Abstract
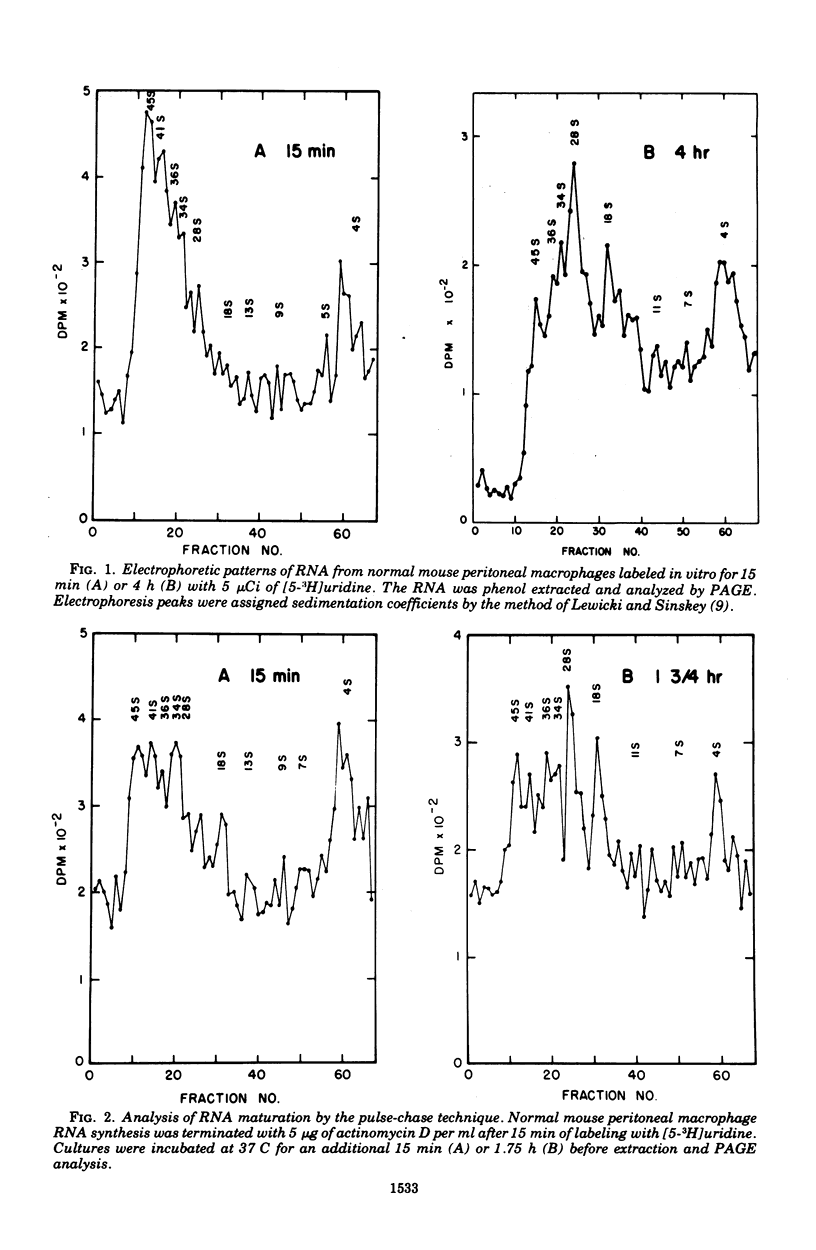
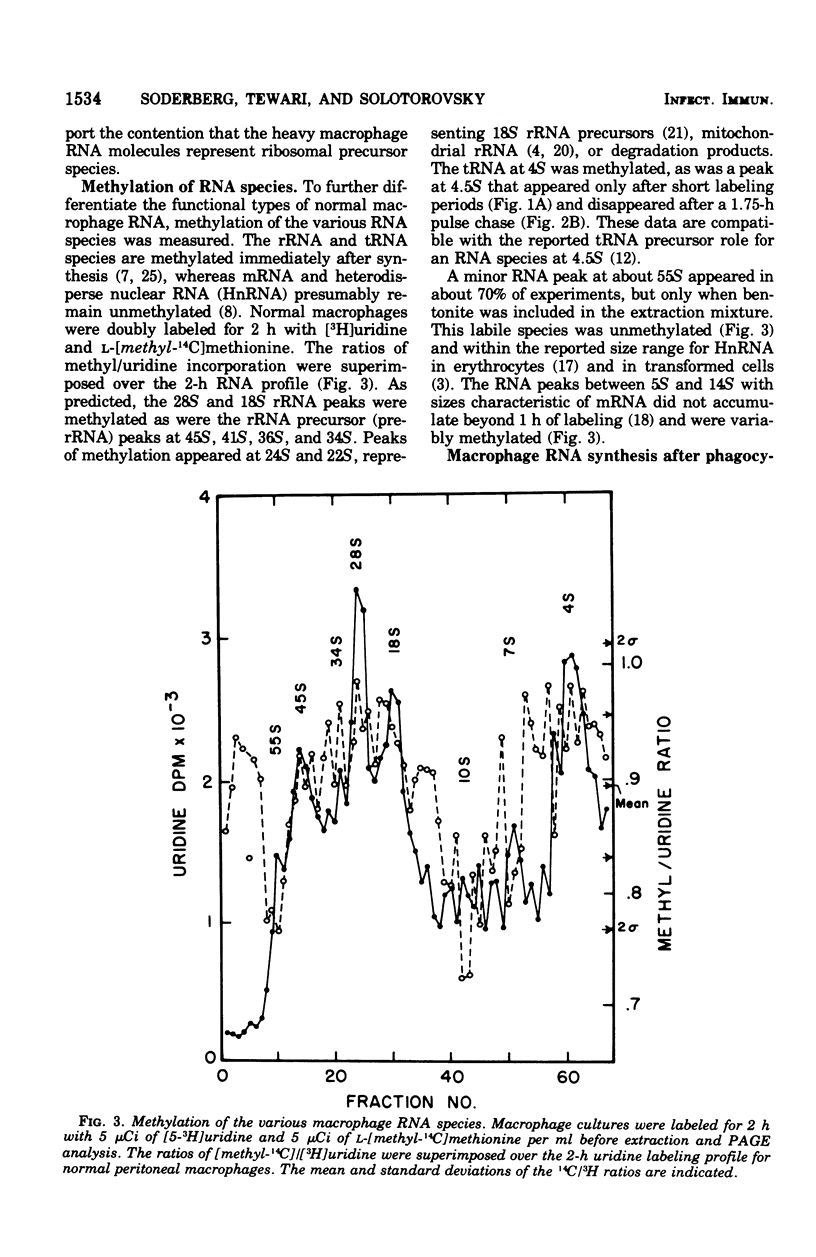
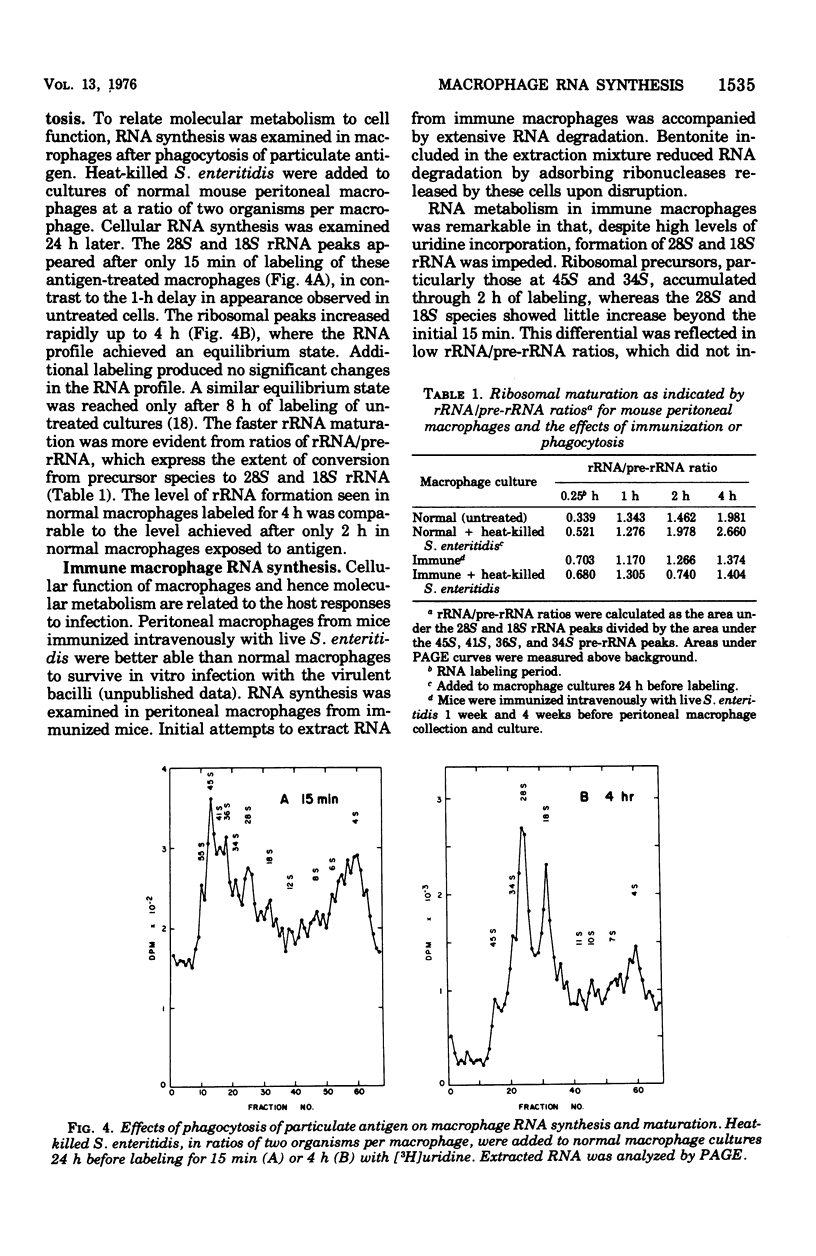
Macrophage ribonucleic acid (RNA) synthesis is an important metabolic process intimately related to the function of these cells. Mouse peritoneal macrophage RNA was extracted with phenol in the presence of bentonite and electrophoresed on composite agarose-polyacrylamide gels. The pulse-chase technique was used to follow the precursor relationships in macrophage ribosomal RNA (rRNA) maturation. The rRNA species at 18S and 28S appeared at 15 and 45 min, respectively, after RNA synthesis was halted. Their appearance corresponded closely to decreases in the rRNA precursors at 45S, 36S, and 34S. Studies of RNA methylation aided in confirming the identity of these ribosomal species. Unmethylated RNA species appeared as messenger RNA between 5S and 15S, and at about 55S probably represented heterodisperse nuclear RNA. When normal macrophages were incubated with heat-killed Salmonella enteritidis, an acceleration in the maturation of RNA was observed. The accelerated maturation was indicated by the earlier appearance of 28S rRNA and the more rapid development of an equilibrium state, where further labeling did not change the RNA profile. In macrophage RNA from mice immunized with S. enteritidis, rRNA species appeared rapidly but did not accumulate to the same extent as observed for normal macrophages. Precursor rRNA and other RNA species developed as usual, suggesting specific degradation of mature rRNA. Such rRNA wastage could indicate a mechanism controlling ribosome assembly in the non-proliferating activated macrophage. The pattern of RNA synthesis in immune macrophages was essentially unchanged by the presence of heat-killed S. enteritidis in vitro.