Abstract
Background
Zanthoxylum buesgenii is a shrub used in Sierra Leone as remedy to cure venereal diseases, arthritis, and rheumatism whereas leaves and barks are employed to treat leprosy and to relieve pain. In South West Region of Cameroon, the plant locally called “Mbem” by Lewoh-Lebang community, is orally given to patients as aphrodisiac decoction and to increase sperm count. Previous chemical studies on Zanthoxylum species reported the identification of lignans, coumarins, diterpenes, sesquiterpenes, steroids, alkaloids and benzopropanoids. Besides, structurally diverse compounds belonging to these classes of secondary metabolites have been reported as trypanocidal, antileishmanial, antimycobacterial and cytotoxic metabolites.
Results
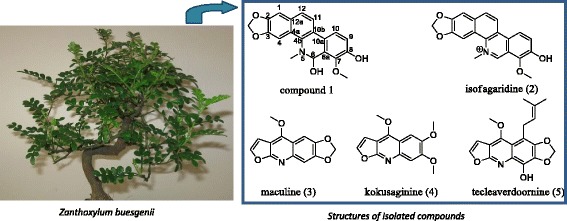
We therefore investigated the alkaloidal constituents of Z. buesgenii. In the course of the study, two benzophenanthridines [1-methoxy-12-methyl-12,13-dihydro-[1,3]dioxolo[4′,5′:4,5]benzo[1,2-c]phenanthridine-2,13-diol (1) and isofagaridine (2)] were identified among them one new. Alongside, three known furoquinolines [maculine (3), kokusaginine (4) and teclearverdoornine (5)] were also obtained and their structures were established on the basis of their NMR data and by comparison with those previously reported. Furthermore, the cytotoxicities of metabolites (1–4) isolated in substantial amount were evaluated against a series of multidrugs-resistant cancer cell lines. While compounds 2–4 showed selective cytotoxicities, compound 1 displayed activities against all cancer cells.
Conclusions
The observed activities corroborate those previously reported on similar benzophenanthridine alkaloids indicating that compounds 1 and 2 can chemically be explored to develop other chemotherapeutic agents.
Graphical abstract.
Cytotoxic Benzophenanthridine and Furoquinoline Alkaloids from Zanthoxylum buesgenii (Rutaceae).
Electronic supplementary material
The online version of this article (doi:10.1186/s13065-014-0061-4) contains supplementary material, which is available to authorized users.
Keywords: Zanthoxylum buesgenii, Benzophenanthridines, Furoquinolines, Cytotoxicity
Background
Formerly named Fagara buesgenii, Zanthoxylum buesgenii is a shrub or small tree of about 4 m height with leaves about 20 to 75 cm long [1,2]. In Sierra Leone, roots are used as remedy to cure venereal diseases, arthritis, and rheumatism whereas leaves and barks are employed to treat leprosy and to relieve pain [2]. In South West Region of Cameroon, Z. buesgenii locally called “Mbem” by Lewoh-Lebang community, is orally given to patients as aphrodisiac decoction and to increase sperm count [3]. Previous chemical studies on Zanthoxylum species reported the identification of lignans, coumarins, diterpenes, sesquiterpenes, steroids, alkaloids [4] and benzopropanoids [5]. Interestingly, alkaloids represent the largest group of secondary metabolites obtained from the genus Zanthoxylum with structurally diverse scaffolds including oxoaporphines [6], aporphines, quinolinones, furoquinolines [4], indolopyridoquinazolines, β-carbolines, and benzophenanthridines [4,7]. Besides bioactivities such as trypanocidal [8], antileishmanial [9], antimycobacterial [10] effects, most of these alkaloids have shown from moderate to significant cytotoxicity against several cancer cell lines [11-13]. Therefore, we investigated the alkaloidal constituents of Z. buesgenii. In the course of the study, two benzophenanthridines were identified among them one new. Alongside, three known furoquinolines were also obtained.
We herein report the structure elucidation of the new compound and the cytotoxic potentiality of the identified secondary metabolites against a series of multidrugs-resistant cancer cell lines.
