Abstract
Objective
To determine the geographical distribution of hemoglobinopathies in the State of Pernambuco, to characterize the children with these diseases and to describe factors associated with their follow-up at the referral center during the period from 2003 to 2010.
Methods
A retrospective, cross-sectional, descriptive study was carried out of 275 medical records from a total of 302 children with hemoglobinopathies diagnosed by the National Neonatal Screening Program in the State of Pernambuco in the study period. Microsoft Excel was used for data processing and analysis. The chi-square and the Fisher test were used for statistical analysis. The level of significance was set at 5%. Terra View software was used to analyze the geographical distribution of hemoglobinopathies in the State.
Results
A total of 8.9% of the cases of hemoglobinopathies detected in the period were not followed up at the referral center. For the mothers of children with diseases, this was their second or third or more pregnancy in 64.2% and 30.2%, respectively. Regarding the influence of region of residence and regular medical appointments, the study demonstrated that children from the Zona da Mata, Sertão and Vale do São Francisco regions did not attend 45.2%, 50% and 55.6% of their appointments in the outpatient department, respectively.
Conclusions
This study shows that a significant number of children do not begin consultations in the outpatient clinic and even those who started treatment early and who have the most severe form of the disease, usually miss medical appointments.
Keywords: Hemoglobinopathies, Hemoglobin SC disease, Neonatal screening
Introduction
Hemoglobinopathies result from mutations in the genes that encode the alpha (α) and beta (β) globin chains of the hemoglobin molecule (Hb). The abnormal Hb S is the result of a mutation that leads to the replacement of a glutamic acid with a valine at position 6 of the β chain, with consequent changes in the Hb molecule.1
In general, all symptomatic clinical forms of the presence of the Hb S gene, either homozygosis or in combination with another variant hemoglobin such as Hb C, or Hb D or in association with the β thalassemia gene are known as sickle cell disease (SCD). These are the most prevalent forms of all hemoglobinopathies and have the greatest impact owing to the seriousness of clinical manifestations.2, 3, 4 Sickle cell anemia is the form of the disease characterized by Hb S homozygosis inherited from both parents (Hb SS).5
The World Health Organization (WHO) estimates that 270 million people carry genes determining variant hemoglobins and that every year 300,000 children are born with SCD.6 It is the most prevalent hereditary disease in Brazil, affecting 25,000–30,000 people, with approximately 3500 children born with SCD annually. In the State of Pernambuco, the incidence is 1:1400.7 The disease is thus a public health issue, associated with high infant morbidity and demands regular medical care, adequate housing and nutrition, and general health care.8
The disease's clinical variability results from a combination of hereditary and environmental factors. A newborn with SCD is generally asymptomatic due to the protective effect of fetal hemoglobin (Hb F) which, during this period of life, represents around 80% of the total hemoglobin. With the replacement of Hb F by an increase in Hb S, clinical manifestations begin to appear,9 usually starting at the age of six months and lasting throughout the patient's life.5, 10
The first years are marked by hemolytic anemia and by vaso-occlusive episodes, dactylitis (inflammation of fingers or toes) or hand-foot syndrome, splenic hypofunction or sequestration, and a higher susceptibility to infections.9
Other clinical manifestations may affect SCD children, such as thoracic syndrome, cognitive problems, priapism (which may lead to sexual impotence) and damage to organs such as the kidneys, the lungs and the eyes, diminishing quality of life, generating incapacitation, and frequent stays in hospital for treatment or surgical procedures.11
Neonatal diagnosis, early prophylactic treatment with penicillin, prophylactic vaccination, follow-up by specialized services with periodic clinical evaluations, hospitalization in situations of risk, and early identification of splenic sequestration by mothers and caregivers have contributed to a reduction in mortality from 25% to approximately 3% in the first five years of life.8, 12
The National Neonatal Screening Program (NNSP), created by Resolution GM/MS no. 822/01,13 enabled early diagnosis, treatment and follow-up of hemoglobinopathies. In the State of Pernambuco, according to Resolution GM no. 452, of October 18, 200114 children with the diagnosis of SCD have been sent to a referral center since 2002 to immediately start treatment in a specialized multidisciplinary outpatient department and a specialized clinic.15
The NNSP includes early treatment and regular outpatient appointments of children after neonatal diagnosis.13, 16 It is, however, difficult to achieve this, as several factors lead patients to miss medical consultations. An understanding of what hinders treatment has proved a challenge, as there has been little research on children's follow-up at the referral center after diagnosis at neonatal screening.
