 (7.3) | 1 (2.8) | 2 (3.6) | 8 (4.5) |
| Nausea | 1 (3.0) | 2 (3.6) | 0 | 4 (7.3) | 7 (3.9) |
| Vomiting | 2 (6.1) | 1 (1.8) | 1 (2.8) | 3 (5.5) | 7 (3.9) |
| Asthenia | 0 | 1 (1.8) | 0 | 2 (3.6) | 3 (1.7) |
| Anorexia | 1 (3.0) | 0 | 0 | 2 (3.6) | 3 (1.7) |
| Palmar–plantar erythrodysesthesia syndrome | 0 | 0 | 0 | 3 (5.5) | 3 (1.7) |
Ca/Mg, calcium/magnesium salts; CO, continuous oxaliplatin; IO, intermittent oxaliplatin.
discussion
The primary objective of CONcePT was to investigate whether an intermittent schedule of FOLFOX/BEV, with 4-month, eight-cycle blocks of oxaliplatin administration alternating with holding that drug, would allow subjects to benefit from oxaliplatin-based therapy for a longer period by reducing discontinuations (e.g. for neurotoxicity). This strategy builds on the Optimized Leucovorin–Fluorouracil–Oxaliplatin (OPTIMOX) approach (six cycles of higher dose oxaliplatin in FOLFOX7 and 12 cycles of 5-FU/LV), but restarts treatment with oxaliplatin every eight cycles, rather than waiting for tumor regrowth to a target percent of baseline (as in OPTIMOX protocol, though seldom achieved in the actual study). To further optimize treatment duration, a nonbolus 5-FU–containing regimen, mFOLFOX7, was used as the chemotherapy backbone to reduce incidence and severity of neutropenia. The secondary objective of this trial was to examine the effect of Ca/Mg on incidence and severity of neurotoxicity (with IO or CO treatment schedule), in a properly randomized, placebo-controlled study. The IO strategy was quite effective in practice as 21 of 22 (95%) patients on study beyond cycle 16 reintroduced oxaliplatin correctly, and all eight beyond cycle 24 stopped and restarted a second or third time per protocol.
In exploratory analysis of the primary efficacy end point, IO was associated with significantly better TTF versus CO (HR = 0.58), and Ca/Mg prophylaxis may have improved chronic neurotoxicity without any impact on efficacy. Analysis of other efficacy end points showed improved TTP (HR = 0.53) favoring the IO schedule and equivalent sequentially confirmed response. While the response rate was lower than reported in other studies, responses plus stable disease rates were 76% and 87% for CO and IO, respectively, possibly reflecting stringent blinded response evaluation; comparison with historical controls may be inaccurate as these response rates were determined by an IRRC which retrospectively reviewed every scan in a blinded fashion. Our overall results are also consistent with those of CAIRO2 (CApecitabine, IRinotecan and Oxaliplatin in advanced CRC) [16], and as predicted, we found the use of mFOLFOX7 to be associated with a low incidence of neutropenia, with efficacy outcomes comparable with the bolus 5-FU-containing FOLFOX + BEV regimens [7].
Despite early termination by the DMC, subsequent IRRC review of the actual scans (collected retrospectively) of all treated patients for both confirmed and unconfirmed response showed no impact of Ca/Mg on response rate [9, 17]. Furthermore, results from the initial cohort of patients randomized to Ca/Mg or placebo suggest that the efficacy of mFOLFOX plus BEV is unaffected by co-administration of Ca/Mg.
Patient-reported outcomes assessing influence of oxaliplatin dosing on both acute and chronic PSN also indicated a degree of benefit for Ca/Mg over placebo in terms of alleviating chronic, but not acute, PSN (see Appendix 2). These findings are supported by results from the ‘North Central Cancer Treatment Group’ N04C7 adjuvant trial, which was also terminated at the same time due to the same concerns raised by the CONcePT DMC [18]; retrospective analysis of CAIRO2 also showed no impact of Ca/Mg infusions on treatment efficacy, whereas neurotoxicity was significantly reduced [19].
Current understanding of optimal palliative therapy in advanced CRC is highlighted by a ‘continuum of care’ [20], with exposure of patients to prolonged antitumor therapy, while limiting treatment-related toxicities. Results from CONcePT provide a clear example of how such an approach can be realized using an oxaliplatin/BEV-containing first-line therapy with 4 month blocks of oxaliplatin administration alternating with breaks.
