Background: To study Plk3-activated γH2AX affecting hyperosmotic stress-induced cell fate.
Results: Plk3 directly catalyzed γH2AX in hyperosmotic stress-induced cells, resulting in an accumulation of G2/M phase, altered population in the G1 and S phases, and increased apoptosis.
Conclusion: Hyperosmotic stress-activated Plk3 elicited γH2AX, which regulates cell cycle progression and cell fate.
Significance: Plk3-mediated γH2AX in stress-induced cells plays important roles to determine cell growth and death.
Keywords: Cell Cycle; Cornea; Epithelial Cell; H2A Histone Family, Member X (H2AFX); Phosphorylation; Signaling
Abstract
Increased concentrations of extracellular solutes affect cell function and fate by stimulating cellular responses, such as evoking MAPK cascades, altering cell cycle progression, and causing apoptosis. Our study results here demonstrate that hyperosmotic stress induced H2AX phosphorylation (γH2AX) by an unrevealed kinase cascade involving polo-like kinase 3 (Plk3) in human corneal epithelial (HCE) cells. We found that hyperosmotic stress induced DNA-double strand breaks and increased γH2AX in HCE cells. Phosphorylation of H2AX at serine 139 was catalyzed by hyperosmotic stress-induced activation of Plk3. Plk3 directly interacted with H2AX and was colocalized with γH2AX in the nuclei of hyperosmotic stress-induced cells. Suppression of Plk3 activity by overexpression of a kinase-silencing mutant or by knocking down Plk3 mRNA effectively reduced γH2AX in hyperosmotic stress-induced cells. This was consistent with results that show γH2AX was markedly suppressed in the Plk3−/− knock-out mouse corneal epithelial layer in response to hyperosmotic stimulation. The effect of hyperosmotic stress-activated Plk3 and increased γH2AX in cell cycle progression showed an accumulation of G2/M phase, altered population in G1 and S phases, and increased apoptosis. Our results for the first time reveal that hyperosmotic stress-activated Plk3 elicited γH2AX. This Plk3-mediated activation of γH2AX subsequently regulates the cell cycle progression and cell fate.
Introduction
Changes of the extracellular osmotic pressure are often found in some pathologic conditions, such as diabetes mellitus, uremia, dehydration after exercise, heat shock, fatal burns, and inflammation sites in various tissues including the cornea (1–4). It has been shown that persistent hyperosmotic stress can induce DNA damage, cell cycle arrest, and apoptosis (5, 6). Hyperosmotic stress extracts water out of the cell to induce cell shrinkage. To restore the volume, cells undergo a regulated volume increase (RVI) process within several minutes by uptake of inorganic ions and water (7–9). Cell shrinkage and increased ionic strength alternate cell architecture compartments, denature proteins, and disturb cell function (5, 10, 11). Hyperosmotic stress is one of the important stimuli for cells on the surface of the body. For example, the corneal epithelial layer in the front of the eye forms the first defense line to protect injuries of eye structures behind the cornea from insults of environmental hazards including hyperosmotic stresses (12). Hyperosmotic stresses from environmental and pathological conditions strike the corneal epithelial cells and alter the fluid balance resulting in cell shrinkage, which may attribute to delayed wound healing and dry eye diseases (13).
Hyperosmotic stress as one of the extracellular stimuli induces a series of changes in intracellular kinase cascades that include activation of JNK, p38, and Polo-like kinase 3 (Plk3)2 signaling pathways to activate the important AP-1 (activating protein-1) transcription factor complex (11, 14–18). Particularly, Plk3, one of the four members in the Polo like kinase family, plays a significant role in hyperosmotic stress-induced signaling cascades in addition to the JNK signaling pathway to transmit the stress signals in corneal epithelial cells. As a protein kinase, it catalyzes downstream phospho-proteins at serine/threonine sites. There are two functional domains in the Plk3 protein including a kinase domain (KD) at the N terminus and a Polo-box domain (PBD) at the C terminus that is able to interact with interactive proteins (19, 20). Following cell cycle progression, Plk3 undergoes substantial alterations in abundance, kinase activity, and subcellular distribution. As a multifunctional protein, active Plk3 induced by hyperosmotic stress plays an important function in the formation of c-Jun and ATF-2 heterodimers and homodimers in the AP-1 complex (21–25). Plk3 also involves the pathways of IR/UV irradiation-induced DNA damage by regulating p53 activity and cell cycle progress in G2/M phase (26, 27). Plk3 activity is rapidly increased upon stress stimulation by ionizing radiation (IR), reactive oxygen species (ROS), methylmethane sulfonate (MMS), UV irradiation, and hypoxia (17, 18, 28–31). Plk3 plays roles in the pathway of DNA damage-dependent activation of p53 downstream from both ATM (mutated in ataxia telangiectasia)/ATR (ATM-related), in the family of phosphatidylinositol 3-kinase-related kinases, and Chk2 (32, 33).
