Background: CO-releasing molecules (CO-RMs) are used to study biological interactions with this toxic gas.
Results: CORM-3 inhibits the NO detoxification activity of the E. coli flavohemoglobin Hmp in vivo but not in vitro.
Conclusion: CO-RMs must be used with regard to their chemistry in biological studies.
Significance: CORM-3 is a useful tool to study concerted effects of NO and CO in vivo.
Keywords: Carbon Monoxide, Escherichia coli (E. coli), Hemoglobin, Microbiology, Nitric Oxide
Abstract
CO and NO are small toxic gaseous molecules that play pivotal roles in biology as gasotransmitters. During bacterial infection, NO, produced by the host via the inducible NO synthase, exerts critical antibacterial effects while CO, generated by heme oxygenases, enhances phagocytosis of macrophages. In Escherichia coli, other bacteria and fungi, the flavohemoglobin Hmp is the most important detoxification mechanism converting NO and O2 to the ion nitrate (NO3−). The protoheme of Hmp binds not only O2 and NO, but also CO so that this ligand is expected to be an inhibitor of NO detoxification in vivo and in vitro. CORM-3 (Ru(CO)3Cl(glycinate)) is a metal carbonyl compound extensively used and recently shown to have potent antibacterial properties. In this study, attenuation of the NO resistance of E. coli by CORM-3 is demonstrated in vivo. However, polarographic measurements showed that CO gas, but not CORM-3, produced inhibition of the NO detoxification activity of Hmp in vitro. Nevertheless, CO release from CORM-3 in the presence of soluble cellular compounds is demonstrated by formation of carboxy-Hmp. We show that the inability of CORM-3 to inhibit the activity of purified Hmp is due to slow release of CO in protein solutions alone i.e. when sodium dithionite, widely used in previous studies of CO release from CORM-3, is excluded. Finally, we measure intracellular CO released from CORM-3 by following the formation of carboxy-Hmp in respiring cells. CORM-3 is a tool to explore the concerted effects of CO and NO in vivo.
Introduction
CO is a poisonous odorless gas that avidly binds to reduced (Fe(II)) hemes, such as hemoglobin (Hb)2 resulting in the formation of carboxy-hemoglobin (CO-Hb). This binding inhibits formation of oxy-hemoglobin (O2-Hb) and inhibits respiration (1). CO also binds to other Fe(II) hemes, notably those of terminal oxidases and other O2-reactive centers, including guanylyl cyclase. Although the reactions with globins and oxidases elicit toxicity, CO has important physiological functions in mammalian systems involving signaling and regulation. The gas is endogenously produced by inducible heme oxygenase and constitutive heme oxygenase-2. These enzymes catalyze the rate-limiting step in the heme degradation pathway, producing biliverdin IXa, CO and free iron (Fe(II)) and the gas modulates a number of key cellular functions, thus acting as an anti-inflammatory, anti-apoptotic, and cytoprotective molecule (2–5). Interestingly, not only animals, but also plants and some pathogenic microorganisms produce CO via heme oxygenase enzymes (6, 7).
The use of CO-releasing molecules (CO-RMs), mostly metal carbonyl compounds, has allowed substantial advances in biological studies without the handling difficulties and health risks associated with the use of CO gas in the laboratory. Numerous and diverse CO-RMs are now available (e.g. with ruthenium, manganese, iron, and boron centers), showing different rates, kinetics and conditions for CO release (2, 8, 9). For instance, [Ru(CO)3Cl (glycinate)], CORM-3 (10), has been successfully exploited in models of vascular dysfunction, inflammation, and ischemic injury (11, 12).
The ability of CO to bind transition metal compounds such as hemes and iron-sulfur clusters (6, 13) led to the suggestion that this compound might also have antibacterial effects by targeting, for instance, terminal oxidases or other heme proteins. Since Nobre et al. (14) demonstrated the lethal effects of CO delivered via organometallic CO-RMs against selected bacteria, many papers reporting the antibacterial properties and transcriptomic and biochemical effects of CO-RMs have appeared (reviewed in Refs. 15, 16). Indeed, CO-RMs appear to have great potential as novel antibacterial agents with targets distinct from those of established antibiotics (4).
CORM-3 is a water-soluble molecule with a very complex chemistry in solution. Full understanding of the mechanism of CO release from CORM-3, particularly in complex biological environments, is a formidable task (16). For instance, the myoglobin (Mb) assay based on the formation of CO-Mb from ferrous Mb (Fe(II)-Mb) in the presence of a CO-RM has been routinely exploited to report and quantify CO release. However, McLean et al. (17) recently reported the release of CO from CORM-3 in the standard assay to be dependent on the reducing agent sodium dithionite, used for the reduction of Mb. In the absence of the reductant, negligible amounts of CO bound to reduced Mb. Since other sulfites also facilitated the release of CO from CORM-3, it has been suggested that cellular components, such as sulfites, might trigger the CO release in vivo.
Like CO, nitric oxide (NO) has vital biological functions but, unlike CO, it is a free radical with extensive biological reactivity (18). Key bacterial proteins are attacked by high and sustained concentrations of NO generated by the immune system. NO diffuses across the bacterial membrane to the cytoplasm where it reacts with terminal oxidases (19), aconitase (20), other hemes (21), other iron-sulfur (Fe-S) clusters (22), and protein thiols (23).
Resistance to NO and reactive nitrogen species (RNS) in bacteria is mainly attributed to the presence of hemoglobins (24, 25). In Escherichia coli and many other bacteria the main mechanism for NO detoxification in aerobic conditions is the NO-inducible flavohemoglobin Hmp (26, 27). The conversion of NO and O2 to the innocuous ion nitrate has been suggested to occur via either a dioxygenase (NOD) or denitrosylase reaction with some lines of evidence supporting each mechanism (reviewed in Ref. 28). The NOD activity involves the reaction of a ferrous-oxy heme (Fe(II) + O2) with NO (29–31), while denitrosylation implies binding of NO to a ferrous heme (FeII) that in turn reacts with O2 (21, 32). In either case, nitrate production by Hmp leads to the oxidation of the globin heme. This is followed by an intra-protein electron transfer from the reductase domain (or FNR, ferredoxin-NADP reductase-like domain) to the N-terminal heme domain in an NAD(P)H-dependent reaction via a non-covalently bound FAD allowing the re-conversion of the oxidized heme (ferric, Fe(III)) to the reduced (ferrous, (FeII)) form and consequent continuation of catalytic activity (26, 32, 33). In the absence of O2, Hmp is able to reduce NO to N2O, but this reaction proceeds at a very low rate (34, 35).
There is a very large body of literature on the reactivity of hemoglobins with gaseous inhibitors. CO binds rapidly to the ferrous form of E. coli Hmp with high affinity (association constant k′ = 22 μm−1s−1, association equilibrium constant K = 386 μm−1). The E. coli protein also has a high O2 association rate constant (k′ = 38 μm−1s−1), but the higher dissociation constant yields a lower association equilibrium constant (K = 86 μm−1). Thus, NO detoxification by Hmp is inhibited by CO in vitro as expected because of the competition between CO and O2 for the ferrous heme (30).
We hypothesized that CO might also inhibit NO detoxification by Hmp in vivo, affecting the survival of pathogenic organisms exposed to both NO (from the macrophage, for example) and CO produced by the host or endogenously by bacteria. Thus, the present work explores the biological interplay of NO and CO in E. coli, by investigating whether CORM-3 inhibits NO detoxification both in vivo and in vitro. During the course of this work, a clear distinction between the utility of CORM-3 in cellular and protein samples was revealed.
EXPERIMENTAL PROCEDURES
Reagents
CORM-3 was synthesized as described previously (10). Inactivated CORM-3 (iCORM) was prepared based on (10, 17). Briefly, a 5 mm stock of CORM-3 in 0.1 m PBS was bubbled with N2 for 5 min every two h for 8 h and then incubated overnight at room temperature followed by further bubbling with N2. To confirm the inability of the iCORM to release CO, a myoglobin assay (10) was performed prior to use the compound. CO-saturated solution was obtained by bubbling potassium phosphate buffer (pH 7.4) with CO gas for 30 min and used immediately. Sodium dithionite, glucose, and NADH were purchased from Sigma. Restriction enzymes were purchased from Promega. 3,3-Bis(aminoethyl)-1-hydroxy-2-oxo-1-triazene (DETA NONOate) (with a half-life of 20 h at 37 °C (pH 7.4) to liberate 2 mol NO per mol parent compound) (36, 37) and 1-(hydroxyl-NNO-azoxy)-l-proline, disodium salt (PROLI-NONOate) (half-life of 1.8 s at 37 °C (pH 7.4) to liberate 2 mol NO per mol parent compound) (38) were purchased from Enzo Life Science.
