Abstract
OBJECTIVE
Despite advances in surgical techniques, neurocognitive decline (NCD) after cardiopulmonary bypass (CPB) remains a common and serious complication. We have previously demonstrated that patients with NCD have unique genetic responses 6 hours after CPB when compared with normal patients (NORM). We used genomic microarray to objectively investigate whether patients with NCD had associated preoperative gene expression profiles, and how these profiles changed up to four days after surgery.
METHODS
Cardiac surgery patients underwent neurocognitive assessments preoperatively and four days after surgery. Skeletal muscle was collected intra-operatively. Whole blood collected pre-CPB, 6 hours post-CPB, and on post-operative day four was hybridized to Affymetrix Gene Chip U133 Plus 2.0 microarrays. Gene expression in patients with NCD was compared with gene expression in the NORM group using JMP Genomics. Only genes that were commonly expressed in the two groups with a false discovery rate of 0.05 and a fold change of >1.5 were carried forward to pathway analysis using Ingenuity Pathway Analysis. Microarray gene expression was validated by Green real–time polymerase chain reaction and western blotting.
RESULTS
17 out of 42 patients developed NCD. 54,675 common transcripts were identified on microarray in each group across all time points. Preoperatively there were 140 genes that were significantly altered between the NORM and NCD groups (p < 0.05). Pathway analysis demonstrated that preoperatively patients with NCD had increased regulation in genes associated with inflammation, cell death, and neurologic dysfunction. Interestingly, the number of significantly regulated genes between the two groups changed over each time point, and decreased from 140 preoperatively, to 64, six hours after CPB, and 25, four days after surgery. There was no correlation in gene expression between the blood and skeletal muscle.
CONCLUSIONS
Patients who developed NCD post-CPB had increased differential gene expression before surgery versus patient who did not develop NCD. While significant differences in gene expression also existed post-operatively, these differences gradually decreased over time. Preoperative gene expression may be associated with neurologic injury after CPB. Further investigation into these genetic pathways may help predict patient outcome and guide patient selection.
Keywords: Neurocognitive Decline, Microarray, Inflammation, Gene Expression, Cardiopulmonary Bypass
Introduction
Neurocognitive dysfunction (NCD) is a common but poorly understood complication of cardiopulmonary bypass (CPB). Depending on the definition, as many as 80% of patients undergoing CPB may manifest neurologic complications postoperatively 1. Neurologic deficits are commonly divided into two categories: Type 1 deficits include focal neurologic events such as stroke, stupor, and coma, while type 2 deficits are more global cognitive deficits such as memory loss, confusion, and deterioration in intellectual function 2. While type 1 deficits can usually be attributed to a specific cause, such as cerebral hypoperfusion or thromboembolic events, the etiology of type 2 events is more vague. However, their incidence is similar to that of type 1 events 3, and they can be equally as devastating. A lack of understanding of the precipitating pathophysiology and inability to predict this type of injury only adds to the strain on both patients and their family members.
A variety of pathologic processes including cerebral hypoperfusion, microembolization, inflammation, temperature changes, genetic predisposition, cerebral edema, or dysfunction of the blood-brain barrier have been implicated in NCD 4, 5. Cardiopulmonary bypass, while an essential component of the cardiac surgeon’s armamentarium, has significant deleterious effects on the human body related to the interaction of blood components with the artificial surfaces of the circuit, including activation of leukocytes, cytokine release, and increase in reactive oxygen species. Our group, as well as others, has previously demonstrated the association between systemic inflammation and NCD after CPB 6, 7. However, a comprehensive understanding of the precipitating and predisposing causes of NCD remains elusive, making accurate diagnosis and treatment difficult.
High-throughput microarray analysis provides insight into the response of nearly the entire human genome to a particular disease, and thus is an intriguing technique for identifying regulatory pathways and genes involved in poorly understood disease processes. Microarray technology has progressed exponentially in the past decade with the completion of the human genome project, development of more comprehensive microchips, and introduction of powerful pathway analysis software. We previously used microarray methods to show that genes associated with inflammation, antigen presentation, and cellular adhesion were differentially regulated in patients exhibiting NCD after CPB. In this prior study same-group comparisons were made both in patients with NCD pre- and postoperatively as well in NORM patients pre- and postoperatively 8. We now compare NORM patients to those with NCD pre and postoperatively to assess whether there are inherent differences pre-operatively leading to differential gene regulation 6 hours and 4 days post-CPB. The present study uses up-to-date microarray analytic techniques to identify specific cellular functions that may be involved in the development of NCD immediately and four days post-CPB.
Materials and Methods
Patient Enrollment
We enrolled forty-three patients scheduled electively or urgently for coronary artery bypass grafting, valvular surgery (aortic or mitral), or a combination of the two requiring CPB in this single-institution (Beth Israel Deaconess Medical Center, Boston, MA) prospective cohort study. All forms and procedures were approved by the Beth Israel Deaconess Medical Center Institutional Review Board/Committee on Clinical Investigations. Preoperative informed consent was obtained from all study participants for surgical procedures performed and additional blood and tissue collection for the purpose of this investigation. Exclusion criteria included: patients undergoing aortic arch/root procedures, patients with known calcified aortas or high-grade carotid stenosis, and patients with recent stroke, severe neurologic deficits, hepatic cirrhosis, or chronic renal failure (serum creatinine > 2.0 mg/dL). Patients who were unable to complete baseline psychological testing due to severe cognitive impairment, psychiatric disease, substance abuse, blindness, or poor English were also excluded. One enrolled patient was excluded due to inability to complete the neuropsychological assessment prior to discharge. Ultimately, forty-two patients were included in the analysis.
Surgical Technique
All operations followed the conventional approach at our institution with regards to induction of general anesthesia, invasive monitoring, midline sternotomy, and systemic heparinization. CPB was initiated via right atrial and ascending aorta cannulae with a nonpulsatile system, membrane oxygenator, and a 40-μm arterial filter. Crystalloid pump prime was used. In all cases, mild hypothermic CPB (32–34 °C) with intermittent cold blood hyperkalemic (25 mmol/L) cardioplegia was used. Serum glucose levels were monitored, and intermittent intravenous insulin injection or insulin infusion was used to target a level of less than 130 mg/dL. While on CPB, pump flow was maintained at 2 to 2.4 L/min/m2 body surface area. Arterial partial oxygen pressure was maintained between 150–250 mmHg. Mean blood pressure was maintained between 50 and 90 mmHg by using conventional vasoactive medications.
Neurocognitive Assessment
Patients underwent evaluation with a battery of neurocognitive tests preoperatively (1–10 days before surgical intervention), on postoperative day 4, and at 3 months postoperatively. All patients also underwent depression assessment with the Geriatric Depression Scale. All evaluations were carried out by trained, blinded psychometricians. 8 validated tools were used to assess memory, executive function, attention, language, and global cognition:
The Hopkins Verbal Learning Test assessed the number of items learned, the number of items recalled after a 20-minute delay divided by the maximum number of items learned, and the number of items correctly identified from a list to measure verbal learning, retention, and recall. The Boston Naming Test was used to measure confrontational naming9. Attention shifting ability was measured by recording time to complete Trailmaking A and B. Digit Span was used to measure working memory and sustained attention span. Fluency was assessed by requiring patients to generate words in a category (semantic fluency) or beginning with a specific letter (phonemic fluency). The Wechsler Test of Adult Reading was used as a test of premorbid intelligence. The Stroop Color-Word Inference Test was used to assess executive function, and the Visual Search and Acuity Test assessed visuospatial abilities and executive function.