Results and discussion
Chemistry
A Dragendorff reagent-guided isolation of the aerial part of Zanthoxylum buesgenii yielded five alkaloids identified as benzophenanthridines (1 and 2) and furoquinolines (3–5).
Compound 1 was obtained as a red powder giving a positive test with the Dragendorff reagent indicative of alkaloids. The HR ESI mass spectrum gave a pseudo-molecular peak at m/z 374.1003 ([M + Na]+, calcd. 374.1004) consistent with the molecular formula C20H17NO5. This formula corresponded to thirteen double bond equivalents. The NMR spectra of compound 1 (Table 1 and the Additional file 1) displayed two pairs of aromatic signals at δ [7.62 (d, J = 8.5 Hz)/124.2, 8.02 (d, J = 8.5 Hz)/121.0] and δ [7.82 (d, J = 8.4 Hz)/120.4, 7.41 (d, J = 8.4 Hz)/118.3) in an ortho arrangement, two aromatic CH groups resonating as singlets at δ 7.31/105.3 and 7.89/101.7, one hemiaminal at δ 6.54 (s)/80.0, one acetalic CH2 group at δ [6.04 (d, J = 1.2 Hz), 6.08 (d, J = 1.2 Hz)/102.0] and two downfield CH3 groups among them one NCH3 group at δ 2.77/40.6 and one OCH3 at δ 4.24/62.2. Moreover, ten aromatic quaternary carbon signals were further revealed, among them, four oxygenated and arranged in pairs of ortho resonances. The aforementioned data suggested 1 to be a benzophenanthridine alkaloid [14]. COSY correlations (Figure 1) between H-12 (δH 7.62) and H-11 (δH 8.02) as well as H-10 (δH 7.82) and H-9 (δH 7.41) supported the presence of two sets of two ortho aromatic protons. The 1,3-dioxole ring was fused to the aromatic ring bearing the singlets at δH 7.31 (H-1) and 7.89 (H-4) according to HMBC correlations (Figure 1) observed from H-1 to C-2 (δC 148.8), and H-4 to C-3 (δC 148.2) as well as those found from the acetalic CH2 group to carbons C-2 and C-3. Furthermore, H-4 showed long range correlations to C-4a (δC 128.2) and C-4b (δC 140.1) while H-1 displayed same interactions with C-12a (δC 131.7) and C-12 (δC 124.2). Similarly, the NCH3 group (δH 2.77) presented HMBC correlations with C-4b and the hemiaminal carbon C-6 (δC 80.0). H-6 (δH 6.54) in turn correlated with C-4b, C-6a (δC 124.3), C-7 (δC 146.9), and C-10a (δC 129.1). Further HMBC correlations were observed from the CH3 group at δH 4.62 to C-7 (δC 146.9), from H-9 (δH 7.41) to C-7, C-8 (δC 150.6) and C-10 (δC 120.4) while H-10 (δH 7.82) correlated with C-8, C-10a, C-10b (δC 124.3). The benzophenanthridine core was formed on the basis of the HMBC cross peaks found between H-11 and C-4b, C-10b, C-12 (δC 124.2), and C-12a (δC 131.7) as well as between H-12 and C-12a, C-4a, C-10b, and C-1.
Table 1.
NMR data of compound 1 (C 5 D 5 N, 400 MHZ)
| Position | δ H (multi, J = Hz) | δ C |
|---|---|---|
| 1 | 7.31, s | 105.3 (CH) |
| 2 | - | 148.8 (C) |
| 3 | - | 148.2 (C) |
| 4 | 7.89, s | 101.7 (CH) |
| 4a | - | 128.2 (C) |
| 4b | - | 140.1 (C) |
| 5 | - | - |
| 6 | 6.54, s | 80.0 (CH) |
| 6a | - | 124.3 (C) |
| 7 | - | 146.9 (C) |
| 8 | - | 150.6 (C) |
| 9 | 7.41, d (8.4) | 118.3 (CH) |
| 10 | 7.82, d (8.4) | 120.4 (CH) |
| 10a | - | 129.1 (C) |
| 10b | - | 124.3 (C) |
| 11 | 8.02, d (8.5) | 121.0 (CH) |
| 12 | 7.62, d (8.5) | 124.2 (CH) |
| 12a | - | 131.7 (C) |
| OCH2O | 6.04, d (1.2) | 102.0 (CH2) |
| 6.08, d (1.2) | ||
| NCH3 | 2.77, s | 40.6 (CH3) |
| OCH3 | 4.24, s | 62.2 (CH3) |
δH, and δC are chemical shifts of protons and carbons, respectively in ppm.