Histone H2AX is one of the nucleosome core histone H2A variants and has been considered as a marker that indicates the occurrence of DNA double strand breaks (DSBs) in mammalian cells (34, 35). One of the early events in IR/UV irradiation-induced DNA DSBs is ATM/ATR-dependent phosphorylation of the histone variant H2AX at the C terminus serine 139 subsequently termed γH2AX (36, 37). In DNA DSBs cells, DNA damage response (DDR)-induced and ATM/ATR-catalyzed phosphorylation of H2AX interacts with checkpoint- and DNA repair-associated proteins such as Rad50, Rad51, and Brca1 as well as the p53 binding protein 1 (53BP1), resulting in cell cycle arrest (38–41). The questions remaining in hyperosmotic stress-induced cells are whether there is a phosphorylation of H2AX and whether extracellular stress-sensitive Plk3 interacts with H2AX in the ATM-Chk2 pathway to regulate cell fate. In the present study, we demonstrate important findings to show that hyperosmotic stress-induced DNA DSBs in corneal epithelial cells increased phosphorylation of H2AX, dependent on activation of Plk3. As an alternative signaling pathway, Plk3 served as a transducer to amplify the hyperosmotic stress-induced cellular response by directly interacting with H2AX at the protein level and to catalyze phosphorylation of H2AX at the position of serine 139, resulting in alteration of cell fate.
EXPERIMENTAL PROCEDURES
Cell Culture and Treatments
Primary human corneal epithelial (PHCE) cells and human telomerase immortalized corneal epithelial (HTCE) cell were cultured in a serum-free keratinocyte medium (Defined Keratinocyte-SFM; Invitrogen, Carlsbad, CA). The human corneal epithelial (HCE) cell line was grown in Dulbecco's modified Eagle's medium/F-12 (1:1) containing 10% fetal bovine serum and 5 μg/ml insulin. Mouse embryonic fibroblasts (MEF) were isolated for wild-type and Plk3−/− mouse embryos. Primary MEFs were derived from embryonic day 14.5 embryos with the respective genotype of both wild-type and PLK3−/− mice (42). The MEF cells were cultured in high glucose DMEM supplemented with 10% FBS and antibiotics (100 μg of penicillin and 50 μg of streptomycin sulfate per ml). In all of the MEF experiments, primary MEFs were restricted only in third to fifth passages. The culture conditions for corneal epithelial cells and MEFs were in an incubator supplied with 95% air and 5% CO2 at 37 °C. The medium was replaced every 2 days, and cells were subcultured by treatment with 0.05% trypsin-EDTA. For experiments of hyperosmotic stress stimulation, various concentrations of sorbitol, sucrose, NaCl, and glucose were added to the culture medium following a time course. Osmotic pressures of hyperosmotic media were measured using a Vapor Pressure Osmometer (VAPRO5520).
Cell Cycle Analysis
Cell cycle analysis was performed using a flow cytometry (BD LSRII, BD Biosciences, San Jose, CA). HCE cells and MEFs were treated with the control condition and hyperosmotic stresses following a time course, and then the attached cells were trypsinized and fixed with 70% ethanol and 50 mm glycine. The cells were resuspended in PBS containing RNase A (100 ng/ml) and propidium iodide (PI, 25 ng/ml). Cell populations in different phases were mapped with BD FAC Diva Software V6.11, and cell cycle progression was analyzed with MedFit LTTM V3.1 (Verity Software House). Statistical analysis was performed as described in the statistical section below.