Patients with NCD were defined as those who demonstrated a 1-standard deviation decline from baseline on 25% of the tasks (2/8 measures), in accordance with the “Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery”10.
Sample Collection and Microarray Processing
For all 42 patients, blood samples were collected from a central venous line preoperatively after induction of anesthesia and before skin incision (Pre-CPB), early or 6 hours (6H) post-CPB in the intensive care unit, and late or 4 days (4D) post-CPB. Blood was drawn directly into PAXgene tubes (QIAGEN Inc, Valencia, CA) for mRNA stabilization and extraction, per the manufacturer’s recommendation. Skeletal muscle samples were collected from twenty patients from the left intercostal muscle bed after cannulation but before the initiation of CPB, and again after removal of the aortic cross clamp and weaning from CPB. Skeletal muscle samples were snap frozen in liquid nitrogen immediately after collection and stored at −80 °C.
RNA extraction and purification, cDNA synthesis, and production of biotin-labeled cRNA were completed by the Beth Isreal Deaconess Medical Center Proteomics Core according to previously described protocols11, 12. cRNA from all samples were hybridized with Affymetrix GeneChip HG-U133 Plus 2.0 (Affymetrix Inc, Santa Clara, CA), which probes for over 38,500 genes. Chips were scanned with an HP G2500A ChipScanner (Affymetrix), and low-level quality control analysis and signal value measurement was performed using dChip software (Wong et al, Boston, MA) 13. No outliers were identified by dChip, so all samples were carried on for subsequent analysis.
Gene Expression and Pathway Analysis
Gene expression analysis was performed on raw microchip data using JMP Genomics 4.0 (SAS, Cary, NC) for quality control, normalization, and statistical analysis. Composite chip data were normalized and compared using the Robust Multichip Average method, which revealed one blood and one skeletal muscle sample to be outliers. These were excluded from subsequent analysis. Gene expression in Pre-CPB and Post-CPB skeletal muscle samples and Pre-CPB, 6H Post-CPB, and 4D Post-CPB blood samples in patients with NCD were compared to the corresponding samples in patients without NCD using one-way ANOVA. A post-hoc false detection rate algorithm with alpha of 0.05 was applied to control for false positives. Genes that were considered significantly regulated met two criteria: 1) mean fold change >1.5 or <−1.5 in NCD patients compared to NORM, and 2) −log (p-value) exceeding threshold calculated by the software for each comparison. All significant genes were uploaded into Ingenuity Pathway Analysis (IPA, Ingenuity Systems, Redwood City, CA) which was used to generate the top canonical pathways involving the differentially regulated genes.
Real-time PCR
Gene expression analysis of whole blood-derived mRNA with HGU 133 Plus 2.0 chips was previously validated by real-time PCR8. We used real-time PCR to validate gene expression analysis of skeletal muscle-derived mRNA as well. Total RNA was extracted from frozen sections of skeletal muscle using a Trizol-based method following the manufacturer’s recommendations (Gibco BRL, Rockville, MD).
Results
Patient Characteristics
As previously reported, 17 out of the 42 patients included for analysis developed early NCD at post-operative day 4. After three months all but one patient returned to their normal cognitive function8. As demonstrated in our prior manuscript, patients had similar baseline preoperative characteristics including age, race, sex and co-morbidities. Similarly, patients intraoperative course was well matched including type of procedure, time on CPB, crossclamp time, use of cell saver and cardiotomy suction. Moreover, there were no differences in observed postoperative complications between the two groups, and there were no documented focal neurologic deficits or cerebrovascular events in any of the enrolled patients during this study period8.
Gene Expression and Confirmation
We have previously published a comprehensive database of gene expression in patients with and without NCD after CPB, including unsupervised hierarchical clustering of samples, and confirmation of microarray gene-expression with real-time PCR8. 54,675 transcripts were identified using our described microarray GeneChip.
Preoperative Gene Expression and Pathway Analysis in Patients with NCD compared with NORM Patients
Preoperatively there were 140 genes that were significantly altered between the NORM and NCD groups, of which 108 were named. Notably all 108 of these genes were upregulated in patients with NCD compared with NORM patients (Figure 1; Table 4). Pathway analysis was used to group genes by potential pathophysiologic function. This analysis demonstrated that preoperatively patients with NCD had a significant increase in several genes involved in inflammation, cell death and neurologic dysfunction. Selected genes have been listed in Table 1. Gene expression in the blood was not correlated with gene expression in the skeletal muscle obtained at the time of surgery.
Figure 1. Number of Genes Significantly Regulated in Patients with NCD versus NORM.
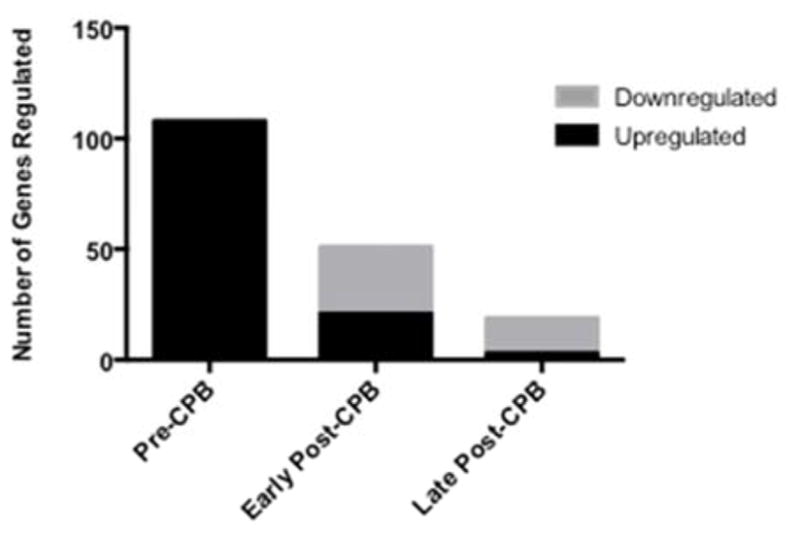
These represent named genes on pathway analysis. Early Post-CPB: represents gene expression 6 hours after cardiopulmonary bypass (CPB); Late Post CPB: represents gene expression 4 days after CPB. NCD: Neurocognitive Decline Post-CPB; NORM: Patient without Neurocognitive Decline Post-CPB.
Table 4.