Sensitivity Tests to NO and CORM-3
Overnight starter cultures of the E. coli wild type strain (MG1655) and its isogenic hmp derivative (RKP3036, MG1655 hmp::KM (39) grown in LB at 37 °C were used to inoculate 10 ml defined minimal medium with glycerol (54 mm) as a carbon source (40) in 250 ml flasks with side arms (4% (v/v)), in the presence or absence of 100 μm DETA NONOate. When cultures reached 40 Klett units, CORM-3 (5 μm or 10 μm) or iCORM (10 μm) were added. Growth was measured by culture turbidity using a Klett-Summerson photoelectric colorimeter (Klett Manufacturing Co., New York, NY), equipped with a no. 66 (red) filter.
E. coli Soluble Extract and Membrane Preparation
LB (200 ml) supplemented with kanamycin (35 μg ml−1) was inoculated with an overnight culture of strain RKP3036 (1% (v/v)) and incubated for 15 h at 37 °C, 240 rpm. After centrifugation at 1000 × g for 15 min, the cellular pellet was resuspended in 10 ml of Tris-HCl 50 mm buffer (pH 7.4), and cells were disrupted by sonication. Undisrupted cells and cell debris were removed by centrifugation. Ultracentrifugation at 225,000 × g for 1 h was used to separate membranes from the soluble fraction. Soluble sample was stored at 4 °C for up to 48 h. Membranes were stored at −70 °C in small aliquots. Protein concentration was determined by the Markwell assay (41).
Gene Cloning and Protein Expression
Primers to amplify the hmpA gene from E. coli MG1655 genomic DNA were: 5′-accccatggttgacgctcaaac-3′ (upper primer) and 5′-gacgtggctcgagtcattcg-3′ (lower primer), containing the restriction sites NcoI and XhoI respectively. The amplified segment was cloned under control of an arabinose-inducible promoter into the commercial vector pBAD/HisA (Invitrogen), and the construction (pBAD-hmp) verified by sequencing. The second amino acid of the cloned Hmp protein, glycine, was changed to valine to introduce the NcoI site. Strain RKP3036 was transformed with pBAD-hmp and the strain named RKP140. To express Hmp from the RKP140 strain, cultures were grown in LB supplemented with FeCl3 (13 μm), δ-aminolevulinic acid (500 μm), ampicillin (100 μg ml−1), and kanamycin (35 μg ml−1) until OD600 nm 0.4 and then supplemented with 0.2% arabinose (v/v) and grown for a further 4 h. For spectroscopic studies of intracellular Hmp, 40 ml cultures of strain RKP140 and of the strain carrying the empty vector as a control (RKP3919) were grown and cells stored at 4 °C overnight. Cells were harvested by centrifugation at 1000 × g for 15 min and resuspended in 10 ml Tris-HCl 50 mm (pH 7.4). Cell suspensions were standardized to similar OD600 nm values and protein concentration was determined by the Markwell assay (41).
Purification of Hmp
Cultures of strain RKP140 expressing Hmp (6 × 500 ml cultures in 2 liters baffled flasks) were centrifuged to harvest the deep brown cell pellets and stored at −70 °C. The method was adapted from Ref. 42. Cell pellets were resuspended in 30 ml of 50 mm Tris-HCl buffer (pH 9.0) and disrupted by sonication (16 μm amplitude on an MSE Soniprep 150 machine, 3 cycles for 20 s each, on ice). Cell debris was removed by centrifugation at 70,000 × g for 10 min. The supernatant containing 400–500 mg of total protein was loaded onto a 20 ml of DEAE Sepharose Fast Flow (GE Healthcare) column equilibrated with 50 mm Tris-HCl buffer (pH 9.0) in an ÄKTA purifier (GE Healthcare Bio-Sciences, Amersham Biosciences Ltd., UK). Elution of the protein was achieved using a NaCl gradient (from 0 to 0.3 m). Fractions containing Hmp, based on a red-orange color, were pooled together and diluted 2.5 fold with ultra pure water. Sample, typically containing 70–90 mg of total protein, was applied on a 6 ml Resource Q column (GE Healthcare) equilibrated in 50 mm MES-NaOH pH 6.5 buffer. Proteins were eluted from the column by 90 ml gradient of NaCl concentration from 50 to 250 mm in the same buffer. Red-orange fractions (7–12 mg of total protein) were combined and concentrated in a Vivaspin concentrator (5,000 Molecular Weight Cut-Off (MWCO)) (VivaScience) to a final volume of 1–2 ml and loaded onto a 16 × 60 mm HiLoad Superdex200 gel filtration column (GE Healthcare), equilibrated with 50 mm Tris-HCl, 500 mm NaCl buffer (pH 8.0) and eluted in the equilibration buffer at a flow rate of 1.5 ml min−1. Peak fractions (2–3 mg) were combined and concentrated to 6–14 mg ml−1. The purified Hmp was stored at 4 °C, and the heme concentration determined by the alkaline pyridine assay (43).
Polarographic Studies of the Hmp Interaction with NO, CORM-3, and CO
O2 consumption and NO accumulation were measured simultaneously following the method based on (34). Briefly, O2 uptake was followed polarographically with a digital Clark-type O2 electrode system (Rank Bros., Bottisham. Cambridge, UK) where the electrode is positioned at the bottom of a water-jacketed Perspex chamber stirred magnetically and maintained at 37 °C. The chamber was sealed with a custom-built adjustable Perspex cap (2 ml working volume) drilled in the center to accept a World Precision Instruments ISO-NOP2 sensor (2 mm diameter) for measurement of NO. Additional fine vertical holes allowed the injection of solutions to reach final concentrations of 500 μm NADH, 25 μm PROLI-NONOate, 200 μm CORM-3, or 100 μm CO (from CO-saturated buffer) by using Hamilton microsyringes through the cap. CO-saturated buffer was used to reach 1 mm CO. The O2 electrode was calibrated with air-saturated Tris-HCl 50 mm, NaCl 50 mm buffer (pH 8), and sodium dithionite to achieve anoxia. The NO electrode was calibrated using acidified KI and NaNO2 as described by the manufacturer.
Optical Spectroscopy
Optical spectra of purified Hmp were recorded using a Cary 50 UV-Visible spectrophotometer against a buffer baseline, unless otherwise stated, at room temperature in a 1-ml quartz cuvette. Purified Hmp (4.8 or 5 μm) was scanned in 50 mm Tris-HCl, 50 mm NaCl (pH 8.0). The protein was reduced by adding a few grains of sodium dithionite to the cuvette followed by gentle mixing, and the Hmp-CO spectrum of the protein was obtained by bubbling CO through the sample for 3 min. For the tests of CO release from CORM-3 in buffer with or without glucose (30 mm), the flavohemoglobin was reduced by adding either a few grains of sodium dithionite or NADH (500 μm) to the cuvette followed by gentle mixing. CORM-3 (10 or 100 μm) was added and the spectral changes followed over time. For anaerobic experiments, samples were prepared in an anaerobic cabinet. For tests of Hmp in soluble extracts (4.3 mg ml−1 total protein) and membrane suspensions (1.2 mg ml−1 total protein), spectra were recorded against a baseline of extract or membrane suspensions containing NADH (1 mm). Hmp (4.8 μm) was added, and the sample incubated for 10 min to promote protein reduction. After addition of CORM-3 (100 μm) the spectral changes were followed for an additional 40 min as described above. Optical spectroscopy of intracellular Hmp was performed in a Johnson Foundation SDB3 dual-wavelength spectrophotometer at room temperature (44). Optical spectra of the Hmp-expressing cell suspensions were recorded in native and reduced states. Reduction was achieved either by addition of a few grains of sodium dithionite or glucose (15 mm). Before the spectroscopic measurements were carried out, the capacity of the cell suspensions to consume O2 upon addition of glucose was followed polarographically in a closed chamber. After O2 depletion, the lid of the chamber was removed, allowing air diffusion into the stirred sample and the O2 levels recorded for a further 1 h. Native and reduced samples with sodium dithionite or glucose were bubbled with CO gas for 2 min and spectroscopic changes recorded immediately. When CORM-3 (300 μm) was added to the reduced samples, changes were recorded every min for 10 min, and every 5 min for an additional 20 min. Difference spectra (CO-reduced minus reduced) were plotted. Cells carrying the empty vector were reduced with dithionite and bubbled with CO gas, and the difference spectrum was plotted for comparison. Hmp absolute spectra (reduced and CO-reduced) were obtained by subtraction of the absorbance values from the samples carrying the empty vector.
RESULTS
Hmp-supported NO Resistance of E. coli Is Inhibited by CORM-3 in Vivo
The effect of CO on the NO detoxification capacity of Hmp was tested by using the water-soluble CO-releasing molecule CORM-3. Initially, E. coli wild type and the isogenic hmp mutant strains growing aerobically were challenged with increasing concentrations of the NO donor DETA NONOate (not shown). A final concentration of 100 μm DETA NONOate inhibited growth of the hmp mutant but not the wild type strain (Fig. 1A), directly implicating the NO detoxification activity of Hmp as the resistance mechanism.