Comet Assay
Hyperosmotic stress-induced DNA damage in the individual HCE cell was detected by the comet assay or the single cell gel electrophoresis assay (SCGE). The classic “comet tail” shapes resulting from damaged cellular DNA (containing fragments and strand breaks) were separated from intact DNA in the electrophoretic field. The assay was performed according to the manufacturer's instructions (Cell Biolabs, San Diego, CA). Briefly, cells were trypsinized to detach from culture dishes and washed once with PBS. Cells were loaded onto a low melting-point agarose gel and spread on the OxiSelect™ Comet Slide. Embedded cells were treated for 30 min at 4 °C in the dark with a lysis buffer and then alkaline solution, which relaxes and denatures the DNA. Electrophoresis was performed for 15–30 min in an electric field with 1 volt/cm. After rinsing the slide three times in distilled water and once in 70% ethanol, DNA was stained with Vista Green DNA Dye. Preparations were photographed using a Nikon Ti fluorescent microscope.
Gene Transfection and Recombinant Proteins
Human corneal epithelial cells were transfected with Plk3 wild type and kinase-defective Plk3K52R mutant (a full-length Plk3 that contains a mutation to substitute the lysine 52 with an arginine) using Lipofectamine reagents (Invitrogen). Transfected cells were subjected to Western analysis and immunocomplex kinase assays. Transfections of Plk3-specific siRNA (Qiagen, SI02223473 and SI02223466) were done by adding Plk3-specific siRNAs with a final concentration of 25 nm mixed with 12 μl of HiPerFect in 100 μl of serum-free culture medium. The mixtures were incubated for 20 min at room temperature. The mixture was evenly added into culture cells. Transfected cells were cultured under normal growth conditions for 48–84 h before performing experiments. Non-silencing siRNA-transfected cells were used as the controls with the same transfection method. In addition, human H2AX full-length cDNA in a pCR2.1-TOPO plasmid was subcloned into the EcoRI cloning sites in vector pFlag-CMV-4 (Sigma). H2AXS139A mutant was generated by site-directed mutagenesis using the QuikChange Lightning Site-directed Mutagenesis Kit (Agilent Technologies, Inc.), and the mutant sequence was confirmed by DNA sequencing. The fusion protein of GST-H2AXwt and GST-H2AXS139A was produced by cloning the wild type H2AX and H2AXS139A mutant into EcoRI sites within the bacterial expression vector pGEX-4T-3. Purification of GST-H2AXwt and GST-H2AXS139A was performed under standard conditions. Briefly, Sf9 cells (ATCC) infected with H2AX baculovirus were cultured in Grace's insect cell culture medium. Infected cells were harvested on day 3 and lysed in a lysis buffer (50 mm NaH2PO4, 300 mm NaCl, 1% Nonidet P-40 20 mm imidazole, 1 mm PMSF, 2 μm pepstatin A, 10 units/ml aprotinin). Cell lysates were incubated with Ni-NTA agarose resins for 3 h at 4 °C. Fusion proteins were eluted from Ni-NTA resins using the lysis buffer containing 200 mm imidazole after extensive wash of the resins with lysis buffer. Fusion proteins were purified by dialyzing in a storage buffer (25 mm Tris, pH 7.4, 5 mm EGTA, 2 mm DTT, 0.1% Triton X-100, and 50% glycerol) and stored at 80 °C for subsequent uses.
Immunocytochemistry
Immunostaining Experiments
corneal epithelial cells were grown on glass slides and hyperosmotic sorbitol solutions were used to treat HCE cells. Mouse corneal sections and HCE cells were fixed for 15 min in 4% paraformaldehyde, and then permeabilized with PBS-0.2% Triton X-100 (PBS-T) for 30 min at room temperature. The cells were blocked by incubation with 10% normal horse serum (NHS) or 10% normal goat serum in PBS-T for 1 h at room temperature, followed by double immunostaining with the corresponding antibodies. Corneal tissue and HCE cell slices were washed with PBS and stained with DAPI. A Nikon fluorescent Ti microscope was used to capture stained tissue imaging. Imaging data were analyzed using a Nikon NIS Element Software program.