Figure 1.
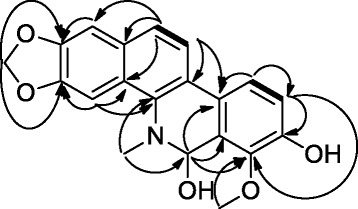
COSY (bold) and HMBC (arrow) correlations of compound 1.
The relative configuration at C-6 could not be established by using NMR information although the methoxy proton (δH 4.24) showed NOE contact (Figure 2) with the hemiaminal proton (δH 6.54) which in turn revealed similar interactions with the NCH3 group. Likewise, the NCH3 group correlated with the aromatic proton H-4 at δH 7.89 while H-1 had spatial correlations with H-12 and H-11 showed similar contact with H-10.
Figure 2.
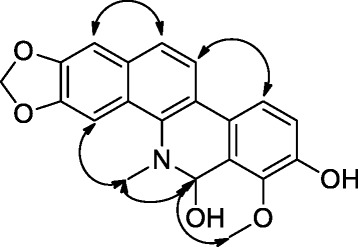
NOESY correlations of compound 1.
The foregoing data led to identification of compound 1 as 1-methoxy-12-methyl-12,13-dihydro-[1,3]dioxolo[4′,5′:4,5]benzo[1,2-c]phenanthridine-2,13-diol which was trivially named buesgenine (Figure 3).
Figure 3.
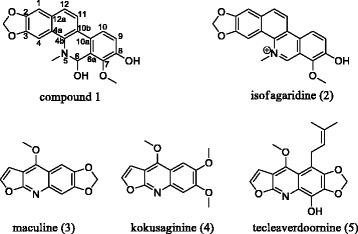
Structure of the isolated compounds.
The known compounds (Figure 3) were identified as isofagaridine 2 [15], maculine 3 [16], kokusaginine 4 [17] and teclearverdoornine 5 [18] based on their NMR data and by comparison with those previously reported.
Biological assay
Compound 1 displayed cytotoxicity towards all the nine tested cancer cell lines with IC50 values below or around 65 μM while other metabolites showed selective activities. The activities of compounds 2–4 were observed on 8/9, 2/9, and 6/9 of the tested cancer cell lines, respectively (Table 2). The lowest IC50 values of 0.24 μM and 0.30 μM were obtained with compounds 1 and 2, respectively towards the leukemia CCRF-CEM cancer cell line. The activities of compounds 1–4 were better than that of doxorubicin towards the resistant CEM/ADR5000 cell line (Table 2). Compound 1 can therefore be considered as a potential cytotoxic candidate agent to fight malignant diseases. Interestingly, compound 1 was active on both sensitive and resistant cell lines. Meanwhile, all tested compounds were generally less toxic on normal AML12 hepatocytes. However, compound 1 was generally less active than the reference drug, doxorubicin but could inspire synthesis of more cytotoxic analogues. This assumption is supported by recent studies showing the ability of some benzophenanthridines to induce apoptosis in colon carcinoma cancer cells HCT116 [12]. Besides, sanguinarine structurally related to compounds 1 and 2 has been previously reported as apoptosis inducer in KB [19], AsPC-1, BxPC-3 [20], U937 [21], and MDA-MB-231 [22] cancer cells via different mechanisms.
Table 2.