Preoperative Gene Expression in Patients with NCD compared with NORM – Complete List
| Accession ID | Gene Name | FC | LCI | UCI | p value |
|---|---|---|---|---|---|
| Upregulated | |||||
| ADA | adenosine deaminase | 1.54 | 1.14 | 2.08 | 0.0059 |
| ANAPC2 | anaphase promoting complex subunit 2 | 1.53 | 1.12 | 2.08 | 0.0081 |
| APOBEC3C | apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3C | 1.73 | 1.24 | 2.40 | 0.0015 |
| ARHGEF12 | Rho guanine nucleotide exchange factor (GEF) 12 | 1.63 | 1.19 | 2.22 | 0.0027 |
| ARHGEF2 | Rho/Rac guanine nucleotide exchange factor (GEF) 2 | 1.73 | 1.21 | 2.46 | 0.0031 |
| ARID1A | AT rich interactive domain 1A (SWI-like) | 1.59 | 1.24 | 2.04 | 0.0004 |
| ARID2 | AT rich interactive domain 2 (ARID, RFX-like) | 1.55 | 1.14 | 2.11 | 0.0060 |
| ARL4C | ADP-ribosylation factor-like 4C | 1.51 | 1.15 | 1.99 | 0.0040 |
| ASB8 | ankyrin repeat and SOCS box containing 8 | 1.73 | 1.23 | 2.43 | 0.0022 |
| ASXL2 | additional sex combs like 2 (Drosophila) | 1.55 | 1.15 | 2.08 | 0.0049 |
| ATP2B4 | ATPase, Ca++ transporting, plasma membrane 4 | 1.72 | 1.21 | 2.44 | 0.0032 |
| BPTF | bromodomain PHD finger transcription factor | 1.68 | 1.16 | 2.43 | 0.0073 |
| CBL | Cbl proto-oncogene, E3 ubiquitin protein ligase | 1.65 | 1.19 | 2.29 | 0.0034 |
| CD3E | CD3e molecule, epsilon (CD3-TCR complex) | 1.85 | 1.30 | 2.63 | 0.0009 |
| CD3G | CD3g molecule, gamma (CD3-TCR complex) | 1.77 | 1.28 | 2.44 | 0.0008 |
| CIZ1 | CDKN1A interacting zinc finger protein 1 | 1.57 | 1.20 | 2.05 | 0.0014 |
| CNPPD1 | cyclin Pas1/PHO80 domain containing 1 | 2.02 | 0.0033 | ||
| CTSB | cathepsin B | 2.44 | 1.43 | 4.16 | 0.0015 |
| DCAF12 | DDB1 and CUL4 associated factor 12 | 2.48 | 1.46 | 4.23 | 0.0011 |
| E2F2 | E2F transcription factor 2 | 1.66 | 1.23 | 2.25 | 0.0014 |
| EIF2AK1 | eukaryotic translation initiation factor 2-alpha kinase 1 | 2.25 | 1.25 | 4.03 | 0.0075 |
| ELOF1 | elongation factor 1 homolog (S. cerevisiae) | 1.57 | 1.13 | 2.18 | 0.0080 |
| EML3 | echinoderm microtubule associated protein like 3 | 1.51 | 1.21 | 1.87 | 0.0004 |
| EPB41 | erythrocyte membrane protein band 4.1 (elliptocytosis 1, RH-linked) | 2.07 | 1.30 | 3.30 | 0.0026 |
| FAM104A | family with sequence similarity 104, member A | 2.20 | 1.43 | 3.38 | 0.0006 |
| FAM117A | family with sequence similarity 117, member A | 1.86 | 1.20 | 2.89 | 0.0068 |
| FAM134A | family with sequence similarity 134, member A | 1.62 | 1.14 | 2.30 | 0.0083 |
| FAM46C | family with sequence similarity 46, member C | 2.62 | 1.31 | 5.26 | 0.0074 |
| FAXDC2 | fatty acid hydroxylase domain containing 2 | 1.67 | 0.0044 | ||
| FBXO9 | F-box protein 9 | 3.05 | 1.77 | 5.26 | 0.0001 |
| FECH | ferrochelatase | 2.81 | 1.42 | 5.56 | 0.0036 |
| FKBP1B | FK506 binding protein 1B, 12.6 kDa | 2.65 | 1.75 | 4.03 | 0.0000 |
| FOXO3 | forkhead box O3 | 1.99 | 1.26 | 3.15 | 0.0038 |
| FTO | fat mass and obesity associated | 1.63 | 1.15 | 2.32 | 0.0069 |
| FUNDC2 | FUN14 domain containing 2 | 1.86 | 1.20 | 2.87 | 0.0059 |
| GDE1 (includes EG:393213) | glycerophosphodiester phosphodiesterase 1 | 2.06 | 1.29 | 3.28 | 0.0030 |
| GSPT1 | G1 to S phase transition 1 | 3.16 | 1.51 | 6.61 | 0.0041 |
| HBZ | hemoglobin, zeta | 1.72 | 1.16 | 2.56 | 0.0082 |
| HECA | headcase homolog (Drosophila) | 1.50 | 1.12 | 2.02 | 0.0079 |
| HECTD3 | HECT domain containing E3 ubiquitin protein ligase 3 | 1.89 | 1.46 | 2.45 | 0.0000 |
| HEMGN | hemogen | 2.04 | 1.23 | 3.38 | 0.0066 |
| IBA57 | IBA57, iron-sulfur cluster assembly homolog (S. cerevisiae) | 1.69 | 0.0007 | ||
| IL2RG | interleukin 2 receptor, gamma | 1.96 | 1.23 | 3.14 | 0.0058 |
| IL32 | interleukin 32 | 2.06 | 1.29 | 3.30 | 0.0032 |
| ITM2A | integral membrane protein 2A | 2.05 | 1.30 | 3.23 | 0.0027 |
| JHDM1D | jumonji C domain containing histone demethylase 1 homolog D (S. cerevisiae) | 1.74 | 1.17 | 2.59 | 0.0067 |
| JUND | jun D proto-oncogene | 1.57 | 1.18 | 2.10 | 0.0024 |
| KIAA1143 | KIAA1143 | 1.78 | 1.22 | 2.59 | 0.0032 |
| KIAA1919 | KIAA1919 | 1.90 | 1.36 | 2.64 | 0.0003 |
| KPNA1 | karyopherin alpha 1 (importin alpha 5) | 1.54 | 1.16 | 2.06 | 0.0037 |
| KPNA6 | karyopherin alpha 6 (importin alpha 7) | 1.51 | 1.15 | 1.99 | 0.0041 |
| MARCH8 | membrane-associated ring finger (C3HC4) 8, E3 ubiquitin protein ligase | 2.52 | 1.43 | 4.43 | 0.0030 |
| MINK1 | misshapen-like kinase 1 | 1.56 | 1.20 | 2.02 | 0.0013 |
| MKRN1 | makorin ring finger protein 1 | 2.26 | 1.30 | 3.93 | 0.0046 |
| MPHOSPH9 | M-phase phosphoprotein 9 | 1.51 | 1.20 | 1.90 | 0.0006 |