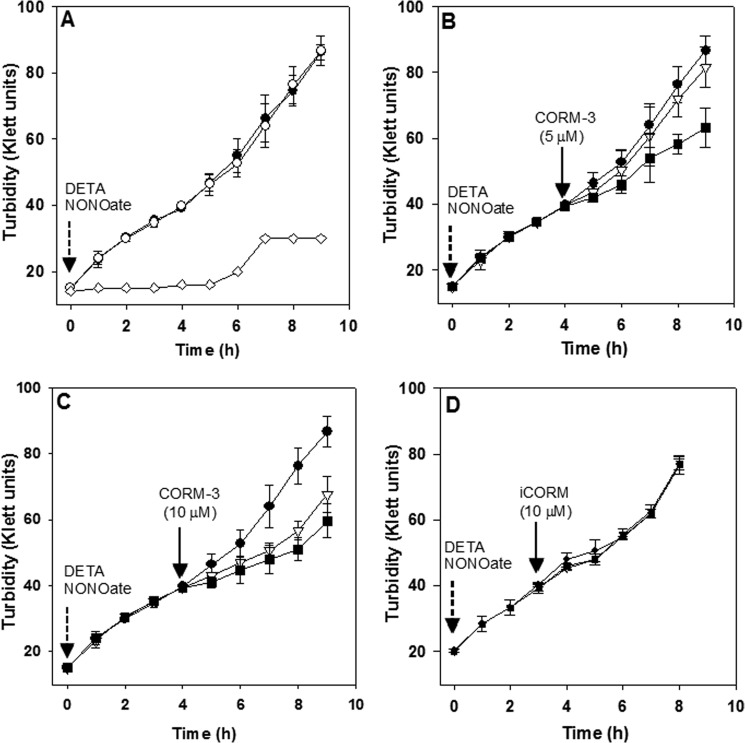
FIGURE 1.
Inhibition of Hmp-supported NO resistance by CORM-3 in E. coli cultures. Growth of aerobic cultures in defined minimal medium was monitored every hour using a Klett meter. A, cultures of the E. coli wild type (●) or an hmp mutant (♢) upon addition of 100 μm DETA NONOate (dotted arrow) were compared with a wild type culture growing in the absence of DETA NONOate (○). B, wild type cultures supplemented with 100 μm DETA NONOate (as in A) plus 5 μm CORM-3 (solid arrow) (■), were compared with cultures containing 100 μm DETA NONOate (●) or 5 μm CORM-3 (▿) only, added in the same conditions. C, as in B, but 10 μm CORM-3 was added. D, as in B but 10 μm iCORM-3 instead of CORM-3 was added. Bars represent the standard deviation of three independent experiments.
The effect of CORM-3 on Hmp-supported NO resistance of the wild type strain was investigated. Addition of CORM-3 (5 μm) to a mid-exponential phase culture growing in the presence of 100 μm DETA NONOate significantly inhibited growth (Fig. 1B). This suggests an additive toxic effect due to the inhibition of the NO detoxification capacity of Hmp, since cultures treated with the same concentration of CORM-3 in the absence of NONOate remained unaffected (Fig. 1B). A higher dose of CORM-3 (10 μm) inhibited the growth even in the absence of NONOate and, even though a slightly higher inhibition was found in the culture containing both NONOate and CORM-3, the difference was not statistically significant (Fig. 1C). On the other hand, addition of the inactive form of CORM-3 abbreviated here as (iCORM) (10) did not inhibit growth in either the absence or presence of DETA NONOate, suggesting that the inhibitory effect resulted from the CO released by CORM-3 and not by additional toxicity related to the CO-free metal compound or from its interaction with DETA NONOate (Fig. 1D).
CO Gas, but Not CORM-3, Inhibits the NO Detoxification Activity of Hmp in Vitro
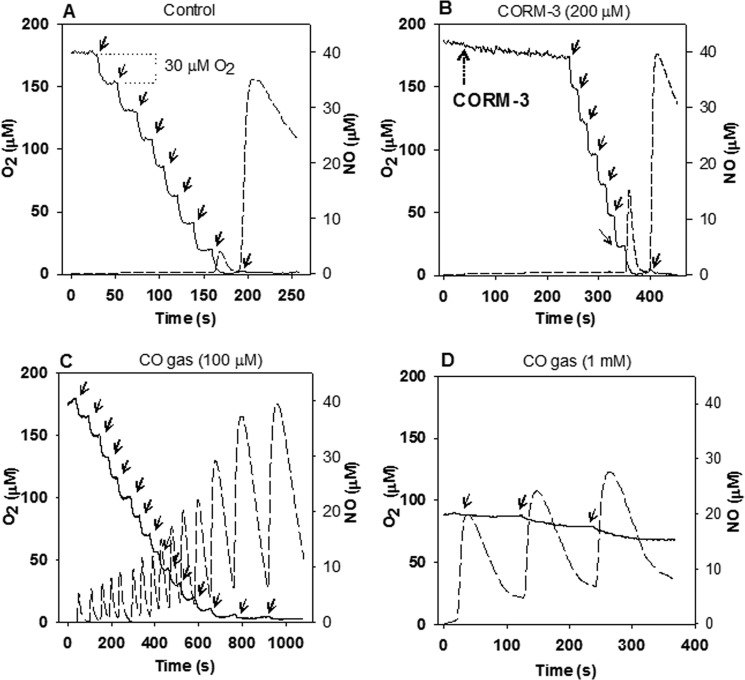
As previously shown, the Hmp heme cofactor binds CO rapidly, inhibiting Hmp-supported NO detoxification activity (Hmp Fe(II) association rate constants for NO and CO being 26 and 22 μm−1 s−1 respectively, and dissociation constants being 0.002 and 0.057 s−1, respectively) (30). In agreement, our in vivo model (Fig. 1) suggests that the decrease of the E. coli growth capacity under nitrosative stress conditions in the presence of CORM-3 is related to the inhibition of Hmp activity. Since CO-RMs are being considered as potential antimicrobial agents (4), and NO detoxification by pathogens is important for their survival (e.g. in macrophages (45)), we tested the effects of CORM-3 on NO detoxification by Hmp. Consumption of NO and O2 were followed polarographically in a reaction mix containing 1 μm purified flavohemoglobin and 500 μm NADH as the electron donor. In a control, NO from the NO fast-releasing molecule PROLI-NONOate was instantaneously and repeatedly consumed by Hmp, since the NO electrode failed to detect the signal even after several additions of 25 μm NONOate to the reaction mix in air-containing Tris-HCl buffer (pH 7.4) at 37 °C. Consumption of ∼30 μm O2 per 25 μm NONOate aliquot was calculated from the O2 electrode measurements, indicating the oxidation of NO to NO3− (∼1 O2 per NO) (46). Only when O2 was depleted after about 200 s was NO detectable in the chamber on adding PROLI-NONOate, showing the much less efficient NO reductase activity of Hmp (35, 46) (Fig. 2A). Inhibition of NO consumption by CORM-3 in the same conditions described for the control was tested. In this case, the reaction mix was incubated for 4 min after addition of a high concentration of CORM-3 (200 μm) and then the first aliquot of PROLI-NONOate was added. Surprisingly, in aerobic conditions, the NO and O2 consumption by Hmp were comparable to the control in the absence of CORM-3 (compare Fig. 2B with 2A). However, at very low oxygen tensions, the NO signal was recorded earlier in the presence of CO-RM than in its absence (compare Fig. 2B with 2A), suggesting a slight inhibition of the NOD activity of Hmp due perhaps to the presence of a small amount of CO released from CORM-3 (see below). Conversely, the presence of CO gas (100 μm CO dissolved in the reaction buffer) clearly inhibited the NO detoxification reaction of Hmp in an O2 concentration dependent manner (Fig. 2C). Furthermore, CO-saturated buffer (1 mm CO final concentration) completely abolished O2 consumption and NO was accumulated at high concentrations after each addition of PROLI-NONOate (Fig. 2D). These results confirm the inhibition of Hmp-supported NO detoxification activity by CO but reveal a failure of CORM-3 to inhibit under these conditions.
FIGURE 2.
CORM-3 fails to inhibit the NO detoxification activity of purified Hmp in vitro. O2 consumption (solid line) and NO accumulation (dashed line) were polarographically recorded simultaneously. Reaction mixes containing 1 μm Hmp and 500 μm NADH final concentrations in 50 mm Tris-HCl, 50 mm NaCl (pH 8.0) at 37 °C were treated with repeated additions of 25 μm PROLI-NONOate (solid arrows) in the absence (A) or presence of 200 μm CORM-3 (dotted arrow) (B), or CO-saturated buffer was added to a final concentration of 100 μm (C) or 1 mm (D). O2 concentration consumed after additions of PROLI-NONOate is shown in A. The experiments were repeated at least three times with similar results.