Cytotoxicity of the studied compounds towards sensitive and drug-resistant cancer cell lines and normal cells as determined by the resazurin assay
| Cell lines | Isolated compounds, doxorubicin and IC 50 values (μM) | ||||
|---|---|---|---|---|---|
| Compounds | Doxorubicin | ||||
| 1 | 2 | 3 | 4 | ||
| CCRF-CEM | 0.24 ± 0.01 | 0.30 ± 0.04 | 89.09 ± 6.22 | 49.81 ± 5.04 | 0.20 ± 0.06 |
| CEM/ADR5000 | 31.58 ± 3.48 | 20.37 ± 2.16 | 63.09 ± 3.75 | 44.56 ± 3.92 | 195.12 ± 14.30 |
| MDA-MB231 | 30.14 ± 4.12 | 41.38 ± 3.44 | >164.61 | 62.01 ± 7.24 | 1.10 ± 0.28 |
| MDA-MB231/BCRP | 65.01 ± 5.37 | 113.98 ± 9.82 | >164.61 | >154.44 | 7.83 ± 0.47 |
| HCT116 (p53 +/+ ) | 42.46 ± 3.22 | 87.08 ± 7.55 | >164.61 | 119.88 ± 13.14 | 1.41 ± 0.29 |
| HCT116 (p53 −/− ) | 62.34 ± 4.41 | >119.76 | >164.61 | >154.44 | 4.06 ± 0.07 |
| U87MG | 60.55 ± 7.29 | 105.19 ± 9.16 | >164.61 | 70.08 ± 6.40 | 1.06 ± 0.15 |
| U87MG. ΔEGFR | 61.84 ± 4.68 | 115.30 ± 13.78 | >164.61 | >154.44 | 6.11 ± 0.57 |
| HepG2 | 22.37 ± 1.97 | 26.69 ± 3.15 | >164.61 | 90.77 ± 8.86 | 3.83 ± 0.94 |
| AML12 | >106.92 | >119.76 | >164.61 | >154.44 | >73.59 |
Conclusions
The purification of the aerial part of Z. buesgenii monitored by TLC and Dragendorff reagent as alkaloids indicator led to the isolation of one new benzophenanthridine (buegenine, 1) along with four known metabolites namely a benzophenanthridine (isofagaridine, 2) and three furoquinolines (maculine 3, kokusaginine 4, and teclearverdoornine 5). Compounds (1–4) in substantial amount were evaluated for cytotoxicity activities and the obtained secondary metabolites showed from moderate to strong bioactivities. The observed activities corroborated those previously reported on similar benzophenanthridine alkaloids [19-22] indicating that compounds 1 and 2 can be chemically explored to develop other chemotherapeutic agents.
Methods
General procedure
Optical rotation: JASCO P-2000 polarimeter; IR (KBr disc): JASCO A-302 spectrophotometer; HR-ESI-MS: JOEL MS apparatus; 1 and 2D NMR: Brüker DRX-400 MHz with TMS as internal reference. Thin layer chromatography (TLC) was performed over silica gel aluminum plates 60 F254. Silica gel 40–63 μm were used for columns chromatography (CC) separation. The melting point (m.p.) was measured by an Electro thermal IA 9000 digital melting point apparatus: uncorrected.
Plant collection
The aerial of Z. buesgenii was collected in Buea, South West region of Cameroon, in January 2014. Voucher specimens (BUD 0510) were deposited in the Herbarium of the Botany Department of the University of Dschang, Cameroon.