The aims of this study were to determine the geographic distribution of hemoglobinopathies in the State of Pernambuco, to characterize the children affected and to describe factors associated to the follow-up at the referral center in the period from 2003 to 2010.
Methods
A retrospective, cross-sectional, descriptive study was carried out of 275 (91.5%) medical records of a total of 302 children with hemoglobinopathies as diagnosed by the NNSP in the State of Pernambuco in the period from 2003 to 2010, who started treatment in the referral center, located at the Pernambuco Hematology and Hemotherapy Foundation (HEMOPE).
According to Datasus,17 Pernambuco has an area of 98,311 km2 and a population of 8,810,318. Between 2003 and 2010, a total of 1,166,189 live births occurred in Pernambuco and 605,632 newborns were screened at public health care clinics.
The drawing of blood samples for neonatal screening is carried out at health care units located around the State. Blood samples are then taken to the Pernambuco Central Laboratory (LACEN), where they are processed and analyzed. Positive results for hemoglobinopathies are sent to the Neonatal Screening Referral Service, which refers the cases to HEMOPE, the only referral center in the state that performs treatment and regular outpatient follow-ups in a specialized multidisciplinary outpatient department.
For this study, data collection initially used the LACEN database; the NNSP identified 302 children with hemoglobinopathies. The records of 275 were found at the referral center and several factors were analyzed: diagnosis, region of residence, socioeconomic status of the family, age at first medical appointment, regularity of medical appointments, number of births to the child's mother, and the existence of siblings with hemoglobinopathies. Subsequently, the geographic distribution of the disease in the State was analyzed.
Microsoft Excel was used for data processing and analysis. The chi-square and the Fisher tests were used for statistical analysis. The level of significance was set at 5% (p-value < 0.05). Terra View software18 was used to analyze the geographical distribution of hemoglobinopathies in the State.
This study was approved by the Human Research Ethics Committee of the Prof. Fernando Figueira Institute for Integral Medicine (IMIP) and by the Pernambuco Hematology and Hemotherapy Foundation (HEMOPE) (protocols no. 2084-11 and 043/2011).
Results
Of all hemoglobinopathy cases detected (n = 302) in the study period, 27 (8.9%) were not being treated in the referral center (HEMOPE). Of these, 14 were found in the LACEN database and the other 13 had been to the Referral Service (Hospital da Restauração), but did not start treatment at HEMOPE. Of all the detected cases, 20 (6.6%) were found at HEMOPE but were missing from the LACEN database.
After applying the Fisher test, no significant association (p-value = 0.1) was found between age at the first HEMOPE medical appointment and the place of residence. Family income had no influence on age at first medical appointment (p-value = 0.464) for families earning up to one minimum wage, and 78.8% of the children were taken to the outpatient department during the first six months of life (Table 1). Again, family income had no influence on missing regular medical appointments at HEMOPE (p-value = 0.227).
Table 1.