Immunoprecipitation and Immunocomplex Kinase Assay
Corneal epithelial cells (5 × 107) were rinsed with PBS and incubated in 1 ml of lysis buffer (20 mm Tris, pH 7.5, 137 mm NaCl, 1.5 mm MgCl2, 2 mm EDTA, 10 mm sodium pyrophosphate, 25 mm glycerophosphate, 10% glycerol, 1% Triton X-100, 1 mm sodium vanadate, 1 mm phenylmethylsulfonyl fluoride, 250 μm 4NPP, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) on ice for 30 min. The cell lysates were spun at 13,000 × g for 10 min at 4 °C and incubated at 4 °C overnight with antibodies against Plk3 and γH2AX, respectively. The immunocomplexes were recovered by incubation with 50 μl of 10% protein A/G-Sepharose (Santa Cruz Biotechnology). The immunocomplex beads were rinsed twice with lysis buffer and once with kinase buffer, and then subject to immunoblotting and kinase assay. The effect of active Plk3 on catalyzing H2AX phosphorylation was measured using immunocomplex kinase assays by incubation of immunoprecipitated Plk3 with H2AX fusion protein in 30 μl of kinase buffer (20 mm HEPES, pH 7.6, 5 mm MgCl2, 10 μm MnCl2, 25 mm glycerophosphate, 1 mm sodium orthovanadate, 2 mm dithiothreitol, 20 μm ATP, and, 10 μCi of [γ-32P]ATP) for 30 min at 37 °C. Kinase reactions were terminated by adding an equal volume of 2× Laemmli buffer and boiling for 5 min. Equal volumes of the samples were displayed on 10–15% SDS-PAGE and visualized by exposure on x-ray films. For cold kinase assay, [γ-32P]ATP was omitted from the kinase buffer. Western blot assay was performed by harvesting 5 × 105 cells in 0.5 ml of lysis buffer. Samples were loaded onto 10–15% SDS-PAGE gels and fractionated by electrophoresis. Proteins in the gel were electro-transferred to a PVDF membrane. The membrane was exposed to blocking buffer (5% nonfat milk in TBS-0.1% Tween 20 (TBS-T) for 1 h at room temperature, and then incubated with respective antibodies overnight at 4 °C. After three washes with TBS-T buffer, the membrane was incubated with horseradish peroxidase (HRP)-linked secondary antibody for 1 h at room temperature. Expression of proteins was detected with a Western blot detection kit (Santa Cruz Biotechnology).
RESULTS
Effects of Hyperosmotic Stresses on DSBs and H2AX Phosphorylation/Activation
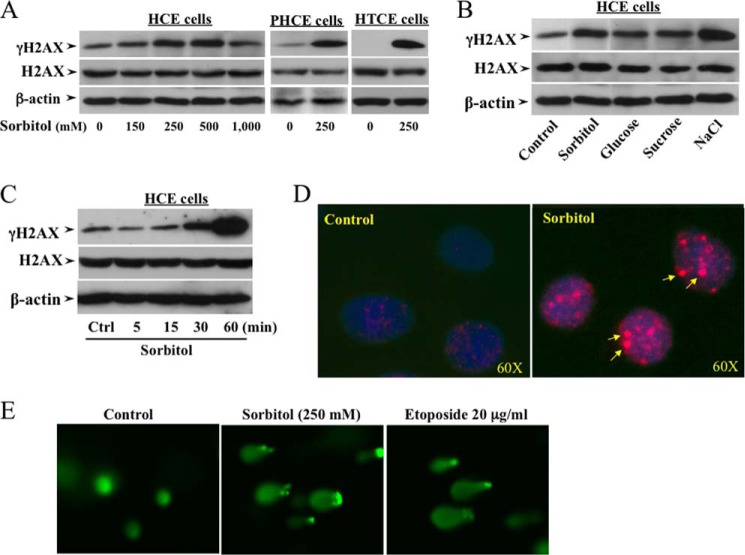
In previous studies, we found that Plk3 activity is one of the major regulators to mediate cellular responses to various stresses including UV irradiation, hypoxic stimulation, and hyperosmotic pressure. The effect of hyperosmotic stress-induced Plk3 activation on downstream events involves increases in phosphorylation and formation of c-Jun and ATF-2 hetero/homodimers in the AP-1 complex. In corneal epithelial cells, hyperosmotic stress-induced H2AX phosphorylation was studied by treating cells with increased extracellular osmotic pressures that were created by adding high concentrations of sorbitol of up to 1,000 mm. Phosphorylation levels of γH2AX were markedly increased at a sorbitol concentration of 250 mm sorbitol in the culture medium in three different origins of human corneal epithelial HCE, PHCE, and HTCE cells (Fig. 1A). In addition, four different solutes of sorbitol, glucose, sucrose, and NaCl at concentrations of 300 mm were applied to cell cultures to verify the hyperosmotic pressure effects on the phosphorylation levels of H2AX in HCE cells (Fig. 1B). Phosphorylation of H2AX was increased by 250 mm sorbitol within 15 min and reached the peak level at 60 min (Fig. 1C). Hyperosmotic stress-induced increases in H2AX phosphorylation were also demonstrated in the nuclei by immunostaining in sorbitol-treated cells (Fig. 1D). It is known that H2AX has been considered as a hallmark for DNA DSBs in mammalian cells. To verify the effect of hyperosmotic stress on DNA damage response in corneal epithelial cells, cells were treated with sorbitol and the antitumor drug etoposide that forms a DNA and topoisomerase II enzyme complex resulting in DNA DSBs (43). DNA DSBs were detected using the Comet assay to compare the effects of hyperosmotic sorbitol (250 mm) and etoposide (20 μg/ml for 4 h) (Fig. 1E). The results of these experiments suggest that hyperosmotic stresses significantly increased H2AX phosphorylation and DNA DSBs in corneal epithelial cells.