Extraction and isolation
The dried aerial part (1.8 kg) of Z. buesgenii was cut into small pieces, crushed and the powder was extracted for two days with a sufficient volume of methylene chloride (DCM)/MeOH (1:1). The solid residue was further extracted with MeOH for 24 h. Both solutions were pooled together and evaporated in vacuo to afford 50 g of crude extract. This latter was subjected to a liquid–solid extraction using successively n-hexane (hex), ethyl acetate (EA) and MeOH as the liquid part. Hex and EA fractions were pooled together based on the TLC profile to give fraction A (35 g). TLC of fractions A and B (MeOH) sprayed with Dragendorff’s reagent, revealed the presence of alkaloids in A. Therefore, this latter was purified by silica gel CC eluted with hex, hex/EA (gradient) and EA yielding six sub-fractions (A1-A6). Maculine (3, 1.5 mg) was isolated from A2 eluted with hex/EA (95:5). A3 [5.2 g, hex/EA (3:1)] was further chromatographed on silica gel column eluted with hex/EA in gradient conditions. 60 sub-fractions were collected and isofagaridine (2, 3.1 mg) was filtered from the sub-fractions 10–15 eluted with hex/EA (9:1) while kokusaginine (4, 5.1 mg) was obtained from the sub-fractions 17–23 eluted with the same mixture of solvent. Compound 1 (3.7 mg) was further isolated from sub-fractions 26–33 eluted with hex/EA (85:15). A4 [10.2 g, hex/EA (1:1)] followed the same purification process under isocratic conditions of hex/EA (3:1) used as eluent to give teclearverdoornine (5, 0.7 mg). This latter (0.21 mg) was further obtained from the purification of A5 [8.7 g, hex/EA (1:3)] by using Hex/EA in the gradient condition.
Buesgenine, 1-methoxy-12-methyl-12,13-dihydro-[1,3]dioxolo[4′,5′:4,5]benzo[1,2-c]phenanthridine-2,13-diol (1)
Red powder, m.p. 177°C; [α]D –7 [c 0.37, CH3OH]; IR (KBr), νmax 3450, 3074, 1540, 1480, 1475, 1404, 1385, 1321, 1284, 1257, 1203, 1161, 1126, 1082 cm−1; 1H (C5D5N, 400 MHZ) and 13C (C5D5N, 100 MHZ) NMR data see Table 1 and these data have been compiled in the Additional file 1 provided as supporting information; HR-ESIMS: m/z 374.1003 [C20H17NO5 + Na]+ (calcd. 374.1004).
Cytotoxicity assay
The resazurin reduction assay [23] was performed to assess the cytotoxicity of compounds and doxorubicin as control drug towards various sensitive and drug-resistant cancer cell lines, including the CCRF-CEM and CEM/ADR5000 leukemia, MDA-MB231 breast cancer cells and its resistant subline MDA-MB231/BCRP, HCT116p53+/+ colon cancer cells and its resistant subline HCT116p53−/−, U87MG glioblastoma cells and its resistant subline U87MG. ΔEGFR and HepG2 hepatocarcinoma cells and normal AML12 hepatocytes. The assay is based on the reduction of the indicator dye, resazurin, to the highly fluorescent resorufin by viable cells. Non-viable cells rapidly lose their metabolic capacity to reduce resazurin and, thus, do not produce fluorescent signals anymore. Briefly, adherent cells were detached by treatment with 0.25% trypsin/EDTA (Invitrogen, Darmstadt Germany) and an aliquot of 1 × 104 cells was placed in each well of a 96-well cell culture plate (Thermo Scientific, Langenselbold, Germany) in a total volume of 200 μL. Cells were allowed to attach overnight and then were treated with different concentrations of compounds. For suspension cells, aliquots of 2 × 104 cells per well were seeded in 96-well-plates in a total volume of 100 μL. The studied compounds were immediately added in varying concentrations in an additional 100 μL of culture medium to obtain a total volume of 200 μL/well. After 72 h, resazurin (Sigma-Aldrich, Schnelldorf, Germany) (20 μL, 0.01% w/v) in distilled H2O was added to each well and the plates were incubated at 37°C for 4 h. Fluorescence was measured on an Infinite M2000 ProTM plate reader (Tecan, Crailsheim, Germany) using an excitation wavelength of 544 nm and an emission wavelength of 590 nm. Each assay was done at least twice with six replicates each. The viability was evaluated based on a comparison with untreated cells. IC50 values represent the compound concentrations required to inhibit 50% of cell proliferation and were calculated from a calibration curve by linear regression using Microsoft Excel [24].
Acknowledgements
V. K. is very grateful to the Alexander von Humboldt foundation for an 18 months fellowship to visit the Department of Prof. Efferth (Johannes Gutenberg-University, Mainz, Germany) through the “Georg Foster Research Fellowship for Experienced Researcher” program for funding this work.