| NDUFV3 | NADH dehydrogenase (ubiquinone) flavoprotein 3, 10kDa | 1.53 | 1.12 | 2.07 | 0.0076 |
| NFATC2 | nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 2 | 1.83 | 1.20 | 2.79 | 0.0058 |
| NTAN1 | N-terminal asparagine amidase | 1.94 | 1.27 | 2.97 | 0.0029 |
| OLA1 | Obg-like ATPase 1 | 1.83 | 1.26 | 2.64 | 0.0018 |
| PAQR8 | progestin and adipoQ receptor family member VIII | 1.58 | 1.20 | 2.09 | 0.0015 |
| PCGF5 | polycomb group ring finger 5 | 1.77 | 1.17 | 2.67 | 0.0081 |
| PCSK5 | proprotein convertase subtilisin/kexin type 5 | 1.52 | 1.13 | 2.05 | 0.0066 |
| PIP4K2A | phosphatidylinositol-5-phosphate 4-kinase, type II, alpha | 2.23 | 1.28 | 3.88 | 0.0055 |
| PITHD1 | PITH (C-terminal proteasome-interacting domain of thioredoxin-like) domain containing 1 | 2.33 | 0.0011 | ||
| PNISR | PNN-interacting serine/arginine-rich protein | 1.85 | 0.0030 | ||
| PRDX2 | peroxiredoxin 2 | 2.41 | 1.34 | 4.31 | 0.0039 |
| PSME4 | proteasome (prosome, macropain) activator subunit 4 | 1.98 | 1.25 | 3.13 | 0.0043 |
| PSMF1 | proteasome (prosome, macropain) inhibitor subunit 1 (PI31) | 1.82 | 1.22 | 2.72 | 0.0043 |
| PTPN4 | protein tyrosine phosphatase, non-receptor type 4 (megakaryocyte) | 1.57 | 1.15 | 2.14 | 0.0054 |
| RAB2B | RAB2B, member RAS oncogene family | 2.95 | 1.72 | 5.08 | 0.0002 |
| RALGDS | ral guanine nucleotide dissociation stimulator | 1.50 | 1.13 | 2.00 | 0.0064 |
| RAPGEF6 | Rap guanine nucleotide exchange factor (GEF) 6 | 1.89 | 1.30 | 2.75 | 0.0011 |
| RGCC | regulator of cell cycle | 2.11 | 0.0021 | ||
| RNF10 | ring finger protein 10 | 2.41 | 1.24 | 3.43 | 0.0059 |
| RNF123 | ring finger protein 123 | 2.03 | 1.25 | 3.31 | 0.0052 |
| RUNDC3A | RUN domain containing 3A | 1.95 | 1.34 | 2.82 | 0.0006 |
| SCML4 | sex comb on midleg-like 4 (Drosophila) | 1.67 | 1.15 | 2.43 | 0.0082 |
| SEC16A | SEC16 homolog A (S. cerevisiae) | 1.86 | 1.20 | 2.89 | 0.0069 |
| SECISBP2 | SECIS binding protein 2 | 1.97 | 1.21 | 3.20 | 0.0070 |
| SEPT6 | septin 6 | 1.50 | 1.14 | 1.97 | 0.0040 |
| SESN3 | sestrin 3 | 2.25 | 1.32 | 3.84 | 0.0035 |
| SF3A2 | splicing factor 3a, subunit 2, 66kDa | 2.03 | 1.46 | 2.84 | 0.0001 |
| SLC25A37 | solute carrier family 25 (mitochondrial iron transporter), member 37 | 2.50 | 1.37 | 4.55 | 0.0034 |
| SLC38A5 | solute carrier family 38, member 5 | 1.57 | 1.15 | 2.14 | 0.0055 |
| SLC41A1 | solute carrier family 41, member 1 | 1.51 | 1.32 | 1.73 | 0.0000 |
| SLC48A1 | solute carrier family 48 (heme transporter), member 1 | 1.63 | 1.32 | 2.03 | 0.0000 |
| SLC6A8 | solute carrier family 6 (neurotransmitter transporter, creatine), member 8 | 2.08 | 1.22 | 3.54 | 0.0080 |
| SNAP29 | synaptosomal-associated protein, 29kDa | 1.59 | 1.22 | 2.08 | 0.0009 |
| SNCA | synuclein, alpha (non A4 component of amyloid precursor) | 2.01 | 1.22 | 3.30 | 0.0068 |
| SPTAN1 | spectrin, alpha, non-erythrocytic 1 | 2.05 | 1.30 | 3.24 | 0.0026 |
| SSBP3 | single stranded DNA binding protein 3 | 1.62 | 1.18 | 2.22 | 0.0036 |
| ST6GALNAC4 | ST6 (alpha-N-acetyl-neuraminyl-2,3-beta-galactosyl-1,3)-N-acetylgalactosaminide alpha-2,6-sialyltransferase 4 | 1.74 | 1.24 | 2.45 | 0.0020 |
| STAT4 | signal transducer and activator of transcription 4 | 1.71 | 1.17 | 2.51 | 0.0062 |
| SUN2 | Sad1 and UNC84 domain containing 2 | 1.63 | 0.0024 | ||
| TMEM245 | transmembrane protein 245 | 1.93 | 0.0031 | ||
| TMEM86B | transmembrane protein 86B | 1.56 | 1.14 | 2.14 | 0.0068 |
| TMOD1 | tropomodulin 1 | 1.75 | 1.18 | 2.58 | 0.0059 |
| TNS1 | tensin 1 | 3.03 | 1.53 | 6.03 | 0.0033 |
| TOLLIP | toll interacting protein | 1.74 | 1.17 | 2.59 | 0.0070 |
| TPGS2 | tubulin polyglutamylase complex subunit 2 | 2.02 | 0.0068 | ||
| TRIM58 | tripartite motif containing 58 | 2.49 | 1.35 | 4.60 | 0.0041 |
| TSPAN5 | tetraspanin 5 | 2.74 | 1.57 | 4.76 | 0.0006 |
| TUBB2A | tubulin, beta 2A class IIa | 4.58 | 1.64 | 12.80 | 0.0041 |
| WDR26 | WD repeat domain 26 | 2.44 | 1.46 | 4.10 | 0.0010 |
| WDR45 | WD repeat domain 45 | 1.89 | 1.22 | 2.87 | 0.0048 |
| WNK1 | WNK lysine deficient protein kinase 1 | 1.92 | 1.22 | 3.02 | 0.0056 |
| YY1 | YY1 transcription factor | 1.97 | 1.31 | 2.97 | 0.0015 |
| ZMAT2 | zinc finger, matrin-type 2 | 1.82 | 1.20 | 2.76 | 0.0058 |
Gene expression listed as fold change (FC) in neurocognitive decline (NCD) patients compared with normal patients (NORM).
All values represented are significant (p < 0.05). LCI: Lower confidence interval; UCI: Upper confidence interval
Table 1.