In conclusion, despite premature closure and limited sample size, results from this study demonstrate increased efficacy and reduced toxicity when IO is used with mFOLFOX/BEV for CRC first-line therapy. The addition of Ca/Mg had no adverse impact on efficacy or response. A reduction of neurotoxicity was also suggested through patient-reported outcomes, but this question could not be addressed definitively due to sample size limitation. CONcePT thus signals an important direction on optimizing oxaliplatin use in first-line treatment of CRC.
funding
This work was supported by Sanofi US.
disclosure
HH has been a consultant/advisor to Sanofi US and Genentech. AG has been a consultant/advisor to Sanofi US and Genentech, and has received research funding from Sanofi US and Genentech. LH's employer, Florida Cancer Specialists, Ft. Myers, FL, has received research funding. RKR has received honoraria and research funding from Sanofi US. MK has been a consultant/advisor to Celgene, and has received honoraria from Celgene and Genentech/Roche. BHC was Vice President of Oncology at Sanofi US during the study and at the time the manuscript was created. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank Liji Shen and Marc Buyce for statistical support and Nicholas Combates for publication support. Editorial support in the preparation of this publication was provided by Colin Evans, PhD, Phase Five Communications, Inc., and sponsored by Sanofi US. The authors were responsible for all content and editorial decisions and received no honoraria related to the development/presentation of this publication.
appendix 1
inclusion/exclusion criteria
Eligibility criteria included age ≥18 years, measurable disease (Response Evaluation Criteria in Solid Tumors Version 1), Eastern Cooperative Oncology Group performance status 0–1, no prior therapy for metastatic or recurrent disease [adjuvant 5-fluorouracil (5-FU)/leucovorin (LV) and/or irinotecan, completed >6 months before entry was allowed], life expectancy >3 months, a normal electrocardiogram, and no other serious concomitant disease. Criteria for exclusion included prior treatment with oxaliplatin and/or bevacizumab (BEV), current digoxin therapy, known dihydropyrimidine dehydrogenase deficiency, peripheral neuropathy (grade >1), and current therapy to prevent/treat neuropathy. Patients with a history of cerebrovascular event, uncontrolled hypertension, clinically significant cardiovascular or peripheral vascular disease, or history of bleeding within 6 months of registration were also excluded.
mFOLFOX regimen
The modified FOLFOX7 regimen consisted of oxaliplatin 85 mg/m2 over 2 h, LV 200 mg/m2 over 2 h, 5-FU 2400 mg/m2 over 46 h, without a bolus; BEV was administered at a dose of 5 mg/kg.
ethical conduct of the study
The protocol complied with recommendations of the 18th World Health Congress (Helsinki, 1964) and all applicable amendments. The informed consent process was carried out in accordance with Good Clinical Practice guidelines, and the study was approved by the institutional review boards at each institution before accrual commenced.
criteria for treatment delays and/or withdrawal
Treatment cycles were delayed for 1 week if any of the following toxicities occurred during the previous cycle: absolute neutrophil count <1200/mm3, platelet count <75 000/mm3, treatment-related diarrhea above baseline, or any other toxicity grade >1 (except neurologic toxicity, hypertension, alopecia, and asthenia). Further 1-week treatment delays were implemented if any of the above toxicities (except asthenia and alopecia) had not resolved to grade ≤1. Dose modifications for 5-FU and oxaliplatin were based on the most severe toxicity observed in the previous cycle. Dose reductions were implemented for grade 3/4 neutropenia (or febrile neutropenia), thrombocytopenia, diarrhea, mucositis/stomatitis, vomiting, and grade 3 other nonhematologic toxicities. For grade ≥2 thromboembolic events, resumption of chemotherapy was at the investigator's discretion. Patients were withdrawn from the study if they experienced grade 4 nonhematologic toxicity (except neurotoxicity, alopecia, or hypersensitivity). Patients who experienced grade 3 neurologic toxicity for >7 days had their oxaliplatin dose reduced to 65 mg/m2. Grade 3 or 4 neurologic toxicity that persisted between treatment cycles led to the patient's withdrawal.
definition of study populations
intent-to-treat population
All randomized subjects, with drug assignment per the original randomization, regardless of whether the subject received any study drug medication or a different drug from that to which they were randomized.
as-treated population
All randomized subjects who had taken at least one dose of any of the study drugs with treatment assignment designated according to what was actually administered and who provided sufficient efficacy data for a given end point; this population could have been different for each selected end point.
safety population
All randomized subjects who received at least one dose of any of the study drugs with treatment assignment designated according to what was actually administered.
quality-of-life assessments
Quality-of-life assessments related to oxaliplatin-specific neurologic symptoms were made using Patient Neurotoxicity Questionnaire–oxaliplatin (completed at baseline, day 1 of each therapy cycle, and end of treatment). Patients' follow-up for survival status was conducted every 3 months from the end of treatment up to 3 years from the date of randomization, though this was discontinued when the study was terminated.
statistical power considerations
Under the assumption that intermittent oxaliplatin (would result in a median time-to-treatment failure of 9.4 months compared with 6 months for continuous oxaliplatin, with concurrent calcium and magnesium salts given to all patients, a sample size of 182 assessable patients (91 for each group) would provide 80% power to detect the above difference in the primary end point with a type I error of ≤5%.