Additional file
NMR spectra of the new compound have been provided as an online file.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RST and SLP isolated the compounds, SLP elucidated the structure and wrote the manuscript, VK carried out the bio-assays, TE and BTN read and brought some corrections to the paper. All authors read and approved the final manuscript.
Contributor Information
Louis P Sandjo, Email: plsandjo@yahoo.fr.
Victor Kuete, Email: kuetevictor@yahoo.fr.
Rodrigue S Tchangna, Email: Rosoprodrigue@yahoo.fr.
Thomas Efferth, Email: efferth@uni-mainz.de.
Bonaventure T Ngadjui, Email: ngadjuibt@yahoo.fr.
References
- 1.Waterman PG. New combinations in Zanthoxylum L. Taxon. 1975;24:361–366. doi: 10.2307/1218347. [DOI] [Google Scholar]
- 2.Burkill HM. The useful plant of west tropical Africa. Royal Botanic Gardens, Kew-UK. 1985;4:4–6. [Google Scholar]
- 3.Fonge BA, Egbe EA, Fongod AGN, Focho DA, Tchetcha DJ, Nkembi L, Tacham WN. Ethnobotany survey and uses of plants in the Lewoh-Lebang communities in the Lebialem highlands, South West Region, Cameroon. J Med Plants Res. 2012;6:855–865. doi: 10.5897/JMPR11.1494. [DOI] [Google Scholar]
- 4.Yang C-H, Cheng M-J, Lee S-J, Yang C-W, Chang H-S, Chen I-S. Secondary metabolites and cytotoxic activities from the stem bark of Zanthoxylum nitidum. Chem Biodivers. 2009;6:846–857. doi: 10.1002/cbdv.200800107. [DOI] [PubMed] [Google Scholar]
- 5.Huang H-Y, Ishikawa T, Peng C-F, Tsai I-L, Chen I-S. Constituents of the root wood of Zanthoxylum wutaiense with antitubercular activity. J Nat Prod. 2008;71:1146–1151. doi: 10.1021/np700719e. [DOI] [PubMed] [Google Scholar]
- 6.Samita FN, Sandjo LP, Ndiege IO, Hassanali A, Lwande W. Zanthoxoaporphines A–C: Three new larvicidal dibenzo[de, g]quinolin-7-one alkaloids from Zanthoxylum paracanthum (Rutaceae) Beilstein J Org Chem. 2013;9:447–452. doi: 10.3762/bjoc.9.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J-J, Fang H-Y, Duh C-Y, Chen I-S. New indolopyridoquinazoline, benzo[c]phenanthridines and cytotoxic constituents from Zanthoxylum integrifoliolum. Planta Med. 2005;71:470–475. doi: 10.1055/s-2005-864144. [DOI] [PubMed] [Google Scholar]
- 8.Costa EV, Pinheiro MLB, AD L d S, Barison A, Campos FR, Valdez RH, Ueda-Nakamura T, Dias Filho BP, Nakamu CV. Trypanocidal activity of oxoaporphine and pyrimidine-β-carboline alkaloids from the branches of Annona foetida Mart. (Annonaceae) Molecules. 2011;16:9714–9720. doi: 10.3390/molecules16119714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Lima JPS, Pinheiro MLB, Santos AMG, Pereira JLS, Santos DMF, Barison A, Silva-Jardim I, Costa EV. In vitro antileishmanial and cytotoxic activities of Annona mucosa (Annonaceae) Rev Virtual Quim. 2012;4:692–702. [Google Scholar]
- 10.Luo X, Pires D, Aínsa JA, Gracia B, Duarte N, Mulhovo S, Anes E, Ferreira M-JU. Zanthoxylum capense constituents with antimycobacterial activity against Mycobacterium tuberculosis in vitro and ex vivo within human macrophages. J Ethnopharmacol. 2013;146:417–422. doi: 10.1016/j.jep.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Garcez FR, da Silva AG F, Garcez WS, Linck G, de Fatima Matos FC, Santos ECS, Queiroz LM. Cytotoxic aporphine alkaloids from Ocotea acutifolia. Planta Med. 2011;77:383–387. doi: 10.1055/s-0030-1250401. [DOI] [PubMed] [Google Scholar]