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper H. L. Control of synthesis and wastage of ribosomal RNA in lymphocytes. Nature. 1970 Sep 12;227(5263):1105–1107. doi: 10.1038/2271105a0. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Ribonucleic acids from animal cells. Bacteriol Rev. 1968 Sep;32(3):262–290. doi: 10.1128/br.32.3.262-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri G. L. Synthesis of mitochondrial RNA in hamster-mouse hybrid cells. Nat New Biol. 1973 Feb 21;241(112):233–234. doi: 10.1038/newbio241233a0. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B., TSUGITA A. Purification of viral RNA by means of bentonite. Virology. 1961 May;14:54–58. doi: 10.1016/0042-6822(61)90131-3. [DOI] [PubMed] [Google Scholar]
- Fishman M. Induction of antibodies in vitro. Annu Rev Microbiol. 1969;23:199–222. doi: 10.1146/annurev.mi.23.100169.001215. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Adesnik M., Salditt M., Sheiness D., Wall R., Molloy G., Philipson L., Darnell J. E. Further evidence on the nuclear origin and transfer to the cytoplasm of polyadenylic acid sequences in mammalian cell RNA. J Mol Biol. 1973 Apr 15;75(3):515–532. doi: 10.1016/0022-2836(73)90458-0. [DOI] [PubMed] [Google Scholar]
- Lewicki P. P., Sinskey A. J. Precision of RNA separation by polyacrylamide gel electrophoresis. Anal Biochem. 1970 Feb;33(2):273–278. doi: 10.1016/0003-2697(70)90297-6. [DOI] [PubMed] [Google Scholar]