Reaction of CO-RM-generated CO with Hmp in Vitro Is Dependent on Sodium Dithionite
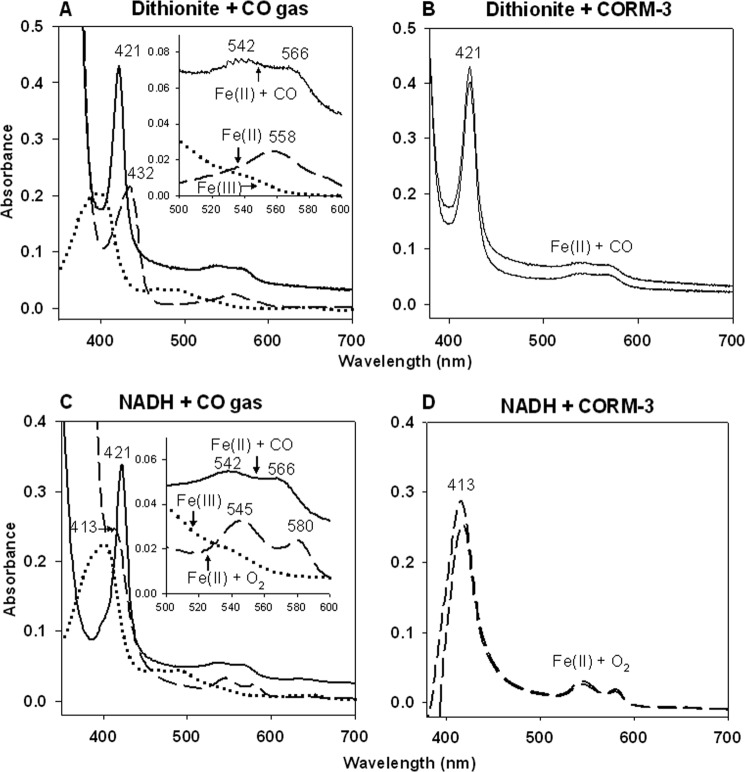
We therefore considered the possibility that CO release from CORM-3 and binding to ferrous Hmp is dependent on sodium dithionite (17), which is routinely used in the literature as a reductant in the standard carboxy-myoglobin assay of CO release from CO-RMs. In order to test this, spectrophotometric changes in the redox state of the Hmp heme cofactor were followed in the Soret and α,β regions of the spectrum in the absence and presence of sodium dithionite and using CO gas as a control. The dithionite-reduced Hmp spectrum (Fe(II), 432, 558 nm (42); Fig. 3A) showed an instantaneous change to the carboxy-ferrous form (Fe(II) + CO, 421, 542, 566 nm (42)) after bubbling with CO gas (Fig. 3A) or addition of 10 μm CORM-3 (Fig. 3B). When the oxy-ferrous form of Hmp was produced by the addition of NADH without dithionite (Fe(II) + O2, 413, 545, 580 nm (42)), CO gas again elicited an immediate shift to the Fe(II) + CO form (Fig. 3C). The presence of dithionite or NADH did not modify Hmp CO binding since bubbling of CO gas to either Fe(II) or Fe(II) + O2 Hmp produced an instantaneous change to the Fe(II) + CO form (Fig. 3, A and C). However, addition of 10 μm CORM-3 to NADH-reduced protein failed to produce changes in the spectrum of the flavohemoglobin (Fig. 3D), in agreement with the results obtained by McLean et al. with Mb (17).
FIGURE 3.
Formation of CO-reduced Hmp from CORM-3 depends on the presence of sodium dithionite in vitro. Absolute spectra showing 4.7 μm Hmp suspended in 50 mm Tris-HCl, 50 mm NaCl buffer (pH 8.0). Upper panel: the native oxidized form (dotted line) was reduced with sodium dithionite (dashed line) and then bubbled with CO gas (A) or supplemented with 10 μm CORM-3 (B) (solid lines). Lower panel: the native oxidized form (dotted line) was reduced by addition of NADH (dashed line) and then bubbled with CO gas (C) (solid line) or supplemented with 10 μm CORM-3 (D) (dashed lines). Measurements at time 0 and then 5 min after or at time 0 and 40 min after addition of CORM-3 are shown (C and D, respectively). Numbers indicate the absorbance maxima (nm). Insets show amplifications of the α,β regions.
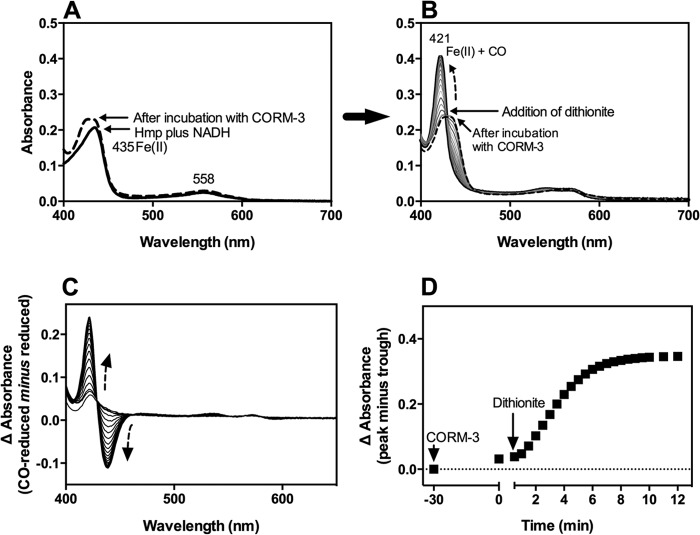
Since the assay was performed in aerobic conditions, it was possible that the presence of O2 in the sample influenced the binding of CO from CORM-3 to the flavohemoglobin. To test this hypothesis, Hmp was incubated with NADH, without dithionite, in an anaerobic cabinet. After 15 min of incubation, the reduction of Hmp to the Fe(II) form was recorded (λmax 432 nm) (Fig. 4A), (instead of the Fe(II) + O2 form that is produced aerobically and that persists until oxygen is depleted (Fig. 3C) (48). Even in the total absence of oxygen, incubation of Fe(II) Hmp with 10 μm CORM-3 for 30 min elicited only a small change in the spectrum at the Soret region (Fig. 4A). However, subsequent addition of sodium dithionite to the sample produced a rapid conversion to the Fe(II) + CO form shown in the absolute spectra (λmax 421 nm) (Fig. 4B) and the difference spectra (CO-reduced minus reduced) (Fig. 4C). When the difference in absorbance was plotted against time, only 9% of CO-Hmp was observed in the absence of dithionite after 30 min incubation (this might account for the effect observed in Fig. 2B), whereas upon addition of the reducing agent, the conversion to the CO-ligated was complete in less than 10 min (100% of Hmp was CO-bound) (Fig. 4D). In conclusion, rapid CO release from CORM-3 requires the presence of dithionite, and it is not influenced by the presence or absence of oxygen.
FIGURE 4.
Effect of sodium dithionite on the formation of CO-reduced Hmp upon addition of CORM-3 in anaerobic conditions. Hmp (5 μm) was suspended in 50 mm Tris-HCl, 50 mm NaCl buffer (pH 8.0) containing NADH (500 μm) anaerobically. Arrow (A to B) indicates subsequent steps performed with the same sample. Reduction of Hmp was promoted by 15 min incubation in NADH-containing buffer (solid line in A) followed by the addition of 10 μm CORM-3. Changes were recorded after 30 min incubation (dashed line in A and B). An excess of sodium dithionite was added, and changes in the spectra were recorded every 0.5 min for 12 min (gray lines in B). C, difference spectra after addition of dithionite. D, CO release time course from CORM-3 before and after addition of dithionite. Dashed arrows indicate the direction of the changes. For the absolute spectrum of reduced Hmp (A), NADH-containing buffer was used as baseline. The experiment was repeated three times with similar results.
Ligation of CO from CORM-3 to Reduced Hmp Is Achieved in Cellular Soluble Extracts but Is Limited in Membrane Suspensions
The antimicrobial effects of CORM-3 have been previously demonstrated in cultures of P. aeruginosa (49) and E. coli (14, 50). Based on recent evidence, showing that CO release from CORM-3 is achievable not only in the presence of dithionite but also other sulfites, it has been suggested that CO release from CORM-3 is likely to be promoted in cellular milieux by the interaction with sulfites or other unidentified biological species (51). To reconcile the differences between the in vivo studies using cultures (Fig. 1) and the in vitro results using purified protein (Figs. 2–4), changes in the redox state of purified Hmp in the presence of cellular extracts from the E. coli hmp mutant were investigated. Initially, 4.7 μm Hmp was added to E. coli soluble cell extracts in the presence of 1 mm NADH. After 10 min of incubation at room temperature, a fully reduced (Fe(II)) globin spectrum was recorded (λmax 436 nm) (Fig. 5A, dashed line). Addition of 100 μm CORM-3 to the Fe(II)-Hmp produced an immediate but transient change to the Fe(II) + O2 state (Fig. 5A, dotted line) (explained by the introduction of some O2 to the mix during the addition of CORM-3), followed by the gradual conversion to the Fe(II) + CO state (Fig. 5A, solid lines). CO binding was clearly observed in either the absolute spectra (Fig. 5A) or the difference spectra (CO-reduced minus reduced) (Fig. 5B). Absorbance difference (peak minus trough) plotted against time showed the course of the reaction of CO with Hmp taking less than 15 min to reach the absorbance maxima (Fig. 5B, lower inset). Interestingly, when E. coli wild type membrane suspensions instead of soluble extracts were tested, and even though addition of NADH elicited full reduction of the globin, only a negligible amount of Fe(II) + CO Hmp was detected after 40 min incubation in the presence of 100 μm CORM-3 (Fig. 6), as when NADH-reduced Hmp was suspended in buffer (Fig. 4). These results suggest that efficient CO release from CORM-3 is promoted in cellular milieu, supporting the role of CO per se as the inhibitor of the Hmp NO detoxification ability of E. coli in our tests in vivo. The data also reveal the caution that needs to be exercised when CORM-3 is used with protein solutions or membrane preparations.