appendix 2
patient neurotoxicity questionnaire–oxaliplatin
Neurotoxicity scoring with Patient Neurotoxicity Questionnaire–oxaliplatin (PNQ-oxali) was affected favorably by intermittent oxaliplatin (IO) for both chronic and acute peripheral sensory neuropathy (PSN), and was affected favorably by concurrent calcium and magnesium salts (Ca/Mg) for chronic PSN. Assessing data for all treatment cycles, the difference between Ca/Mg and placebo was not significant for item 1 (chronic PSN; supplementary Figure S2A, available at Annals of Oncology online) or item 2 (acute PSN; supplementary Figure S2B, available at Annals of Oncology online) of PNQ-oxali.
references
- 1. NCCN Clinical Practice Guidelines in Oncology for Colon Cancer. V.1.2011.
- 2.Goldberg RM. N9741: a phase III study comparing irinotecan to oxaliplatin-containing regimens in advanced colorectal cancer. Clin Colorectal Cancer. 2002;2:81. doi: 10.1016/S1533-0028(11)70509-6. [DOI] [PubMed] [Google Scholar]
- 3.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 4.Giacchetti S, Perpoint B, Zidani R, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–147. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 5.Grothey A, Deschler B, Kroening H, et al. Phase III study of bolus 5-fluorouracil (5-FU)/folinic acid (FA) (Mayo) vs weekly high-dose 24 h 5-FU infusion/FA + oxaliplatin (OXA) (FUFOX) in advanced colorectal cancer (ACRC) Proc Am Soc Clin Oncol. 2002;21:129a. Abstr 512. [Google Scholar]
- 6.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 7.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. Erratum in: J Clin Oncol 2008; 26: 3110; J Clin Oncol 2009; 27: 653. [DOI] [PubMed] [Google Scholar]
- 8.Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 9.Gamelin L, Boisdron-Celle M, Morel A, et al. Oxaliplatin-related neurotoxicity: interest of calcium-magnesium infusion and no impact on its efficacy. J Clin Oncol. 2008;26:1188–1189. doi: 10.1200/JCO.2007.15.3767. [DOI] [PubMed] [Google Scholar]
- 10.Ali BH. Amelioration of oxaliplatin neurotoxicity by drugs in humans and experimental animals: a minireview of recent literature. Basic Clin Pharmacol Toxicol. 2010;106:272–279. doi: 10.1111/j.1742-7843.2009.00512.x. [DOI] [PubMed] [Google Scholar]
- 11.de Gramont A, Buyse M, Abrahantes JC, et al. Reintroduction of oxaliplatin is associated with improved survival in advanced colorectal cancer. J Clin Oncol. 2007;25:3224–3229. doi: 10.1200/JCO.2006.10.4380. [DOI] [PubMed] [Google Scholar]
- 12.Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer—a GERCOR study. J Clin Oncol. 2006;24:394–400. doi: 10.1200/JCO.2005.03.0106. [DOI] [PubMed] [Google Scholar]
- 13.Grothey A. Clinical management of oxaliplatin-associated neurotoxicity. Clin Colorectal Cancer. 2005;5(suppl 1):S38–S46. doi: 10.3816/ccc.2005.s.006. [DOI] [PubMed] [Google Scholar]
- 14.Eng C. Toxic effects and their management: daily clinical challenges in the treatment of colorectal cancer. Nat Rev Clin Oncol. 2009;6:207–218. doi: 10.1038/nrclinonc.2009.16. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events Version 3.0.
- 16.Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. Erratum in: N Engl J Med 2010; 363: 2573. [DOI] [PubMed] [Google Scholar]
- 17.Hochster H, Grothey A, Shpilsky A, et al. Effect of intravenous (IV) calcium and magnesium (Ca/Mg) versus placebo on response to FOLFOX+bevacizumab (BEV) in the CONcePT trial. Presented at the 2008 American Society of Clinical Oncology Gastrointestinal Cancers Symposium; Orlando, FL, USA. January 25–27, 2008; Abstr 280. [Google Scholar]
- 18.Grothey A, Nikcevich DA, Sloan JA, et al. Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. J Clin Oncol. 2011;29:421–427. doi: 10.1200/JCO.2010.31.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knijn N, Tol J, Koopman M, et al. The effect of prophylactic calcium and magnesium infusions on the incidence of neurotoxicity and clinical outcome of oxaliplatin-based systemic treatment in advanced colorectal cancer patients. Eur J Cancer. 2011;47:369–374. doi: 10.1016/j.ejca.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg RM, Rothenberg ML, Van Cutsem E, et al. The continuum of care: a paradigm for the management of metastatic colorectal cancer. Oncologist. 2007;12:38–50. doi: 10.1634/theoncologist.12-1-38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