Preoperative Gene Expression Exhibiting Significant Regulation in Patients with NCD compared with NORM - Selected Genes Grouped by Potential Pathophysiologic Function
| Accession ID | Gene Name | FC | LCI | UCI | p value |
|---|---|---|---|---|---|
| Inflammation | |||||
| ADA | adenosine deaminase | 1.54 | 1.14 | 2.08 | 0.0059 |
| CD3E | CD3e molecule, epsilon (CD3-TCR complex) | 1.85 | 1.30 | 2.63 | 0.0009 |
| CD3G | CD3g molecule, gamma (CD3-TCR complex) | 1.77 | 1.28 | 2.44 | 0.0008 |
| IL2RG | interleukin 2 receptor, gamma | 1.96 | 1.23 | 3.14 | 0.0058 |
| IL32 | interleukin 32 | 2.06 | 1.29 | 3.30 | 0.0032 |
| NFATC2 | nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 2 | 1.83 | 1.20 | 2.79 | 0.0058 |
| STAT4 | signal transducer and activator of transcription 4 | 1.71 | 1.17 | 2.51 | 0.0062 |
| Cell death | |||||
| CTSB | cathepsin B | 2.44 | 1.43 | 4.16 | 0.0015 |
| E2F2 | E2F transcription factor 2 | 1.66 | 1.23 | 2.25 | 0.0014 |
| EIF2AK1 | eukaryotic translation initiation factor 2-alpha kinase 1 | 2.25 | 1.25 | 4.03 | 0.0075 |
| FOXO3 | forkhead box O3 | 1.99 | 1.26 | 3.15 | 0.0038 |
| Neurologic dysfunction | |||||
| SNCA | synuclein, alpha (non A4 component of amyloid precursor) | 2.01 | 1.22 | 3.30 | 0.0068 |
| FTO | fat mass and obesity associated | 1.63 | 1.15 | 2.32 | 0.0069 |
| TUBB2A | tubulin, beta 2A class IIa | 4.58 | 1.64 | 12.80 | 0.0041 |
| YY1 | YY1 transcription factor | 1.97 | 1.31 | 2.97 | 0.0015 |
| SNAP29 | synaptosomal-associated protein, 29kDa | 1.59 | 1.22 | 2.08 | 0.0009 |
Gene expression listed as fold change (FC) in neurocognitive decline (NCD) patients compared with normal patients (NORM).
All values represented are significant (p < 0.05). LCI: Lower confidence interval; UCI: Upper confidence interval
Postoperative Gene Expression and Pathway Analysis in Patients with NCD compared with NORM Patients
Early postoperatively (6H) the number of significantly regulated genes decreased to 64 compared with preoperative gene regulation, of which 51 were named. 21 of these 51 genes were significantly upregulated, while 30 were downregulated in patients with NCD compared with NORM patients (Figure 1; Table 5). Though the selected genes regulated were different than those regulated preoperatively, pathway analysis demonstrated regulation in several genes associated with inflammation, cell death, and neurologic dysfunction in patients with NCD compared with NORM patients (Table 2). Late postoperatively (4D) the number of significantly regulated genes decreased to 25, of which 19 were named (Figure 1; Table 6). Three of these 19 genes were upregulated and the remaining 16 genes were downregulated in patients with NCD compared with the NORM patients (Table 6). Selected genes involved with inflammation, cell death and neurologic dysfunction are listed in Table 3, of which all were all actually downregulated in patients with NCD compared with NORM patients.
Table 5.
Early Post-CPB (6 hours post-CPB) Gene Expression in Patients with NCD compared with NORM - Complete List
| Accession ID | Gen Name | FC | LCI | UCI | p value |
|---|---|---|---|---|---|
| Upregulated | |||||
| CBL | Cbl proto-oncogene, E3 ubiquitin protein ligase | 1.61 | 1.17 | 2.23 | 0.0046 |
| CCNJL | cyclin J-like | 1.70 | 1.21 | 2.37 | 0.0025 |
| CDK5RAP2 | CDK5 regulatory subunit associated protein 2 | 1.51 | 1.12 | 2.04 | 0.0074 |
| CEP19 | centrosomal protein 19kDa | 1.50 | 0.0071 | ||
| CLEC1B | C-type lectin domain family 1, member B | 1.56 | 0.0013 | ||
| DACH1 | dachshund homolog 1 (Drosophila) | 1.74 | 1.33 | 2.28 | 0.0008 |
| DHRS12 | dehydrogenase/reductase (SDR family) member 12 | 1.67 | 1.25 | 2.23 | 0.0002 |
| EPAS1 | endothelial PAS domain protein 1 | 1.73 | 1.31 | 2.27 | 0.0039 |
| FKBP1B | FK506 binding protein 1B, 12.6 kDa | 2.04 | 1.35 | 3.09 | 0.0010 |
| GPSM3 | G-protein signaling modulator 3 | 2.02 | 1.37 | 2.99 | 0.0006 |
| GRB10 | growth factor receptor-bound protein 10 | 1.54 | 1.26 | 1.88 | 0.0001 |
| KBTBD6 | kelch repeat and BTB (POZ) domain containing 6 | 1.78 | 1.27 | 2.48 | 0.0010 |
| MARCH8 | membrane-associated ring finger (C3HC4) 8, E3 ubiquitin protein ligase | 1.64 | 1.16 | 2.32 | 0.0058 |
| METTL21D | methltransferase-like protein 21D | 1.51 | 0.0032 | ||
| OLAH | oleoyl-ACP hydrolase | 1.88 | 1.41 | 2.50 | 0.0000 |
| OSBP2 | oxysterol binding protein 2 | 1.85 | 1.31 | 2.62 | 0.0008 |
| PDZK1IP1 | PDZK1 interacting protein 1 | 2.37 | 1.32 | 4.24 | 0.0045 |
| TLR4 | toll-like receptor 4 | 1.78 | 1.18 | 2.67 | 0.0070 |
| TPK1 | thiamin pyrophosphokinase 1 | 1.67 | 1.24 | 2.24 | 0.0011 |
| TSPAN5 | tetraspanin 5 | 2.17 | 1.25 | 3.77 | 0.0065 |
| ZBTB16 | zinc finger and BTB domain containing 16 | 1.94 | 1.32 | 2.86 | 0.0010 |
| Downregulated | |||||
| ACADM | acyl-CoA dehydrogenase, C-4 to C-12 straight chain | 0.58 | 0.40 | 0.85 | 0.0065 |
| ALG13 | ALG13, UDP-N-acetylglucosaminyltransferase subunit | 0.51 | 0.36 | 0.73 | 0.0005 |
| ANKRD10 | ankyrin repeat domain 10 | 0.58 | 0.41 | 0.81 | 0.0021 |
| ARGLU1 | arginine and glutamate rich 1 | 0.39 | 0.20 | 0.76 | 0.0067 |
| ATF1 | activating transcription factor 1 | 0.63 | 0.45 | 0.88 | 0.0079 |
| C2orf49 | chromosome 2 open reading frame 49 | 0.66 | 0.51 | 0.86 | 0.0025 |