Region of residence and family income stratified by age at first medical appointment.
| Age on first medical appointment (months) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1–6 |
7–12 |
13–24 |
25 or more |
Total |
||||||
| n | % | n | % | n | % | n | % | n | % | |
| Region of residence | ||||||||||
| Recife | 58 | 79.5 | 6 | 8.2 | 9 | 12.3 | 0 | 0.0 | 73 | 100.0 |
| Greater Recife | 75 | 78.9 | 9 | 9.5 | 8 | 8.4 | 3 | 3.2 | 95 | 100.0 |
| Zona da Mata | 34 | 81.0 | 6 | 14.3 | 2 | 4.8 | 0 | 0.0 | 42 | 100.0 |
| Agreste | 25 | 73.5 | 8 | 23.5 | 0 | 0.0 | 1 | 2.9 | 34 | 100.0 |
| Sertão | 12 | 85.7 | 1 | 7.1 | 1 | 7.1 | 0 | 7.1 | 14 | 100.0 |
| São Francisco Valley | 16 | 94.1 | 1 | 5.9 | 0 | 0.0 | 0 | 0.0 | 17 | 100.0 |
| Family Income (min. wages) | ||||||||||
| 0–1.0 | 145 | 78.8 | 25 | 13.6 | 12 | 6.5 | 2 | 1.1 | 184 | 100.0 |
| 1.01–2.0 | 51 | 79.7 | 6 | 9.4 | 6 | 9.4 | 1 | 1.6 | 64 | 100.0 |
| Over 2.0 | 21 | 95.5 | 0 | 0.0 | 1 | 4.5 | 0 | 0.0 | 22 | 100.0 |
Note: Information on family income was missing from five medical records.
The present study shows that, even in cities far from the State capital, such as the ones in the São Francisco Valley, 94.1% of children have their first medical appointment in the outpatient department within their first six months of life. Of those residing in Recife, 12.3%, have the first consultation between 13 and 24 months after diagnosis.
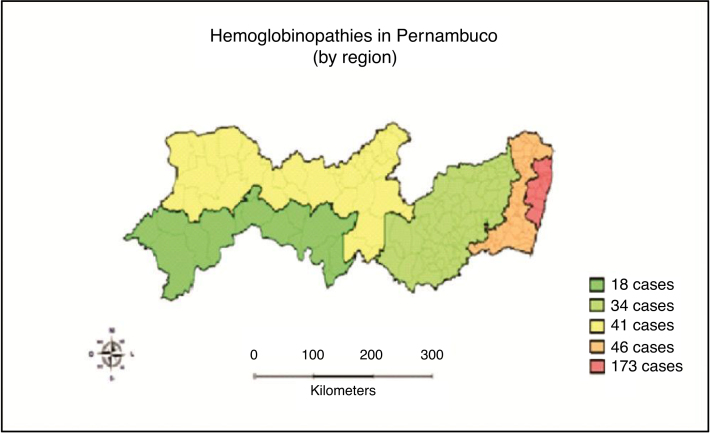
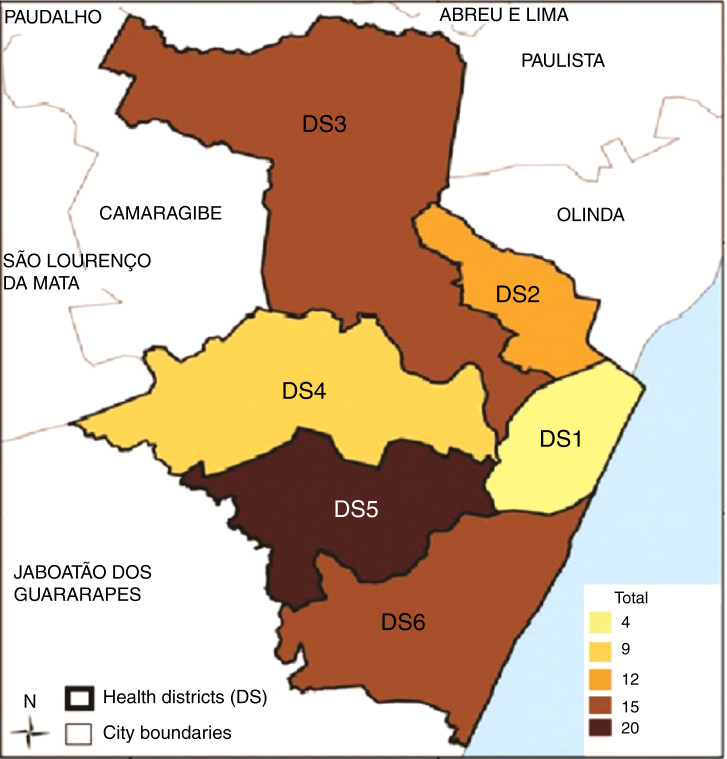
Figure 1, Figure 2 show the geographic distribution of hemoglobinopathies in the State of Pernambuco, according to the child's place of residence stratified by region of the State and by Recife City Health District, respectively.