FIGURE 5.
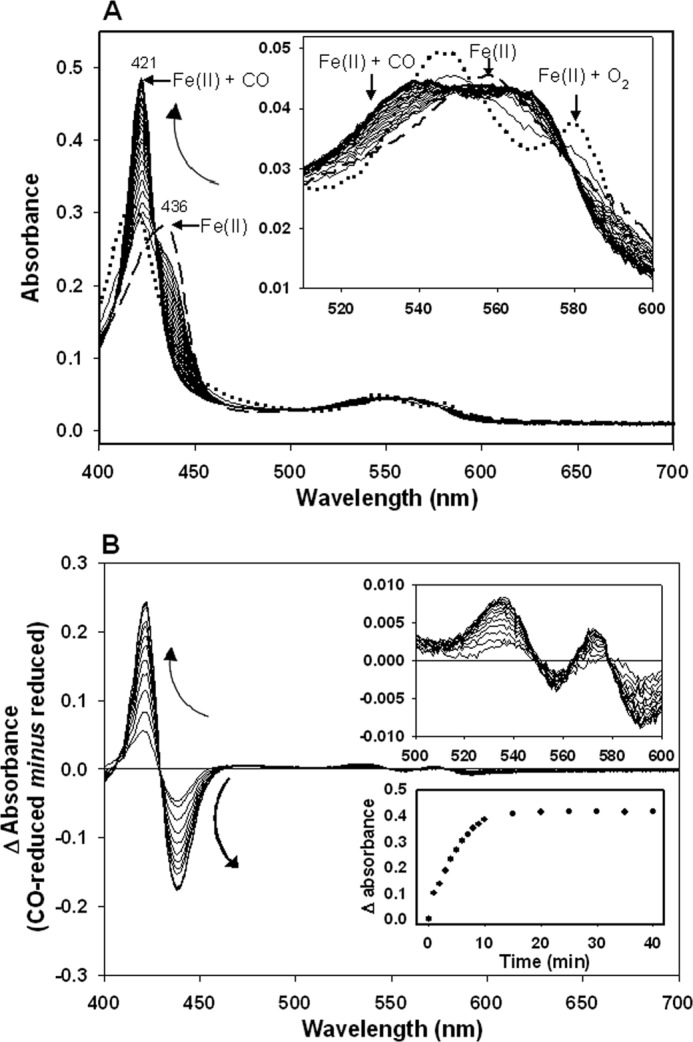
Formation of CO-reduced Hmp upon addition of CORM-3 in E. coli cellular extracts. Hmp (4.7 μm) was added to E. coli hmp soluble extracts containing 500 μm NADH. A, Hmp absolute spectra were recorded after 10 min incubation at room temperature to promote reduction (dashed line), then immediately after addition of 100 μm CORM-3 (dotted line) and every min for 20 min and every 5 min for an additional 20 min (solid lines). Inset shows the amplification of the α,β region. B, difference spectra after addition of CORM-3. Insets show amplification of the α,β region (above), and the CO release time course from CORM-3 (below). Curved arrows indicate the direction of the changes.
FIGURE 6.
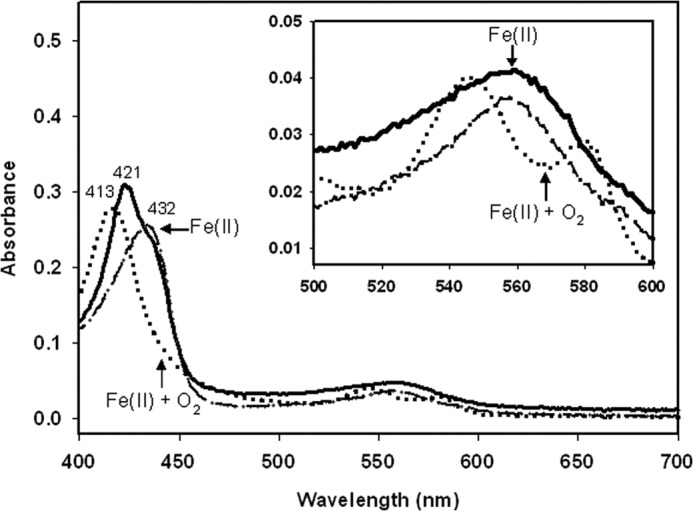
E. coli membrane suspensions fail to promote CO releasing from CORM-3. Hmp (4.7 μm) was added to E. coli membrane suspension containing 1 mm NADH. Hmp absolute spectra were recorded after 10 min incubation at room temperature to promote reduction (dashed line), then immediately after addition of 100 μm CORM-3 (dotted line), and after 40 min (solid line). Inset shows the amplification of the α,β region.
Intracellular Fe(II) + CO Hmp Formation by CORM-3
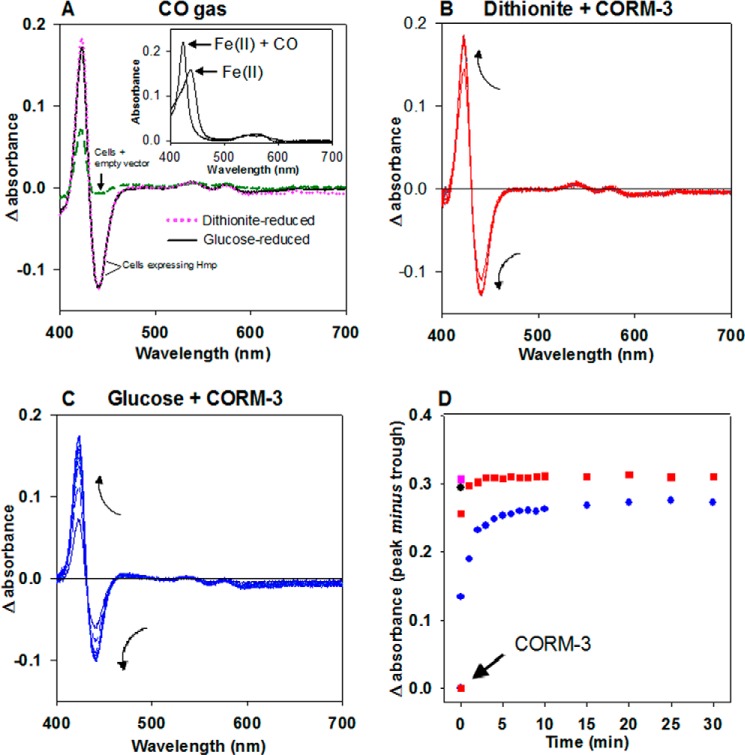
To demonstrate the CO binding of Hmp in the presence of CORM-3 in vivo, an expression vector containing the hmp gene under control of an arabinose inducible promoter was constructed based on pBAD/HisA (Invitrogen). Spectroscopic measurements of E. coli cell suspensions over-expressing Hmp showed a clear signal corresponding to the presence of the globin. Hmp-expressing cell suspensions reduced by sodium dithionite and then bubbled with CO gas until saturation showed a difference spectrum with an intense signal due to Hmp compared with cell suspensions harboring the empty vector (Fig. 7A, magenta dotted line and dashed green lines, respectively). Moreover, when the absorbance values of the sample lacking Hmp were subtracted from the samples overexpressing the globin, the absolute spectra of the ferrous (Fe(II)) (upon addition of sodium dithionite) or the CO-ferrous (Fe(II) + CO) form (by bubbling the dithionite-reduced samples with CO gas) were clearly distinguishable (Fig. 7A, inset). Since dithionite constitutes an undesirable component for the purpose of testing the CO release from CORM-3 in physiological conditions, CO binding to the intracellular Hmp native form of cells suspended in buffer (Fe(II) + O2) was investigated. Saturating concentrations of CO gas failed to produce the characteristic difference spectra (not shown), suggesting the inability of CO to displace the O2 bound to Hmp in the absence of electron input and the need for reducing equivalents as demonstrated in our tests in vitro (Fig. 3C).
FIGURE 7.
Intracellular formation of CO-reduced Hmp by CORM-3. A, E. coli cell suspensions overexpressing Hmp were reduced with sodium dithionite (magenta dotted line) or glucose (15 mm) (black solid line) and bubbled with CO gas. The difference spectra (CO-reduced minus reduced) were plotted against the difference spectra of glucose-reduced cells carrying the empty vector (dashed green line). The absolute spectra of intracellular glucose-reduced (Fe(II)) and glucose-reduced plus CO gas (Fe(II) + CO) Hmp were obtained by subtraction of the absorbance values of similarly treated cells carrying the empty vector (inset). B and C, cells were reduced with dithionite or glucose, respectively, and supplemented with 300 μm CORM-3. Changes in the spectra were followed every minute for 10 min and every 5 min for an additional 20 min. Arrows indicate the direction of the changes. D, differences in the absorption maxima (peak minus trough) were plotted for every time point. Data from B (red squares) and C (blue dots) were plotted together with the single points from A where the difference in absorbance was obtained from the Hmp-expressing samples reduced with dithionite (magenta square) or glucose (black dot) in the presence of CO gas.