| CLIP4 | CAP-GLY domain containing linker protein family, member 4 | 0.65 | 0.48 | 0.88 | 0.0059 |
| CPEB2 | cytoplasmic polyadenylation element binding protein 2 | 0.54 | 0.37 | 0.79 | 0.0020 |
| CRISP2 | cysteine-rich secretory protein 2 | 0.63 | 0.50 | 0.79 | 0.0001 |
| CSE1L | CSE1 chromosome segregation 1-like (yeast) | 0.64 | 0.48 | 0.86 | 0.0035 |
| GPR84 | G protein-coupled receptor 84 | 0.53 | 0.34 | 0.83 | 0.0062 |
| KANSL2 | KAT8 regulatory NSL complex subunit 2 | 0.64 | 0.0032 | ||
| MALT1 | mucosa associated lymphoid tissue lymphoma translocation gene 1 | 0.63 | 0.51 | 0.78 | 0.0001 |
| MBNL2 | muscleblind-like splicing regulator 2 | 0.65 | 0.50 | 0.85 | 0.0017 |
| MIR22HG | MIR22 host gene (non-protein coding) | 0.62 | 0.0008 | ||
| MON2 | MON2 homolog (S. cerevisiae) | 0.63 | 0.47 | 0.84 | 0.0024 |
| MPHOSPH6 | M-phase phosphoprotein 6 | 0.59 | 0.46 | 0.76 | 0.0001 |
| NADSYN1 | NAD synthetase 1 | 0.60 | 0.42 | 0.85 | 0.0051 |
| PIK3C2A | phosphatidylinositol-4-phosphate 3-kinase, catalytic subunit type 2 alpha | 0.60 | 0.42 | 0.86 | 0.0057 |
| RALGAPA1 | Ral GTPase activating protein, alpha subunit 1 (catalytic) | 0.65 | 0.0015 | ||
| RNF144B | ring finger protein 144B | 0.66 | 0.51 | 0.84 | 0.0012 |
| RWDD4 | RWD domain containing 4 | 0.65 | 1.20 | 2.65 | 0.0013 |
| SH3GL3 | SH3-domain GRB2-like 3 | 0.56 | 0.50 | 0.84 | 0.0045 |
| SREK1 | splicing regulatory glutamine/lysine-rich protein 1 | 0.62 | 0.0011 | ||
| TAP1 | transporter 1, ATP-binding cassette, sub-family B (MDR/TAP) | 0.61 | 0.43 | 0.87 | 0.0075 |
| TFEC | transcription factor EC | 0.62 | 0.45 | 0.84 | 0.0025 |
| TMEM168 | transmembrane protein 168 | 0.66 | 0.52 | 0.84 | 0.0011 |
| ZCCHC2 | zinc finger, CCHC domain containing 2 | 0.57 | 0.39 | 0.82 | 0.0033 |
| ZCCHC8 | zinc finger, CCHC domain containing 8 | 0.65 | 0.49 | 0.86 | 0.0033 |
| ZMYND11 | zinc finger, MYND-type containing 11 | 0.65 | 0.49 | 0.85 | 0.0027 |
Gene expression listed as fold change (FC) in neurocognitive decline (NCD) patients compared with normal patients (NORM).
All values represented are significant (p < 0.05). LCI: Lower confidence interval; UCI: Upper confidence interval
Table 2.
Early Post-CPB (6 hours post-CPB) Gene Expression in Patients with NCD compared with NORM – Selected Genes Grouped by Potential Pathophysiologic Function
| Accession ID | Gene Name | FC | LCI | UCI | p value |
|---|---|---|---|---|---|
| Inflammation | |||||
| CLEC1B | C-type lectin domain family 1, member B | 1.56 | 0.0013 | ||
| TLR4 | toll-like receptor 4 | 1.78 | 1.18 | 2.67 | 0.0070 |
| TAP1 | transporter 1, ATP-binding cassette, sub-family B (MDR/TAP) | 0.61 | 0.43 | 0.87 | 0.0075 |
| MALT1 | mucosa associated lymphoid tissue lymphoma translocation gene 1 | 0.63 | 0.51 | 0.78 | 0.0001 |
| Cell death | |||||
| EPAS1 | endothelial PAS domain protein 1 | 1.73 | 1.31 | 2.27 | 0.0002 |
| ZBTB16 | zinc finger and BTB domain containing 16 | 1.94 | 1.32 | 2.86 | 0.0010 |
| Neurologic dysfunction | |||||
| CDK5RAP2 | CDK5 regulatory subunit associated protein 2 | 1.51 | 1.12 | 2.04 | 0.0074 |
Gene expression listed as fold change (FC) in neurocognitive decline (NCD) patients compared with normal patients (NORM).
All values represented are significant (p < 0.05). LCI: Lower confidence interval; UCI: Upper confidence interval
Table 6.
Late Post-CPB (4 days post-CPB) Gene Expression in Patients NCD compared with NORM – Complete List
| Accession ID | Gen Name | FC | LCI | UCI | p value |
|---|---|---|---|---|---|
| Upregulated | |||||
| DDX17 | DEAD (Asp-Glu-Ala-Asp) box helicase 17 | 1.89 | 1.34 | 2.26 | 0.0001 |
| MLLT10 | myeloid/lymphoid or mixed-lineage leukemia (trithorax homolog, Drosophila); translocated to, 10 | 1.57 | 1.31 | 1.88 | 0.0000 |
| PGM2L1 | phosphoglucomutase 2-like 1 | 1.69 | 1.19 | 2.41 | 0.0042 |
| Downregulated | |||||
| C11orf31 | chromosome 11 open reading frame 31 | 0.65 | 0.49 | 0.88 | 0.0057 |
| DIABLO | diablo, IAP-binding mitochondrial protein | 0.61 | 0.0013 | ||
| GIMAP4 | GTPase, IMAP family member 4 | 0.46 | 0.43 | 0.87 | 0.0067 |
| GPR183 | G protein-coupled receptor 183 | 0.56 | 0.27 | 0.79 | 0.0059 |
| HDAC9 | histone deacetylase 9 | 0.67 | 0.39 | 0.80 | 0.0021 |
| MAT2A | methionine adenosyltransferase II, alpha | 0.64 | 0.0075 | ||
| MEF2C | myocyte enhancer factor 2C | 0.47 | 0.47 | 0.88 | 0.0075 |
| PPIA | peptidylprolyl isomerase A (cyclophilin A) | 0.66 | 0.28 | 0.77 | 0.0060 |
| PTMA | prothymosin, alpha | 0.56 | 0.38 | 0.83 | 0.0045 |
| RPL10 | ribosomal protein L10 | 0.60 | 0.41 | 0.87 | 0.0073 |
| RPL12 | ribosomal protein L12 | 0.60 | 0.41 | 0.87 | 0.0079 |
| RPL15 | ribosomal protein L15 | 0.57 | 0.38 | 0.86 | 0.0078 |
| RPS13 | ribosomal protein S13 | 0.56 | 0.39 | 0.81 | 0.0030 |
| Sept9 | septin 9 | 0.62 | 0.44 | 0.87 | 0.0072 |
| SPCS3 | signal peptidase complex subunit 3 homolog (S. cerevisiae) | 0.64 | 0.48 | 0.85 | 0.0031 |
| WWP1 | WW domain containing E3 ubiquitin protein ligase 1 | 0.66 | 0.52 | 0.83 | 0.0008 |
Gene expression listed as fold change (FC) in neurocognitive decline (NCD) patients compared with normal patients (NORM).
All values represented are significant (p < 0.05). LCI: Lower confidence interval; UCI: Upper confidence interval
Table 3.