Figure 1.
Geographic distribution of children with hemoglobinopathies by region of residence.
Figure 2.
Geographic distribution of children with hemoglobinopathies in Recife City Health Districts.
Of the children commencing follow-up in the first six months of life, most had sickle cell anemia (Hb SS); this corresponds to 79.6% of all cases of hemoglobinopathies. The level of schooling of parents (p-value = 0.168) had no influence on the child going to the outpatient unit or on not missing subsequent medical appointments (p-value = 0.691).
The fact that a sibling had a hemoglobinopathy was not significantly associated (p-value = 0.213) with the age of the child at the time of the first medical appointment.
Regular return visits to the referral outpatient unit for medical appointments was considered in respect to the number of appointments according to type of disease, as well as the agenda of the referral center.
Children with Hb SS and Hb SC returned more frequently to the outpatient unit, although they missed appointments rather often. Those with Sß thalassemia or Hb CC missed appointments regularly (Table 2).
Table 2.
Regular return visits to referral center for medical appointments by type of hemoglobinopathy.
| Type of hemoglobinopathy |
||||||||
|---|---|---|---|---|---|---|---|---|
| Hb SS |
Hb SC |
Sß thalassemia |
Hb CC |
|||||
| n | % | n | % | n | % | n | % | |
| Regular medical appointments | ||||||||
| Yes | 141.0 | 73.8 | 39.0 | 65.0 | 10.0 | 50.0 | 1.0 | 25.0 |
| No | 50.0 | 26.2 | 21.0 | 35.0 | 10.0 | 50.0 | 3.0 | 75.0 |
| Total | 191 | 100.0 | 60.0 | 100.0 | 20.0 | 100.0 | 4.0 | 100.0 |
A total of 74.5% of the families with a child with a hemoglobinopathy did not receive the family allowance given by the government to poor, elderly or disabled persons. Of the families that received it, 77.1% had a child with Hb SS. The financial help had no influence on the regularity of return visits to the outpatient clinic (p-value = 0.543), although those who received it hardly ever missed medical appointments.
Of all the children covered by the study (n = 275), 76.4% were first-borns. Of the mothers who already had children with the disease, 64.2% were pregnant for a second time and 30.2% were pregnant for at least the third time.
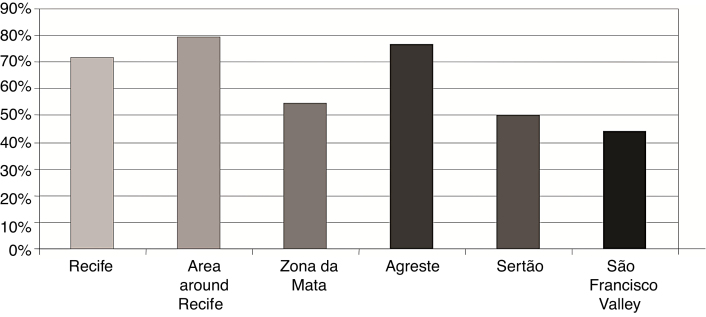
As for the influence of the region of residence on regular return visits, the study showed that children living in the Zona da Mata region missed 45.2% of HEMOPE appointments, compared with 50% of those living in the Sertão and 55.6% of those from in the São Francisco Valley region (Figure 3).