In an attempt to reduce the intracellular globin in the absence of dithionite, glucose (15 mm) was added to the cell suspensions to promote respiration and, consequently, remove the O2 from the sample and generate reducing equivalents. To determine the incubation times needed for O2 depletion by respiration, cellular O2 consumption was followed polarographically in a closed chamber. When anoxic conditions were reached (∼3 min), the chamber was opened, mimicking the experimental condition in the spectrophotometer. The samples remained anoxic for at least a further 45 min (not shown) showing that the rapid removal of O2 via cellular respiration was compatible with spectroscopic measurements. Difference spectra of the Hmp-expressing cell suspensions reduced by addition of glucose and then bubbled with CO gas (Fig. 7A, black solid line) were almost identical to the spectra recorded when sodium dithionite was used instead (magenta dotted line). A sample of the purified Hmp in buffer containing NADH plus 30 mm glucose did not result in Fe(II) + CO Hmp production upon addition of 100 μm CORM-3, demonstrating that glucose per se is unlikely to promote the intracellular CO releasing by CORM-3 (not shown). Thus, this method using glucose to promote intracellular reduction was implemented to investigate the intracellular reaction of Hmp with CORM-3-derived CO.
In a control sample, addition of 300 μm CORM-3 to dithionite-reduced cells showed rapid generation of intracellular Fe(II) + CO Hmp, reaching maximal heme occupancy at ∼3 min (peak minus trough in the difference spectra) (Fig. 7, B and D, red squares). On the other hand, the same concentration of CORM-3 added to glucose-reduced cells resulted in 3-fold slower CO binding (∼10 min for completion) (Fig. 7, C and D, blue circles). Surprisingly, the absorption maxima in the glucose-reduced sample were smaller than in the sample containing dithionite. This difference cannot be explained by a deficiency in reduction by glucose since the sample reduced with either dithionite or glucose and bubbled with CO gas (Fig. 7A) showed similar difference absorbance values (Fig. 7D, magenta square and black circle, respectively).
DISCUSSION
The development of new strategies to combat antibiotic-resistant pathogens is a worldwide priority (52). Endogenously generated CO and CO from CO-RMs possess important antibacterial effects such as increasing phagocytosis (53, 54) and protecting against lethality during bacterial sepsis (54, 55). It seems plausible that the antibacterial effects of CO are related, among other factors, to the inhibition of bacterial defense mechanisms that are critical during infection and colonization of the host. Even though the simultaneous production of NO by NO synthases and CO by heme oxygenases, interacting as cootransmitters (56) and cooregulators (57) has been recognized, it is unclear what levels of these gases occur in either physiological or pathological situations. Importantly, the present work demonstrates that CO generated from CORM-3 compromises the ability of the flavohemoglobin Hmp to detoxify NO in vivo (Fig. 1). We attribute the inhibition of Hmp directly to CO released from CORM-3. Although Ru(III) is known to bind and activate NO (58, 59), we consider this to be unlikely because of the lack of effect observed by the iCORM (Fig. 1D).
CO-RMs have been shown to be effective vehicles for supplying CO to biological samples - cells, organelles, tissues, and whole animals. It is tacitly assumed that the CO-RM acts purely as a delivery vehicle; indeed, control molecules lacking CO have shown that many of the established biological effects of CO, such as the modulation of vascular tone, diminution of inflammation and antimicrobial properties, are absent in the iCORMs (4, 15, 60, 61). However, the first careful transcriptomic comparison of the effects of a CO-RM and an iCORM in E. coli have revealed that iCORMs retain significant biological outcomes altering energy metabolism, motility, membrane transport and the metabolism of cysteine and methionine among other sulfur-containing species (51). Therefore, great caution is needed in using these compounds especially when the metal carbonyl contains a metal that is 'foreign' to biological systems, such as Ru.
A further note of caution comes from the demonstration that the almost universal method for assessing release of CO from CO-RMs, that is, the trapping of CO in vitro by ferrous myoglobin is flawed, since the loss of CO from one of the most widely used CO-RMs, CORM-3, is dependent in the usual assay on the reductant sodium dithionite. An alternative assay that obviates the need for dithionite, namely the trapping of CO by oxyhemoglobin, which is stable, was described by McLean (17).
Here we uncover another potential pitfall in the use of CORM-3. Although this compound inhibits NO detoxification in vivo (Fig. 1) and releases CO in cell extracts measured by the formation of carboxy-Hmp (Fig. 5), it is ineffective in solutions of the protein (Fig. 2B) or in assays with minimal cellular components (Fig. 6). We interpret this as further evidence of the need for CO release via molecules that include dithionite and sulfites (17). The full spectrum of compounds able to elicit CO release and the molecular mechanism of CO release from CORM-3 in the presence of dithionite remain unknown. However, it might be explained by the fact that dithionite is not pure and contains a significant quantity of sulfite which is in equilibrium with sulfur dioxide, a good ligand for transition metals (62). Dithionite is used as a reductant for the O2 adduct of heme and any Fe(III) heme present. Sulfites are also reductants but not as powerful as dithionite. It is unlikely that sulfites will reduce CORM-3 and if it did, it is implausible that CO would be released, as low oxidation states stabilize metal carbonyls. The chemistry of CORM-3 is very complex, making it difficult to elucidate the mechanism of CO release (16, 63). Moreover, since the kinetics of CO-release from CORM-3 has been mostly determined in the presence of sodium dithionite, careful evaluation of experimental conditions is needed. Identification of the cellular components involved in CO-release from CORM-3 as well as investigation of the effect of strong reductants other than dithionite may provide useful information.
We demonstrate here that growing NO-challenged cells are inhibited by CORM-3 (16, 49, 50) and the ability of Hmp to detoxify NO in culture is also decreased in the presence of the CO-releaser (Fig. 1). However, intracellular oxyferrous Hmp does not bind CO gas when cells are resuspended in buffer in the absence of NADH (not shown). It appears that metabolically active cells are able to maintain a sufficiently reduced inner environment, allowing CO binding to ferrous-heme proteins (such as hemoglobins) and terminal oxidases even in aerobic growth conditions. By overexpressing the flavohemoglobin Hmp in E. coli, we demonstrated intracellular CO release from CORM-3 by following the formation of carboxy-flavohemoglobin. Respiring E. coli cells exposed to air in the open electrode were able to constantly consume O2 so efficiently that the electrode failed to detect it for at least 45 min (not shown) promoting the complete reduction of Hmp. Thus, it is clear that the reductant power supplied by respiration can replace the use of dithionite (Fig. 7). However, the presence of membranes, even when supplemented with NADH to promote respiration, was insufficient to promote CO loss from CORM-3 (Fig. 6), suggesting that washed membranes lack the sulfites, sulfur dioxide, or other species discussed in Ref. 17 that promote CO loss.
The use of CO-RMs may be part of a new era in the treatment of bacterial infection where these compounds may be used in conjunction with the classical or new generation antibiotics. One of the most attractive features of CO-RMs is the fact that they appear to be more effective than CO gas. Indeed, bacterial growing cells are inhibited by CORM-3 but not by the same concentration of dissolved CO gas, suggesting that the CO-RM is taken up by the cells delivering the CO in situ (50, 64, 65); this has been called “The Trojan horse mechanism” (64, 66). However, data concerning the modes of action of CO and CO-RMs in biological systems are still very limited. The recent development of novel technology to measure gasotransmitters such as fluorescent probes to sense intracellular CO (67, 68) and the development of a sensitive electrochemical microsensor for the simultaneous detection of CO and NO (47) offers great promise for investigating the physiological and toxic effects of these gases in microbial cells.
This work was supported by Consejo Nacional de Ciencia y Tecnologia (Mexico) through Grant Number 99171 and Consejo Estatal de Ciencia, Tecnología e Innovación de Michoacán through Grant Number 007 (to M. T.-T.) and the UK Biotechnology and Biological Sciences Research Council through Grant Number BB/HO16805/1 (to R. K. P.).
- Hb
- hemoglobin
- CO-RM
- CO-releasing molecule
- CORM-3
- (Ru[CO]3Cl(glycinate))
- iCORM
- inactive CORM
- DETA NONOate
- 3,3-bis(aminoethyl)-1-hydroxy-2-oxo-1-triazene
- PROLI-NONOate
- 1-(hydroxyl-NNO-azoxy)-l-proline, disodium salt
- NOD
- NO dioxygenase.
