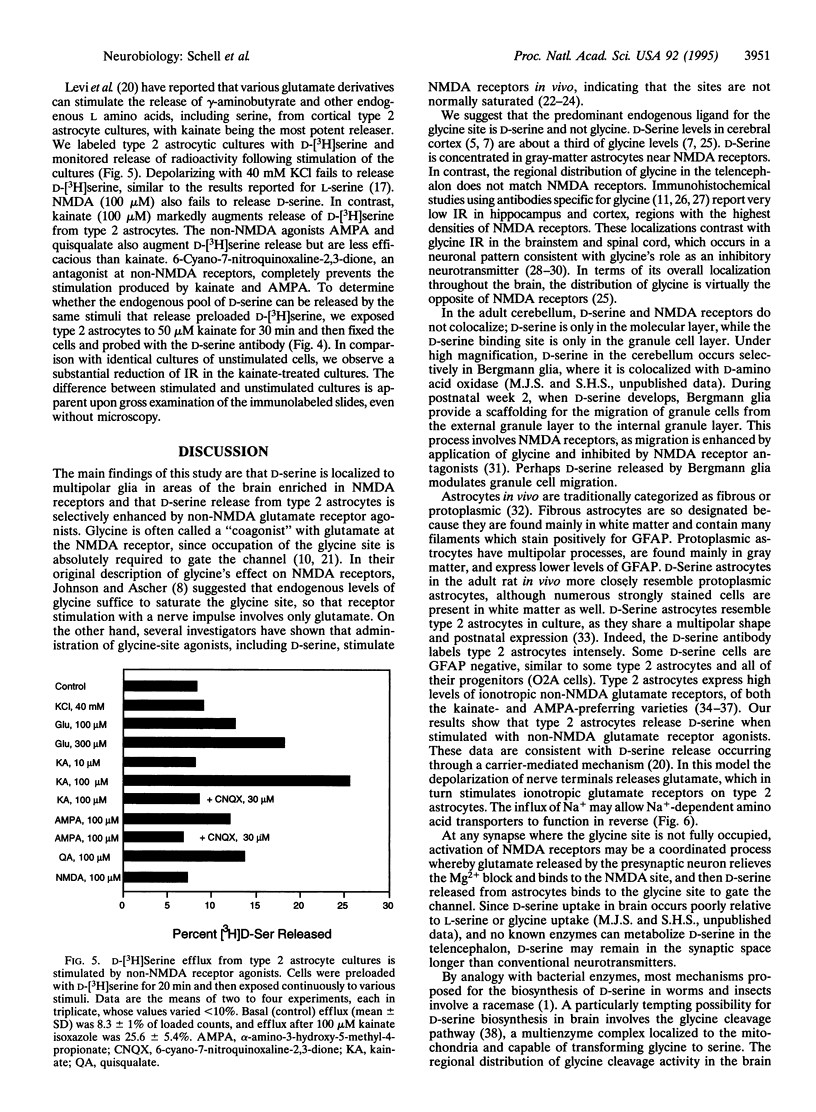
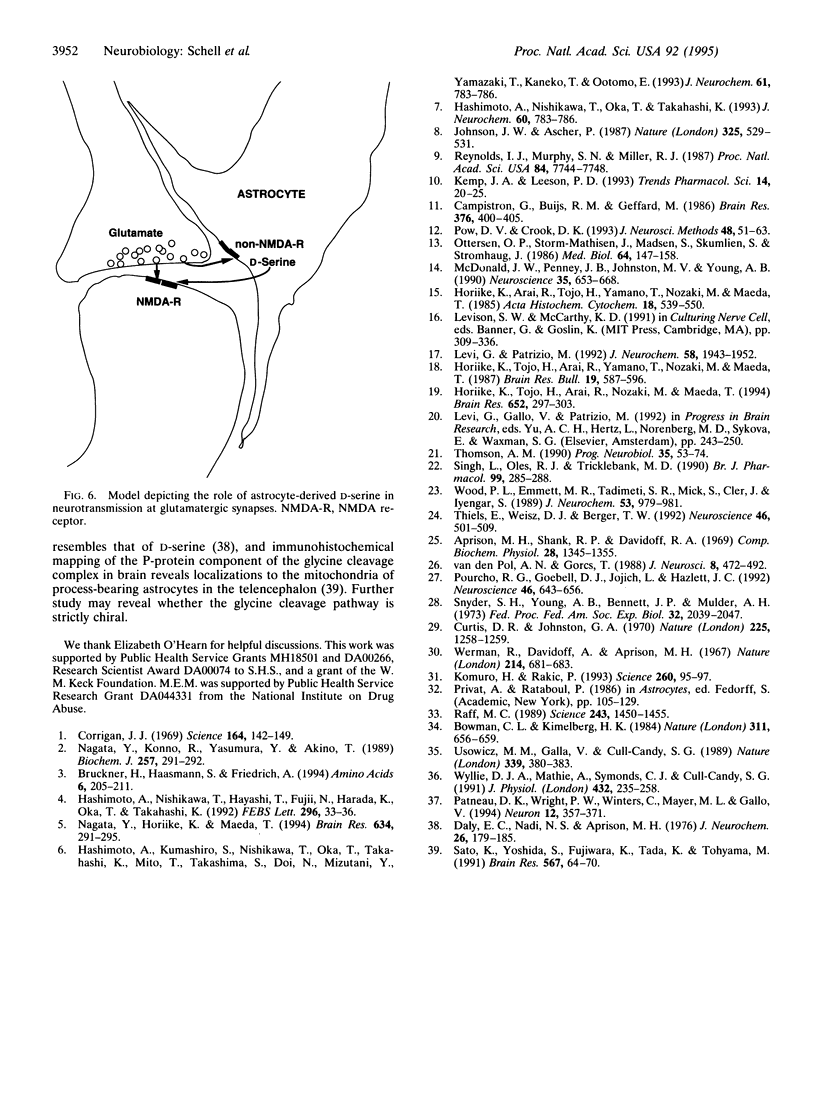
Abstract
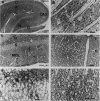
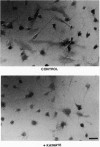
Using an antibody highly specific for D-serine conjugated to glutaraldehyde, we have localized endogenous D-serine in rat brain. Highest levels of D-serine immunoreactivity occur in the gray matter of the cerebral cortex, hippocampus, anterior olfactory nucleus, olfactory tubercle, and amygdala. Localizations of D-serine immunoreactivity correlate closely with those of D-serine binding to the glycine modulatory site of the N-methyl-D-aspartate (NMDA) receptor as visualized by autoradiography and are inversely correlated to the presence of D-amino acid oxidase. D-Serine is enriched in process-bearing glial cells in neuropil with the morphology of protoplasmic astrocytes. In glial cultures of rat cerebral cortex, D-serine is enriched in type 2 astrocytes. The release of D-serine from these cultures is stimulated by agonists of non-NMDA glutamate receptors, suggesting a mechanism by which astrocyte-derived D-serine could modulate neurotransmission. D-Serine appears to be the endogenous ligand for the glycine site of NMDA receptors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aprison M. H., Shank R. P., Davidoff R. A. A comparison of the concentration of glycine, a transmitter suspect, in different areas of the brain and spinal cord in seven different vertebrates. Comp Biochem Physiol. 1969 Mar;28(3):1345–1355. doi: 10.1016/0010-406x(69)90571-4. [DOI] [PubMed] [Google Scholar]
- Bowman C. L., Kimelberg H. K. Excitatory amino acids directly depolarize rat brain astrocytes in primary culture. Nature. 1984 Oct 18;311(5987):656–659. doi: 10.1038/311656a0. [DOI] [PubMed] [Google Scholar]
- Campistron G., Buijs R. M., Geffard M. Glycine neurons in the brain and spinal cord. Antibody production and immunocytochemical localization. Brain Res. 1986 Jun 25;376(2):400–405. doi: 10.1016/0006-8993(86)90208-8. [DOI] [PubMed] [Google Scholar]
- Corrigan J. J. D-amino acids in animals. Science. 1969 Apr 11;164(3876):142–149. doi: 10.1126/science.164.3876.142. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Johnston D. A. Strychnine, glycine and vertebrate postsynaptic inhibition. Nature. 1970 Mar 28;225(5239):1258–1259. doi: 10.1038/2251258a0. [DOI] [PubMed] [Google Scholar]