Figure 3.
Region of residence and regular return visits to the referral center.
Discussion
This study shows that diagnosis of hemoglobinopathies using the Guthrie test does not guarantee treatment for all affected children, because 8.9% of positive cases are not being followed up at the referral center. This may be due to the fact that fetal hemoglobin strongly inhibits the sickling of red cells and is present in high percentages during the first months of life, thereby protecting children from the clinical effects of the disease. As there are no apparent symptoms, the parents do not go to the outpatient clinic, although they are informed of the importance of prophylactics and early follow-up.19, 20
Other factors that explain why children miss appointments are the quality of information on the disease, the availability of transport and the severity of the hemoglobinopathy diagnosed. Furthermore, it is difficult to carry out the whole procedure proposed by the NNSP in Pernambuco, as children with the disease receive the test results at the Central Laboratory, go to the referral service and then to the referral center, which is a complicated procedure.
HEMOPE is the only referral center for the treatment of children with hemoglobinopathies in the State. Once the disease has been diagnosed, children living in Pernambuco must go for specialized follow-up in the State capital of Recife. The present study shows that the region of residence of the children had no influence on the date of the first medical appointment at the outpatient unit, as 12.3% of the children living in Recife only showed up nearly a year after diagnosis.
It was also clear that the children living in cities/towns far from Recife started treatment in the first months of life, but regularly missed subsequent appointments. This result corroborates other studies,15, 21 which suggests that the distance between the place of residence and the referral center, together with the cost of transportation and information on the disease and its treatment make it hard not to miss medical appointments.
The current study also shows that children with Hb SS (79.6%), the most severe form of the disease, were taken to the outpatient unit within the first six months of life. Such results have been confirmed by other studies.22, 23, 24, 25 However, although these children started follow-up early, 26.2% regularly missed appointments or did not follow the treatment schedule properly. The same occurred in children with other hemoglobinopathies, such as Hb SC, Sβ thalassemia and Hb CC.
The reasons for children not going to the outpatient unit may be because of the difficulty to access the service, delayed diagnosis, a lack of understanding of the disease by the family and the asymptomatic character of the less severe types of hemoglobinopathies.24, 25, 26
The current study shows that indicators such as the level of schooling of parents, having a sibling with a hemoglobinopathy, and receipt of financial or family support have no influence on the time of the child's first medical appointment or on the regularity of return visits to the outpatient unit.
These data differ from the results of other studies,19, 25, 27, 28 which showed the influence of socioeconomic status on the outpatient follow-up, continued prophylactics and treatment of the disease. This difference suggests that the quality of information about the disease and its treatment rather than the family's socioeconomic status may determine whether medical appointments will be missed or not.
An analysis of the number of pregnancies of mothers with children affected by the disease shows that this was the second child for most of them (64.2%) while 30.2% had no less than two other children with at least one with SCD. This result may reflect the parents’ lack of understanding of genetic counseling on the probability of generating a sick child.
Some studies highlight the importance of training health care professionals on how to responsibly and clearly inform parents so that conscious reproductive decisions can be taken.28, 29, 30
The current study faced a number of limitations including insufficient information on socioeconomic aspects, difficulties in finding the clinical records for children who were in different neonatal screening units, and data collection from clinical records without using other sources such as interviews with users and professionals, which could have provided further data.
This study shows that a significant number of diagnosed children do not start follow-up at the referral center. Even those who received early treatment missed some subsequent medical appointments. In spite of parents having received genetic counseling, many children are born after the second pregnancy, which suggests that there is a lack of understanding on the part of the families in relation to the information provided by healthcare staff.