- 12.Mansoor TA, Borralho PM, Luo X, Mulhovo S, Rodrigues CMP, Ferreira M-JU. Apoptosis inducing activity of benzophenanthridine-type alkaloids and 2-arylbenzofuran neolignans in HCT116 colon carcinoma cells. Phytomedicine. 2013;20:923–929. doi: 10.1016/j.phymed.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 2002;19:742–760. doi: 10.1039/b104971m. [DOI] [PubMed] [Google Scholar]
- 14.Tarus PK, Coombes PH, Crouch NR, Mulholland DA. Benzo[c]phenanthridine alkaloids from stem bark of the Forest Knobwood, Zanthoxylum davyi (Rutaceae) S Afr J Bot. 2006;72:555–558. doi: 10.1016/j.sajb.2006.03.014. [DOI] [Google Scholar]
- 15.Nakanishi T, Suzuki M. Revision of the structure of fagaridine based on the comparison of UV and NMR data of synthetic compounds. J Nat Prod. 1998;61:1263–1267. doi: 10.1021/np980193s. [DOI] [PubMed] [Google Scholar]
- 16.Nunes FM, Barros-Filho BA, de Oliveira MCF, Andrade-Neto M, de Mattos MC, Mafezoli J, Piran JR. 1H and 13CNMR spectra of 3,8-dimethoxyfuro[3,2-g]coumarin and maculine from Esenbeckia grandiflora Martius (Rutaceae) Magn Reson Chem. 2005;43:864–866. doi: 10.1002/mrc.1621. [DOI] [PubMed] [Google Scholar]
- 17.Cardoso-Lopes EM, Maier JA, da Silva MR, Regasini LO, Simote SY, Lopes NP, Pirani JR, da Silva BV, Young MCM. Alkaloids from stems of Esenbeckia leiocarpa Engl. (Rutaceae) as potential treatment for alzheimer disease. Molecules. 2010;15:9205–9213. doi: 10.3390/molecules15129205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okogun JI, Ayafor JF: Tecleaverdoornine: a new C-prenylated phenylated furoquinoline.J Chem Soc Chem Commun 1977, 652–653 (doi:10.1039/C39770000652)
- 19.Chang M-C, Chan C-P, Wang Y-J, Lee P-H, Chen L-I, Tsai Y-L, Lin B-R, Wang Y-L, Jeng J-H. Induction of necrosis and apoptosis to KB cancer cells by sanguinarine is associated with reactive oxygen species production and mitochondrial membrane depolarization. Toxicol Appl Pharmacol. 2007;128:143–151. doi: 10.1016/j.taap.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Ahsan H, Reagan-Shaw S, Breur J, Ahmad N. Sanguinarine induces apoptosis of human pancreatic carcinoma AsPC-1 and BxPC-3 cells via modulations in Bcl-2 family proteins. Cancer Lett. 2007;249:198–208. doi: 10.1016/j.canlet.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Han MH, Yoo YH, Choi YH. Sanguinarine-Induced apoptosis in human leukemia U937 Cells via Bcl-2 down regulation and caspase-3 activation. Chemotherapy. 2008;54:157–165. doi: 10.1159/000140359. [DOI] [PubMed] [Google Scholar]
- 22.Choi WY, Kim G-Y, Lee WH, Choi YH. Sanguinarine, a benzophenanthridine alkaloid, Induces apoptosis in MDA-MB-231 human breast carcinoma cells through a reactive oxygen species-mediated mitochondrial pathway. Chemotherapy. 2008;54:279–287. doi: 10.1159/000149719. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 24.Kuete V, Krusche B, Youns M, Voukeng I, Fankam AG, Tankeo S, Lacmata S, Efferth T. Cytotoxicity of some Cameroonian spices and selected medicinal plant extracts. J Ethnopharmacol. 2011;134:803–812. doi: 10.1016/j.jep.2011.01.035. [DOI] [PubMed] [Google Scholar]