REFERENCES
- 1. Keilin D. (1966) The History of Cell Respiration and Cytochrome, Cambridge University Press, Cambridge [Google Scholar]
- 2. Mann B. E. (2010) Carbon monoxide: an essential signalling molecule. Top. Organomet. Chem. 32, 247–285 [Google Scholar]
- 3. Marks G. S., Brien J. F., Nakatsu K., McLaughlin B. E. (1991) Does carbon monoxide have a physiological function? Trends Pharmacol. Sci. 12, 185–188 [DOI] [PubMed] [Google Scholar]
- 4. Motterlini R., Otterbein L. E. (2010) The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 9, 728–743 [DOI] [PubMed] [Google Scholar]
- 5. Ryter S. W., Alam J., Choi A. M. K. (2006) Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 86, 583–650 [DOI] [PubMed] [Google Scholar]
- 6. Boczkowski J., Poderoso J. J., Motterlini R. (2006) CO-metal interaction: vital signaling from a lethal gas. Trends Biochem. Sci. 31, 614–621 [DOI] [PubMed] [Google Scholar]
- 7. Shekhawat G. S., Verma K. (2010) Haem oxygenase (HO): an overlooked enzyme of plant metabolism and defence. J. Exp. Bot. 61, 2255–2270 [DOI] [PubMed] [Google Scholar]
- 8. Desmard M., Foresti R., Morin D., Dagoussat M., Berdeaux A., Denamur E., Crook S. H., Mann B. E., Scapens D., Montravers P., Boczkowski J., Motterlini R. (2012) Differential antibacterial activity against Pseudomonas aeruginosa by carbon monoxide-releasing molecules. Antiox. Redox Signal. 16, 153–163 [DOI] [PubMed] [Google Scholar]
- 9. Schatzschneider U. (2011) PhotoCORMs: Light-triggered release of carbon monoxide from the coordination sphere of transition metal complexes for biological applications. Inorg. Chim. Acta 374, 19–23 [Google Scholar]
- 10. Clark J. E., Naughton P., Shurey S., Green C. J., Johnson T. R., Mann B. E., Foresti R., Motterlini R. (2003) Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ. Res. 93, e2–8 [DOI] [PubMed] [Google Scholar]
- 11. Alcaraz M. J., Guillen M. I., Ferrandiz M. L., Megías J., Motterlini R. (2008) Carbon monoxide-releasing molecules: A pharmacological expedient to counteract inflammation. Curr. Pharm. Des. 14, 465–472 [DOI] [PubMed] [Google Scholar]
- 12. Motterlini R., Mann B. E., Foresti R. (2005) Therapeutic applications of carbon monoxide-releasing molecules. Expert Opin. Investig. Drugs. 14, 1305–1318 [DOI] [PubMed] [Google Scholar]
- 13. Roberts G. P., Youn H., Kerby R. L. (2004) CO-sensing mechanisms. Microbiol. Mol. Biol. Rev. 68, 453–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nobre L. S., Seixas J. D., Romão C. C., Saraiva L. M. (2007) Antimicrobial action of carbon monoxide-releasing compounds. Antimicrob. Agents Chemother. 51, 4303–4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson J. L., Jesse H. E., Poole R. K., Davidge K. S. (2012) Antibacterial effects of carbon monoxide. Curr. Pharm. Biotechnol. 13, 760–768 [DOI] [PubMed] [Google Scholar]
- 16. Davidge K. S., Motterlini R., Mann B. E., Wilson J. L., Poole R. K. (2009) Carbon monoxide in biology and microbiology: surprising roles for the “Detroit perfume”. Adv. Microb. Physiol. 56, 85–167 [DOI] [PubMed] [Google Scholar]
- 17. McLean S., Mann B. E., Poole R. K. (2012) Sulfite species enhance carbon monoxide release from CO-releasing molecules: implications for the deoxymyoglobin assay of activity. Anal. Biochem. 427, 36–40 [DOI] [PubMed] [Google Scholar]
- 18. Poole R. K., Hughes M. N. (2000) New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol. Microbiol. 36, 775–783 [DOI] [PubMed] [Google Scholar]
- 19. Stevanin T. M., Ioannidis N., Mills C. E., Kim S. O., Hughes M. N., Poole R. K. (2000) Flavohemoglobin Hmp affords inducible protection for Escherichia coli respiration, catalyzed by cytochromes bo' or bd, from nitric oxide. J. Biol. Chem. 275, 35868–35875 [DOI] [PubMed] [Google Scholar]
- 20. Gardner P. R., Costantino G., Szabó C., Salzman A. L. (1997) Nitric oxide sensitivity of the aconitases. J. Biol. Chem. 272, 25071–25076 [DOI] [PubMed] [Google Scholar]
- 21. Hausladen A., Gow A., Stamler J. S. (2001) Flavohemoglobin denitrosylase catalyzes the reaction of a nitroxyl equivalent with molecular oxygen. Proc. Natl. Acad. Sci. U.S.A. 98, 10108–10112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cruz-Ramos H., Crack J., Wu G., Hughes M. N., Scott C., Thomson A. J., Green J., Poole R. K. (2002) NO sensing by FNR: regulation of the Escherichia coli NO-detoxifying flavohaemoglobin, Hmp. EMBO J. 21, 3235–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hess D. T., Matsumoto A., Kim S.-O., Marshall H. E., Stamler J. S. (2005) Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 6, 150–166 [DOI] [PubMed] [Google Scholar]
- 24. Poole R. K. (2005) Nitric oxide and nitrosative stress tolerance in bacteria. Biochem. Soc. Trans. 33, 176–180 [DOI] [PubMed] [Google Scholar]
- 25. Vinogradov S. N., Tinajero-Trejo M., Poole R. K., Hoogewijs D. (2013) Bacterial and archaeal globins - A revised perspective. Biochim. Biophys. Acta 1834, 1789–1800 [DOI] [PubMed] [Google Scholar]
- 26. Gardner P. R., Costantino G., Salzman A. L. (1998) Constitutive and adaptive detoxification of nitric oxide in Escherichia coli. Role of nitric-oxide dioxygenase in the protection of aconitase. J. Biol. Chem. 273, 26528–26533 [DOI] [PubMed] [Google Scholar]
- 27. Poole R. K., Anjum M. F., Membrillo-Hernández J., Kim S. O., Hughes M. N., Stewart V. (1996) Nitric oxide, nitrite, and Fnr regulation of hmp (flavohemoglobin) gene expression in Escherichia coli K-12. J. Bacteriol. 178, 5487–5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Forrester M. T., Foster M. W. (2012) Protection from nitrosative stress: a central role for microbial flavohemoglobin. Free Radic. Biol. Med. 52, 1620–1633 [DOI] [PubMed] [Google Scholar]
- 29. Gardner P. R., Gardner A. M., Brashear W. T., Suzuki T., Hvitved A. N., Setchell K. D., Olson J. S. (2006) Hemoglobins dioxygenate nitric oxide with high fidelity. J. Inorg. Biochem. 100, 542–550 [DOI] [PubMed] [Google Scholar]
- 30. Gardner P. R., Gardner A. M., Martin L. A., Dou Y., Li T., Olson J. S., Zhu H., Riggs A. F. (2000) Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon monoxide inhibition. J. Biol. Chem. 275, 31581–31587 [DOI] [PubMed] [Google Scholar]
- 31. Gardner P. R., Gardner A. M., Martin L. A., Salzman A. L. (1998) Nitric oxide dioxygenase: an enzymic function for flavohemoglobin. Proc. Natl. Acad. Sci. U.S.A. 95, 10378–10383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hausladen A., Gow A. J., Stamler J. S. (1998) Nitrosative stress: metabolic pathways involving a flavohemoglobin (denitrosilase). Arch. Physiol. Biochem. 2, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hernández-Urzúa E., Mills C. E., White G. P., Contreras-Zentella M. L., Escamilla E., Vasudevan S. G., Membrillo-Hernández J., Poole R. K. (2003) Flavohemoglobin Hmp, but not its individual domains, confers protection from respiratory inhibition by nitric oxide in Escherichia coli. J. Biol. Chem. 278, 34975–34982 [DOI] [PubMed] [Google Scholar]
- 34. Mills C. E., Sedelnikova S., Søballe B., Hughes M. N., Poole R. K. (2001) Escherichia coli flavohaemoglobin (Hmp) with equistoichiometric FAD and haem contents has a low affinity for dioxygen in the absence or presence of nitric oxide. Biochem. J. 353, 207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim S. O., Orii Y., Lloyd D., Hughes M. N., Poole R. K. (1999) Anoxic function for the Escherichia coli flavohaemoglobin (Hmp): reversible binding of nitric oxide and reduction to nitrous oxide. FEBS Lett. 445, 389–394 [DOI] [PubMed] [Google Scholar]
- 36. Keefer L. K., Nims R. W., Davies K. M., Wink D. A. (1996) “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods Enzymol. 268, 281–293 [DOI] [PubMed] [Google Scholar]
- 37. Hrabie J. A., Klose J. R., Wink D. A., Keefer L. K. (1993) New nitric oxide-releasing zwitterions derived from polyamines. J. Org. Chem. 58, 1472–1476 [Google Scholar]