This demonstrates the need to decentralize follow-up services for the disease to other cities/towns in the State, to train teams of the government healthcare clinics on how to counsel families in respect to prevention and early treatment, and to change the procedures followed by children after diagnosis, so that parents will be more willing to visit the referral center.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Silva W.S., Lastra A., Oliveira S.F., Guimarães N.K., Grisolia C.K. Avaliação da cobertura do programa de triagem neonatal de hemoglobinopatias em populações do Recôncavo Baiano, Brasil. Cad Saude Publica. 2006;22:2561–2566. doi: 10.1590/s0102-311x2006001200006. [DOI] [PubMed] [Google Scholar]
- 2.Naoum P.C. Prevalência e controle da hemoglobina S. Rev Bras Hematol Hemoter. 2000;22:142–148. [Google Scholar]
- 3.Zago M.A., Falcão P.R., Pasquini R. 4 ed. Atheneu; São Paulo: 2001. Hematologia: fundamentos e prática. [Google Scholar]
- 4.Miller S.T., Sleeper L.A., Pegelow C.H., Enos L.E., Wang W.C., Weiner S.J. Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med. 2000;2:83–89. doi: 10.1056/NEJM200001133420203. [DOI] [PubMed] [Google Scholar]
- 5.Anvisa; Brasília: 2001. Manual de diagnóstico e tratamento de doenças falciformes. [Google Scholar]
- 6.World Health Organization . 2005. Sickle cell anaemia. Report by the secretariat, Executive Board 117th session; p. 5. [Google Scholar]
- 7.Ministério da Saúde . 2014. Doença Falciforme. [online] [cited: 2012 Jul. 10] Available from: http://portal.saude.gov.br/portal/saude/Gestor/visualizar_texto.cfm?idtxt=27777. [Google Scholar]
- 8.Cançado R.D., Jesus J.A. A doença falciforme no Brasil. Res Bras Hematol Hemoter. 2007;29:203–206. [Google Scholar]
- 9.Bandeira F.M., Santos M.N., Bezerra M.A., Gomes Y.M., Araújo A.S., Braga M.C. Triagem familiar ampliada para o gene da hemoglobina S. Rev Saude Publica. 2008;42:1–7. [PubMed] [Google Scholar]
- 10.Loureiro M.M., Rozenfeld S. Epidemiologia de internações por doença falciforme no Brasil. Rev Saude Publica. 2005;39:943–949. doi: 10.1590/s0034-89102005000600012. [DOI] [PubMed] [Google Scholar]
- 11.Zago M.A., Pinto A.C. Fisiopatologia das doenças falciformes: da mutação genética à insuficiência de múltiplos órgãos. Rev Bras Hematol Hemoter. 2007;29:207. [Google Scholar]
- 12.Biancalana H. Faculdade de Ciências Médicas da Universidade Estadual de Campinas; Campinas: 2006. Manifestações bucais em crianças com doença falciforme. [MSc dissertation] [Google Scholar]
- 13.Ministério da Saúde . 2001. Portaria n 822/GM em 6 de junho de 2001. Instituição do Programa Nacional de Triagem Neonatal, no âmbito do Sistema Único de Saúde, para fenilcetonúria, hipotiroidismo congênito, fibrose cística e hemoglobinopatias. Diário Oficial da União. [Google Scholar]
- 14.Secretaria de Assistência a Saúde Portaria no 452 em 18 de outubro de 2001. Habilitação do Programa Nacional de Triagem Neonatal no estado de Pernambuco na Fase II de Implantação. Diario Oficial do Estado de Pernambuco. 2001;18 [Google Scholar]