- Maden B. E., Salim M., Summers D. F. Maturation pathway for ribosomal RNA in the Hela cell nucleolus. Nat New Biol. 1972 May 3;237(70):5–9. doi: 10.1038/newbio237005a0. [DOI] [PubMed] [Google Scholar]
- Mikami H., Kurashige S., Kawakami M., Mitsuhashi S. Fractionation of immune ribonucleic acid capable of inducing immunologic memory. Jpn J Microbiol. 1972 Mar;16(2):159–160. doi: 10.1111/j.1348-0421.1972.tb00643.x. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Prestayko A. W., Busch H. Studies on nucleolar 4 to 6 S ribonucleic acid of Novikoff hepatoma cells. J Biol Chem. 1968 Apr 10;243(7):1368–1375. [PubMed] [Google Scholar]
- Prestayko A. W., Busch H. Low molecular weight RNA of the chromatin fraction from Novikoff hepatoma and rat liver nuclei. Biochim Biophys Acta. 1968 Dec 17;169(2):327–337. doi: 10.1016/0005-2787(68)90041-5. [DOI] [PubMed] [Google Scholar]
- Rubin A. D. Ribosome biosynthesis in cultured lymphocytes: II. The role of ribosomal RNA production in the initiation and maintenance of lymphocyte growth. Blood. 1970 May;35(5):708–720. [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Savage H. E., Kuchler R. J. A versatile composite gel column for the electrophoretic separation of ribonucleic acid molecules. Prep Biochem. 1971;1(4):345–360. doi: 10.1080/00327487108081949. [DOI] [PubMed] [Google Scholar]