- Daly E. C., Nadi N. S., Aprison M. H. Regional distribution and properties of the glycine cleavage system within the central nervous system of the rat: evidence for an endogenous inhibitor during in vitro assay. J Neurochem. 1976 Jan;26(1):179–185. [PubMed] [Google Scholar]
- Hashimoto A., Nishikawa T., Hayashi T., Fujii N., Harada K., Oka T., Takahashi K. The presence of free D-serine in rat brain. FEBS Lett. 1992 Jan 13;296(1):33–36. doi: 10.1016/0014-5793(92)80397-y. [DOI] [PubMed] [Google Scholar]
- Hashimoto A., Nishikawa T., Oka T., Takahashi K. Endogenous D-serine in rat brain: N-methyl-D-aspartate receptor-related distribution and aging. J Neurochem. 1993 Feb;60(2):783–786. doi: 10.1111/j.1471-4159.1993.tb03219.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto A., Nishikawa T., Oka T., Takahashi K. Endogenous D-serine in rat brain: N-methyl-D-aspartate receptor-related distribution and aging. J Neurochem. 1993 Feb;60(2):783–786. doi: 10.1111/j.1471-4159.1993.tb03219.x. [DOI] [PubMed] [Google Scholar]
- Horiike K., Tojo H., Arai R., Nozaki M., Maeda T. D-amino-acid oxidase is confined to the lower brain stem and cerebellum in rat brain: regional differentiation of astrocytes. Brain Res. 1994 Aug 1;652(2):297–303. doi: 10.1016/0006-8993(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Horiike K., Tojo H., Arai R., Yamano T., Nozaki M., Maeda T. Localization of D-amino acid oxidase in Bergmann glial cells and astrocytes of rat cerebellum. Brain Res Bull. 1987 Nov;19(5):587–596. doi: 10.1016/0361-9230(87)90076-1. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kemp J. A., Leeson P. D. The glycine site of the NMDA receptor--five years on. Trends Pharmacol Sci. 1993 Jan;14(1):20–25. doi: 10.1016/0165-6147(93)90108-v. [DOI] [PubMed] [Google Scholar]
- Komuro H., Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993 Apr 2;260(5104):95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- Levi G., Patrizio M. Astrocyte heterogeneity: endogenous amino acid levels and release evoked by non-N-methyl-D-aspartate receptor agonists and by potassium-induced swelling in type-1 and type-2 astrocytes. J Neurochem. 1992 May;58(5):1943–1952. doi: 10.1111/j.1471-4159.1992.tb10073.x. [DOI] [PubMed] [Google Scholar]