Late Post-CPB (4 days post-CPB) Gene Expression in Patients NCD compared with NORM – Selected Genes Grouped by Potential Pathophysiologic Function
| Accession ID | Gene Name | FC | LCI | UCI | p value |
|---|---|---|---|---|---|
| Inflammation | |||||
| GIMAP4 | GTPase, IMAP family member 4 | 0.45 | 0.27 | 0.79 | 0.0059 |
| PTMA | prothymosin, alpha | 0.56 | 0.38 | 0.83 | 0.0045 |
| GPR183 | G protein-coupled receptor 183 | 0.56 | 0.39 | 0.80 | 0.0021 |
| Cell death | |||||
| DIABLO | diablo, IAP-binding mitochondrial protein | 0.61 | 0.43 | 0.87 | 0.0045 |
| HDAC9 | Histone deacetylase 9 | 0.67 | 0.0075 | ||
| Neurologic dysfunction | |||||
| MEF2C | myocyte enhancer factor 2C | 0.47 | 0.28 | 0.77 | 0.0039 |
Gene expression listed as fold change (FC) in neurocognitive decline (NCD) patients compared with normal patients (NORM).
All values represented are significant (p < 0.05). LCI: Lower confidence interval; UCI: Upper confidence interval
Discussion
The current study demonstrates that patients who developed NCD post-CPB have differential gene expression before surgery versus patients who did not develop NCD. While significant differences in gene expression exist post-CPB they decreased over time. These findings suggest that patients may be inherently predisposed to NCD after CPB independent of surgical or anesthetic technique. This notion is certainly supported by the failure to reduce the incidence of Type 2 NCD, despite improvements in operative techniques 14. In order to improve these outcomes novel diagnostic and therapeutic techniques will need to be employed with a focus on identifying individual genetic variants associated with disease susceptibility and therapeutic response. The use of up-to-date microarray and bioinformatics analysis is an important step in beginning to address these challenges.
Pre-CPB, 108 named genes were significantly regulated in patients with neurocognitive dysfunction. Several genes involved with inflammation, cell death and neurologic dysfunction were increased in patients who would later develop NCD. Systemic inflammation has been shown to contribute to neurocognitive decline after CPB 7, 15, 16. In a previous study we demonstrated that while an increase in preoperative inflammatory chemokines did not affect outcome, postoperative elevations in chemokines were associated with the development of delirium after CPB 17. Chemokines act as potent immune mediators and may attract inflammatory cells, resulting in a disruption of the blood-brain barrier and cognitive dysfunction. In our current study we demonstrate an elevation in several genes associated with T-cell activation and signaling preoperatively in patients that would later develop NCD. For instance, patients who developed NCD postoperatively had significantly elevated regulation in genes implicated in T-cell activation, maturation, and cytokine signaling including ADA, CD3E, CD3G, IL2RG, IL32, NFATC2, and STAT418–20. Perhaps these inherent elevations result in accentuated inflammatory response and resultant increase in chemokine production. These patients also had a significant increase in genes associated with cell death and oxidative stress, like E2F2, EIF2AK1, and FOX0321–23. Furthermore, patients who developed NCD also had an increase in genes more directly associated with neurologic dysfunction like SNCA, FTO, TUBB2A, YY1 and SNAP2924–28. Though these genes are not directly related to one-another in a specific pathway, bioinformatics analysis demonstrates that they do share important roles in neurologic function and cognition. SNCA is abundantly expressed in the brain and a major component of amyloid plaques in Alzheimer’s disease24. FTO, which has been shown to be inversely associated with brain volume, is also associated with Alzheimer’s disease as well as reduced verbal fluency in obese patients 25, 26. While TUBB2A is involved in microtubule and axonal guidance, SNAP29 has been shown to mediate synaptic membrane docking and may slow neurotransmitter release 28. YY1 has many roles in neuronal development and dysfunction and often plays a larger role in activating or repressing gene expression27.
Interestingly, there was a relative decrease in the number of genes regulated postoperatively when comparing patients with NCD and those without. Again, these findings suggest that patients may be inherently predisposed to developing NCD after CPB. Further investigations may reveal predictive patterns in gene expression and ultimately result in improved preoperative planning and care of patients undergoing cardiac surgery.
Limitation and Future Directions
There are limitations to this study. Though our baseline patient characteristics and operative techniques were similar in this single institution study, the number of patients in the study was limited. A larger sample of patients would help provide greater insight into the unique gene expression profiles associated with NCD and would allow for a more extensive mapping of gene pathways, as opposed to just placing genes in functional groups, as we have done. Another limitation is that we did not directly sample brain tissue for our mRNA extraction. We could not biopsy brain tissue in patients, and even if this were done it would not be feasible as a regular diagnostic or screening tool in a clinical setting. Of note, many of the regulated genes, which have been discussed in this, are associated with on inflammatory processes in the blood, which could secondarily affect the brain. We did sample skeletal muscle, which like brain tissue would not be exposed to CP, but CPB alone, however there were actually no correlations in gene regulation between the blood and muscle samples.
It is also important to note that this study would need to be repeated before any claim can be made as to whether the aforementioned genes were indeed predictive of NCD in patient populations. Although the results of this current study highly suggest that preoperative gene expression is associated with postoperative NCD, we must also be cautious with the interpretation of microarray. In order to actually demonstrate predictive gene expression patterns another study would need to be designed with a new group of patients, where genes would be checked in a prospective manner preoperatively to determine whether any of the genes identified in the current study were actually a predictor of later NCD in new patient cohorts.
Another common pitfall with the interpretation of microarray is errors with the statistical treatment of the data. Since microarray identifies tens-of-thousands of individual genes, random chance alone can often result in significant p-values when simple statistical analysis is performed. To account for this potential error in false discovery, using specialized statistical software we performed ANOVA testing with multiple comparison correction and limited our false discovery rate (FDR) to less than 0.05. This is widely accepted as a stringent method to help prevent an error in multiple comparisons, and though it is not a universal application in the interpretation of microarray, it does improve the likely reproducibility of the results.
Conclusions
This work represents a follow-up study of microarray database compiled in 2007. While our prior study identified differences in gene expression after CPB in patients with NCD and in patients without, the current study is the first to directly investigate differences in genetic regulation of patients with NCD compared with NORM pre- and post-CPB. Currently, these studies should serve primarily as a database to guide further genetic studies in different patient cohorts. The overarching goal of this project is to guide novel diagnostic techniques to help identify inherent genetic variations associated with susceptibility of disease, and ultimately to improve preoperative patient selection and individualized therapeutic techniques.
Acknowledgments
Funding: Funding for this research was provided by the National Heart, Lung, and Blood Institute (HL46716, Dr. Sellke), NIH Training grant 5T32-HL094300-03, (Dr. Sabe Dr. Elmadhun, and Dr. Chu).