- Scherrer K., Marcaud L., Zajdela F., London I. M., Gros F. Patterns of RNA metabolism in a differentiated cell: a rapidly labeled, unstable 60S RNA with messenger properties in duck erythroblasts. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1571–1578. doi: 10.1073/pnas.56.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg L. S., Rubin A., Kuchler R. J., Solotorovsky M. Ribonucleic acid synthesis in mouse peritoneal macrophages in vitro. Cell Immunol. 1972 Apr;3(4):672–679. doi: 10.1016/0008-8749(72)90129-3. [DOI] [PubMed] [Google Scholar]
- Vesco C., Penman S. The cytoplasmic RNA of HeLa cells: new discrete species associated with mitochondria. Proc Natl Acad Sci U S A. 1969 Jan;62(1):218–225. doi: 10.1073/pnas.62.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Loening U., Willems M., Penman S. Acrylamide gel electrophoresis of HeLa cell nucleolar RNA. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1088–1095. doi: 10.1073/pnas.58.3.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Small molecular weight monodisperse nuclear RNA. J Mol Biol. 1968 Dec;38(3):289–304. doi: 10.1016/0022-2836(68)90387-2. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of RNA: processing of HeLa ribosomal RNA. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2827–2831. doi: 10.1073/pnas.70.10.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton J. M., Rosenstreich D. L., Oppenheim J. J. Activation of guinea pig macrophages by bacterial lipopolysaccharide requires bone marrow-derived lymphocytes. J Immunol. 1975 Jan;114(1 Pt 2):388–393. [PubMed] [Google Scholar]
- Zimmerman E. F., Holler B. W. Methylation of 45 s ribosomal RNA precursor in HeLa cells. J Mol Biol. 1967 Jan 28;23(2):149–161. doi: 10.1016/s0022-2836(67)80023-8. [DOI] [PubMed] [Google Scholar]