- McDonald J. W., Penney J. B., Johnston M. V., Young A. B. Characterization and regional distribution of strychnine-insensitive [3H]glycine binding sites in rat brain by quantitative receptor autoradiography. Neuroscience. 1990;35(3):653–668. doi: 10.1016/0306-4522(90)90336-3. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Horiike K., Maeda T. Distribution of free D-serine in vertebrate brains. Brain Res. 1994 Jan 21;634(2):291–295. doi: 10.1016/0006-8993(94)91932-1. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Konno R., Yasumura Y., Akino T. Involvement of D-amino acid oxidase in elimination of free D-amino acids in mice. Biochem J. 1989 Jan 1;257(1):291–292. doi: 10.1042/bj2570291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen O. P., Storm-Mathisen J., Madsen S., Skumlien S., Strømhaug J. Evaluation of the immunocytochemical method for amino acids. Med Biol. 1986;64(2-3):147–158. [PubMed] [Google Scholar]
- Patneau D. K., Wright P. W., Winters C., Mayer M. L., Gallo V. Glial cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor. Neuron. 1994 Feb;12(2):357–371. doi: 10.1016/0896-6273(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Pourcho R. G., Goebel D. J., Jojich L., Hazlett J. C. Immunocytochemical evidence for the involvement of glycine in sensory centers of the rat brain. Neuroscience. 1992;46(3):643–656. doi: 10.1016/0306-4522(92)90151-q. [DOI] [PubMed] [Google Scholar]
- Pow D. V., Crook D. K. Extremely high titre polyclonal antisera against small neurotransmitter molecules: rapid production, characterisation and use in light- and electron-microscopic immunocytochemistry. J Neurosci Methods. 1993 Jun;48(1-2):51–63. doi: 10.1016/s0165-0270(05)80007-x. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Glial cell diversification in the rat optic nerve. Science. 1989 Mar 17;243(4897):1450–1455. doi: 10.1126/science.2648568. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Murphy S. N., Miller R. J. 3H-labeled MK-801 binding to the excitatory amino acid receptor complex from rat brain is enhanced by glycine. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7744–7748. doi: 10.1073/pnas.84.21.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Yoshida S., Fujiwara K., Tada K., Tohyama M. Glycine cleavage system in astrocytes. Brain Res. 1991 Dec 13;567(1):64–70. doi: 10.1016/0006-8993(91)91436-5. [DOI] [PubMed] [Google Scholar]