Footnotes
Disclosures: No conflict of interests exists for any of the authors
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gao L, Taha R, Gauvin D, Othmen LB, Wang Y, Blaise G. Postoperative cognitive dysfunction after cardiac surgery. Chest. 2005;128:3664–70. doi: 10.1378/chest.128.5.3664. [DOI] [PubMed] [Google Scholar]
- 2.Eagle KA, Guyton RA, Davidoff R, Edwards FH, Ewy GA, Gardner TJ, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery) Circulation. 2004;110:e340–437. [PubMed] [Google Scholar]
- 3.Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. The New England journal of medicine. 1996;335:1857–63. doi: 10.1056/NEJM199612193352501. [DOI] [PubMed] [Google Scholar]
- 4.Murkin JM. Etiology and incidence of brain dysfunction after cardiac surgery. Journal of cardiothoracic and vascular anesthesia. 1999;13:12–7. discussion 36-7. [PubMed] [Google Scholar]
- 5.Selnes OA, McKhann GM. Neurocognitive complications after coronary artery bypass surgery. Annals of neurology. 2005;57:615–21. doi: 10.1002/ana.20481. [DOI] [PubMed] [Google Scholar]
- 6.Baufreton C, Allain P, Chevailler A, Etcharry-Bouyx F, Corbeau JJ, Legall D, et al. Brain injury and neuropsychological outcome after coronary artery surgery are affected by complement activation. The Annals of thoracic surgery. 2005;79:1597–605. doi: 10.1016/j.athoracsur.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 7.Ramlawi B, Rudolph JL, Mieno S, Feng J, Boodhwani M, Khabbaz K, et al. C-Reactive protein and inflammatory response associated to neurocognitive decline following cardiac surgery. Surgery. 2006;140:221–6. doi: 10.1016/j.surg.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Ramlawi B, Otu H, Rudolph JL, Mieno S, Kohane IS, Can H, et al. Genomic expression pathways associated with brain injury after cardiopulmonary bypass. The Journal of thoracic and cardiovascular surgery. 2007;134:996–1005. doi: 10.1016/j.jtcvs.2007.01.096. [DOI] [PubMed] [Google Scholar]
- 9.Mack WJ, Freed DM, Williams BW, Henderson VW. Boston Naming Test: shortened versions for use in Alzheimer’s disease. Journal of gerontology. 1992;47:P154–8. doi: 10.1093/geronj/47.3.p154. [DOI] [PubMed] [Google Scholar]
- 10.Murkin JM, Newman SP, Stump DA, Blumenthal JA. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. The Annals of thoracic surgery. 1995;59:1289–95. doi: 10.1016/0003-4975(95)00106-u. [DOI] [PubMed] [Google Scholar]
- 11.Jones J, Otu H, Spentzos D, Kolia S, Inan M, Beecken WD, et al. Gene signatures of progression and metastasis in renal cell cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11:5730–9. doi: 10.1158/1078-0432.CCR-04-2225. [DOI] [PubMed] [Google Scholar]
- 12.Ruel M, Bianchi C, Khan TA, Xu S, Liddicoat JR, Voisine P, et al. Gene expression profile after cardiopulmonary bypass and cardioplegic arrest. The Journal of thoracic and cardiovascular surgery. 2003;126:1521–30. doi: 10.1016/s0022-5223(03)00969-3. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:31–6. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goto T, Maekawa K. Cerebral dysfunction after coronary artery bypass surgery. Journal of anesthesia. 2014;28:242–8. doi: 10.1007/s00540-013-1699-0. [DOI] [PubMed] [Google Scholar]
- 15.Jungwirth B, Kellermann K, Qing M, Mackensen GB, Blobner M, Kochs EF. Cerebral tumor necrosis factor alpha expression and long-term neurocognitive performance after cardiopulmonary bypass in rats. The Journal of thoracic and cardiovascular surgery. 2009;138:1002–7. doi: 10.1016/j.jtcvs.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Hogan AM, Shipolini A, Brown MM, Hurley R, Cormack F. Fixing hearts and protecting minds: a review of the multiple, interacting factors influencing cognitive function after coronary artery bypass graft surgery. Circulation. 2013;128:162–71. doi: 10.1161/CIRCULATIONAHA.112.000701. [DOI] [PubMed] [Google Scholar]
- 17.Rudolph JL, Ramlawi B, Kuchel GA, McElhaney JE, Xie D, Sellke FW, et al. Chemokines are associated with delirium after cardiac surgery. The journals of gerontology Series A, Biological sciences and medical sciences. 2008;63:184–9. doi: 10.1093/gerona/63.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Navio JM, Climent N, Gallart T, Lluis C, Franco R. An old enzyme for current needs: adenosine deaminase and a dendritic cell vaccine for HIV. Immunology and cell biology. 2012;90:594–600. doi: 10.1038/icb.2011.81. [DOI] [PubMed] [Google Scholar]
- 19.Batista A, Millan J, Mittelbrunn M, Sanchez-Madrid F, Alonso MA. Recruitment of transferrin receptor to immunological synapse in response to TCR engagement. Journal of immunology. 2004;172:6709–14. doi: 10.4049/jimmunol.172.11.6709. [DOI] [PubMed] [Google Scholar]
- 20.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature immunology. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller H, Bracken AP, Vernell R, Moroni MC, Christians F, Grassilli E, et al. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes & development. 2001;15:267–85. doi: 10.1101/gad.864201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S, Suragani RN, Wang F, Han A, Zhao W, Andrews NC, et al. The function of heme-regulated eIF2alpha kinase in murine iron homeostasis and macrophage maturation. The Journal of clinical investigation. 2007;117:3296–305. doi: 10.1172/JCI32084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagenbuchner J, Ausserlechner MJ. Mitochondria and FOXO3: breath or die. Frontiers in physiology. 2013;4:147. doi: 10.3389/fphys.2013.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van der Schyf CJ, Geldenhuys WJ, Youdim MB. Multifunctional drugs with different CNS targets for neuropsychiatric disorders. Journal of neurochemistry. 2006;99:1033–48. doi: 10.1111/j.1471-4159.2006.04141.x. [DOI] [PubMed] [Google Scholar]
- 25.Keller L, Xu W, Wang HX, Winblad B, Fratiglioni L, Graff C. The obesity related gene, FTO, interacts with APOE, and is associated with Alzheimer’s disease risk: a prospective cohort study. Journal of Alzheimer’s disease: JAD. 2011;23:461–9. doi: 10.3233/JAD-2010-101068. [DOI] [PubMed] [Google Scholar]
- 26.Benedict C, Jacobsson JA, Ronnemaa E, Sallman-Almen M, Brooks S, Schultes B, et al. The fat mass and obesity gene is linked to reduced verbal fluency in overweight and obese elderly men. Neurobiology of aging. 2011;32:1159, e1–5. doi: 10.1016/j.neurobiolaging.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 27.He Y, Casaccia-Bonnefil P. The Yin and Yang of YY1 in the nervous system. Journal of neurochemistry. 2008;106:1493–502. doi: 10.1111/j.1471-4159.2008.05486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan PY, Cai Q, Lin L, Lu PH, Duan S, Sheng ZH. SNAP-29-mediated modulation of synaptic transmission in cultured hippocampal neurons. The Journal of biological chemistry. 2005;280:25769–79. doi: 10.1074/jbc.M502356200. [DOI] [PMC free article] [PubMed] [Google Scholar]