- 15.Almeida A.M., Godinho T.M., Teles M.S., Rehen A.P., Jalil H.M., Fukuda T.G. Avaliação do Programa de Triagem Neonatal na Bahia no ano de 2003. Rev Bras Saúde Matern Infant. 2006;6:85–91. [Google Scholar]
- 16.Meirelles R.M. Triagem neonatal: ficção ou realidade? Arq Bras Endocrinol Metabol. 2000;44:119–120. [Google Scholar]
- 17.Ministério da Saúde . 2014. Departamento de informática do SUS–DATASUS. [online]. [cited 2012 Jun 10] Available from: http://www2.datasus.gov.br/DATASUS/index.php?area=02. [Google Scholar]
- 18.Instituto Nacional de Pesquisas Espaciais (Brasil) INPE; 1991. Divisão de Processamento de Imagens. Software Terra View. Versão 2. [Google Scholar]
- 19.Silva K.R., Yamaguchi M.U. Os benefícios da inclusão das hemoglobinopatias na triagem neonatal. Arq Cienc Saúde Unipar. 2007;11:67–73. [Google Scholar]
- 20.Adamkiewicz T.V., Silk B.J., Howgate J., Baughman W., Strayhorn G., Sullivan K. Effectiveness of the 7-Valent pneumococcal conjugate vaccine in children with sickle cell disease in the first decade of life. Pediatrics. 2008;121:562–569. doi: 10.1542/peds.2007-0018. [DOI] [PubMed] [Google Scholar]
- 21.Souza R.A., Pratesi R., Fonseca S.F. Análise crítica do Programa de Triagem Neonatal para detecção de hemoglobinopatias em Dourados–MS. Rev Bras Hematol Hemoter. 2010;32:126–130. [Google Scholar]
- 22.Lobo C.L., Bueno L.M., Moura P., Ogeda L.L., Castilho S., Carvalho S.M. Triagem Neonatal para Hemoglobinopatias no Rio de Janeiro. Rev Panam Salud Publica. 2003;13:154–159. doi: 10.1590/s1020-49892003000200018. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho T.M. Triagem Neonatal no Brasil. Rev Med de Minas Gerais. 2005;15:20–22. [Google Scholar]
- 24.Almeida A., Godinho T.M., Teles M.S., Rehem A.P., Jalil H.M., Fukuda T.G. Avaliação do programa de triagem neonatal na Bahia no ano de 2003. Rev Bras Saude Mater Infant. 2006;6:85–91. [Google Scholar]
- 25.Fernandes A.P., Januário J.N., Cangussus C.B., Macedos D.L., Viana M.B. Mortality of children with sickle cell disease: a population study. J Pediatr. 2010;86:279–284. doi: 10.2223/JPED.2005. [DOI] [PubMed] [Google Scholar]
- 26.Stranieri I., Takano O.A. Avaliação do Serviço de Referência em Triagem Neonatal para hipotireoidismo congênito e fenilcetonúria no Estado de Mato Grosso, Brasil. Arq Bras Endocrinol Metabol. 2009;53:446–452. doi: 10.1590/s0004-27302009000400010. [DOI] [PubMed] [Google Scholar]
- 27.Botler J., Camacho L.A., Cruz M.M., George P. Triagem neonatal – o desafio de uma cobertura universal e efetiva. Cien Saude Colet. 2010;15(2):493–508. doi: 10.1590/S1413-81232010000200026. [DOI] [PubMed] [Google Scholar]
- 28.Felix A.A., Souza H.M., Ribeiro S.B. Aspectos epidemiológicos e sociais da doença falciforme. Rev Bras Hematol Hemoter. 2010;32:203–208. [Google Scholar]
- 29.Moraes K.C., Galioti J.B. A doença falciforme: um estudo genético-populacional a partir de doadores de sangue em São José dos Campos, São Paulo, Brasil. Rev Bras Hematol Hemoter. 2010;32:286–290. [Google Scholar]
- 30.Guimarães C.T., Coelho G.O. A importância do aconselhamento genético na anemia falciforme. Cien Saude Coletiva. 2010;15(1):1733–1740. doi: 10.1590/s1413-81232010000700085. [DOI] [PubMed] [Google Scholar]