- Singh L., Oles R. J., Tricklebank M. D. Modulation of seizure susceptibility in the mouse by the strychnine-insensitive glycine recognition site of the NMDA receptor/ion channel complex. Br J Pharmacol. 1990 Feb;99(2):285–288. doi: 10.1111/j.1476-5381.1990.tb14695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H., Young A. B., Bennett J. P., Mulder A. H. Synaptic biochemistry of amino acids. Fed Proc. 1973 Oct;32(10):2039–2047. [PubMed] [Google Scholar]
- Thiels E., Weisz D. J., Berger T. W. In vivo modulation of N-methyl-D-aspartate receptor-dependent long-term potentiation by the glycine modulatory site. Neuroscience. 1992;46(3):501–509. doi: 10.1016/0306-4522(92)90139-s. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. Glycine is a coagonist at the NMDA receptor/channel complex. Prog Neurobiol. 1990;35(1):53–74. doi: 10.1016/0301-0082(90)90040-n. [DOI] [PubMed] [Google Scholar]
- Usowicz M. M., Gallo V., Cull-Candy S. G. Multiple conductance channels in type-2 cerebellar astrocytes activated by excitatory amino acids. Nature. 1989 Jun 1;339(6223):380–383. doi: 10.1038/339380a0. [DOI] [PubMed] [Google Scholar]
- Werman R., Davidoff R. A., Aprison M. H. Inhibition of motoneurones by iontophoresis of glycine. Nature. 1967 May 13;214(5089):681–683. doi: 10.1038/214681a0. [DOI] [PubMed] [Google Scholar]
- Wood P. L., Emmett M. R., Rao T. S., Mick S., Cler J., Iyengar S. In vivo modulation of the N-methyl-D-aspartate receptor complex by D-serine: potentiation of ongoing neuronal activity as evidenced by increased cerebellar cyclic GMP. J Neurochem. 1989 Sep;53(3):979–981. doi: 10.1111/j.1471-4159.1989.tb11803.x. [DOI] [PubMed] [Google Scholar]
- Wyllie D. J., Mathie A., Symonds C. J., Cull-Candy S. G. Activation of glutamate receptors and glutamate uptake in identified macroglial cells in rat cerebellar cultures. J Physiol. 1991 Jan;432:235–258. doi: 10.1113/jphysiol.1991.sp018383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A. N., Gorcs T. Glycine and glycine receptor immunoreactivity in brain and spinal cord. J Neurosci. 1988 Feb;8(2):472–492. doi: 10.1523/JNEUROSCI.08-02-00472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]