- 38. Saavedra J. E., Southan G. J., Davies K. M., Lundell A., Markou C., Hanson S. R., Adrie C., Hurford W. E., Zapol W. M., Keefer L. K. (1996) Localizing antithrombotic and vasodilatory activity with a novel, ultrafast nitric oxide donor. J. Med. Chem. 39, 4361–4365 [DOI] [PubMed] [Google Scholar]
- 39. Blattner F. R., Plunkett G., 3rd, Bloch C. A., Perna N. T., Burland V., Riley M., Collado-Vides J., Glasner J. D., Rode C. K., Mayhew G. F., Gregor J., Davis N. W., Kirkpatrick H. A., Goeden M. A., Rose D. J., Mau B., Shao Y. (1997) The complete genome sequence of Escherichia coli K-12. Science 277, 1453–1462 [DOI] [PubMed] [Google Scholar]
- 40. Flatley J., Barrett J., Pullan S. T., Hughes M. N., Green J., Poole R. K. (2005) Transcriptional responses of Escherichia coli to S-nitrosoglutathione under defined chemostat conditions reveal major changes in methionine biosynthesis. J. Biol. Chem. 280, 10065–10072 [DOI] [PubMed] [Google Scholar]
- 41. Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. (1978) A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87, 206–210 [DOI] [PubMed] [Google Scholar]
- 42. Ioannidis N., Cooper C. E., Poole R. K. (1992) Spectroscopic studies on an oxygen-binding haemoglobin-like flavohaemoprotein from Escherichia coli. Biochem. J. 288, 649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Poole R. K., Baines B. S., Appleby C. A. (1986) Haemoprotein b-590 (Escherichia coli), a reducible catalase and peroxidase: evidence for its close relationship to hydroperoxidase l and a 'cytochrome a1b ' preparation. J. Gen. Microbiol. 132, 1525–1539 [DOI] [PubMed] [Google Scholar]
- 44. Kalnenieks U., Galinina N., Bringer-Meyer S., Poole R. K. (1998) Membrane D-lactate oxidase in Zymomonas mobilis: evidence for a branched respiratory chain. FEMS Microbiol. Lett. 168, 91–97 [DOI] [PubMed] [Google Scholar]
- 45. Stevanin T. M., Moir J. W. B., Read R. C. (2005) Nitric oxide detoxification systems enhance survival of Neisseria meningitidis in human macrophages and in nasopharyngeal mucosa. Infect. Immun. 73, 3322–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gardner A. M., Gardner P. R. (2002) Flavohemoglobin detoxifies nitric oxide in aerobic, but not anaerobic, Escherichia coli. Evidence for a novel inducible anaerobic nitric oxide-scavenging activity. J. Biol. Chem. 277, 8166–8171 [DOI] [PubMed] [Google Scholar]
- 47. Park S. S., Kim J., Lee Y. (2012) Improved electrochemical microsensor for the real-time simultaneous analysis of endogenous nitric oxide and carbon monoxide generation. Anal. Chem. 84, 1792–1796 [DOI] [PubMed] [Google Scholar]
- 48. Poole R. K., Ioannidis N., Orii Y. (1994) Reactions of the Escherichia coli flavohaemoglobin (Hmp) with oxygen and reduced nicotinamide adenine dinucleotide: evidence for oxygen switching of flavin oxidoreduction and a mechanism for oxygen sensing. Proc. Roy. Soc. Lond. B Biol. 255, 251–258 [DOI] [PubMed] [Google Scholar]
- 49. Desmard M., Davidge K. S., Bouvet O., Morin D., Roux D., Foresti R., Ricard J. D., Denamur E., Poole R. K., Montravers P., Motterlini R., Boczkowski J. (2009) A carbon monoxide-releasing molecule (CORM-3) exerts bactericidal activity against Pseudomonas aeruginosa and improves survival in an animal model of bacteraemia. FASEB J. 23, 1023–1031 [DOI] [PubMed] [Google Scholar]
- 50. Davidge K. S., Sanguinetti G., Yee C. H., Cox A. G., McLeod C. W., Monk C. E., Mann B. E., Motterlini R., Poole R. K. (2009) Carbon monoxide-releasing antibacterial molecules target respiration and global transcriptional regulators. J. Biol. Chem. 284, 4516–4524 [DOI] [PubMed] [Google Scholar]
- 51. McLean S., Begg R., Jesse H. E., Mann B. E., Sanguinetti G., Poole R. K. (2013) Analysis of the bacterial response to Ru(CO)3Cl(Glycinate) (CORM-3) and the inactivated compound identifies the role played by the ruthenium compound and reveals sulfur-containing species as a major target of CORM-3 action. Antioxid. Redox Signal. 19, 1999–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lewis K. (2013) Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 12, 371–387 [DOI] [PubMed] [Google Scholar]
- 53. Otterbein L. E., May A., Chin B. Y. (2005) Carbon monoxide increases macrophage bacterial clearance through toll-like receptor (TLR)4 expression. Cell Mol. Biol. 51, 433–440 [PubMed] [Google Scholar]
- 54. Chung S. W., Liu X., Macias A. A., Baron R. M., Perrella M. A. (2008) Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J. Clin. Invest. 118, 239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Takaki S., Takeyama N., Kajita Y., Yabuki T., Noguchi H., Miki Y., Inoue Y., Nakagawa T., Noguchi H. (2010) Beneficial effects of the heme oxygenase-1/carbon monoxide system in patients with severe sepsis/septic shock. Intensive Care Med. 36, 42–48 [DOI] [PubMed] [Google Scholar]
- 56. Xue L., Farrugia G., Miller S. M., Ferris C. D., Snyder S. H., Szurszewski J. H. (2000) Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proc. Natl. Acad. Sci. U.S.A. 97, 1851–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thorup C., Jones C. L., Gross S. S., Moore L. C., Goligorsky M. S. (1999) Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am. J. Physiol. 277, F882–F889 [DOI] [PubMed] [Google Scholar]
- 58. Clarke M. J. (2002) Ruthenium metallopharmaceuticals. Coord. Chem. Rev. 232, 69–93 [Google Scholar]
- 59. Franke A., van Eldik R. (2013) Factors that determine the mechanism of NO activation by metal complexes of biological and environmental relevance. Eur. J. Inorg. Chem. 2013, 460–480 [Google Scholar]
- 60. Johnson T. R., Mann B. E., Clark J. E., Foresti R., Green C. J., Motterlini R. (2003) Metal carbonyls: a new class of pharmaceuticals. Angew. Chem. Int. Ed. 42, 3722–3729 [DOI] [PubMed] [Google Scholar]
- 61. Romão C. C., Blättler W. A., Seixas J. D., Bernardes G. J. L. (2012) Developing drug molecules for therapy with carbon monoxide. Chem. Soc. Rev. 41, 3571–3583 [DOI] [PubMed] [Google Scholar]
- 62. Ryan R. R., Kubas G. J., Moody D. C., Eller P. G. (1981) Structure and bonding of transition metal-sulfur dioxide complexes in Inorg. Chem., pp. 47–100, Springer; Berlin Heidelberg [Google Scholar]
- 63. Johnson T. R., Mann B. E., Teasdale I. P., Adams H., Foresti R., Green C. J., Motterlini R. (2007) Metal carbonyls as pharmaceuticals? [Ru(CO)3Cl(glycinate)], a CO-releasing molecule with an extensive aqueous solution chemistry. Dalton Trans. 1500–1508 [DOI] [PubMed] [Google Scholar]
- 64. Wilson J. L., Jesse H. E., Hughes B., Lund V., Naylor K., Davidge K. S., Cook G. M., Mann B. E., Poole R. K. (2013) Ru(CO)3Cl(Glycinate) (CORM-3): a carbon monoxide-releasing molecule with broad-spectrum antimicrobial and photosensitive activities against respiration and cation transport in Escherichia coli. Antioxid. Redox. Signal. 19, 497–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jesse H. E., Nye T. L., McLean S., Green J., Mann B. E., Poole R. K. (2013) Cytochrome bd-I in Escherichia coli is less sensitive than cytochromes bd-II or bo'' to inhibition by the carbon monoxide-releasing molecule, CORM-3: N-acetylcysteine reduces CO-RM uptake and inhibition of respiration. Biochim. Biophys. Acta 1834, 1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tinajero-Trejo M., Jesse H. E., Poole R. K. (2013) Gasotransmitters, poisons, and antimicrobials: it's a gas, gas, gas!. F1000prime reports 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Michel B. W., Lippert A. R., Chang C. J. (2012) A reaction-based fluorescent probe for selective imaging of carbon monoxide in living cells using a palladium-mediated carbonylation. J. Am. Chem. Soc. 134, 15668–15671 [DOI] [PubMed] [Google Scholar]
- 68. Wang J., Karpus J., Zhao B. S., Luo Z., Chen P. R., He C. (2012) A selective fluorescent probe for carbon monoxide imaging in living cells. Angew. Chem. Int. Ed. Engl. 51, 9652–9656 [DOI] [PubMed] [Google Scholar]