FIGURE 1.
Effects of altered osmotic stresses on DNA DSBs and H2AX phosphorylation. A, increases in γH2AX following increased sorbitol concentrations in various human corneal epithelial cells. B, effects of high concentrations of extracellular solutes: sorbitol (300 mm), glucose (300 mm), sucrose (300 mm), and NaCl (300 mm) on increases in γH2AX in HCE cells. C, high concentration sorbitol-induced γH2AX following a time course in HCE cells. D, localization of hyperosmotic stress-induced γH2AX in nuclei of HCE cells. E, hyperosmotic stress-induced DNA DSBs in HCE cells. DNA DSBs were detected with Comet assays in sorbitol (250 mm)-treated cells, and the anti-tumor drug etoposide (20 μg/ml)-treated cells served as positive controls.
Effect of Hyperosmotic Stress-induced Active Plk3 on H2AX Phosphorylation
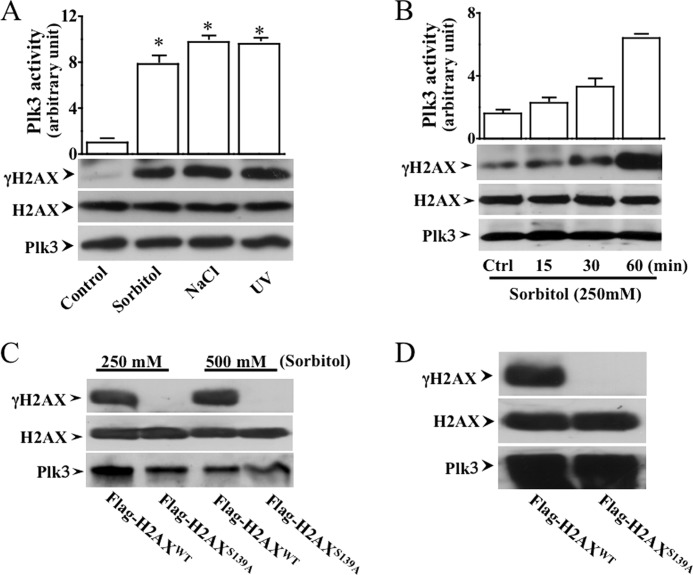
One of the important questions in the present study is to determine whether hyperosmotic stress-induced Plk3 activity is associated with H2AX phosphorylation. To detect interactions between Plk3 and H2AX, H2AX fusion proteins were purified and used as substrates in immunoprecipitation kinase assays to test the effect of hyperosmotic stress-induced Plk3 kinase activity on H2AX phosphorylation. Hyperosmotic stress-induced active Plk3 was immunoprecipitated and used to catalyze phosphorylation of the H2AX fusion proteins. Stress stimuli were applied by treating cells with UV irradiation, 250 mm sorbitol, and adding 300 mm NaCl to corneal epithelial cells. Plk3 that was immunoprecipitated from stress-induced cells phosphorylated the wild-type H2AX fusion protein at Ser-139 recognized by a γH2AX Ser-139 phosphorylation-specific antibody (Fig. 2A). Hyperosmotic stress-induced Plk3 activation and H2AX phosphorylation occurred within 15 min and reached the peak effect at 60 min following a time course (Fig. 2B). A mutant of Flag-H2AXS139A fusion proteins made by replacing serine139 with an alanine residue was subject to immunoprecipitation kinase assay for detecting hyperosmotic stress (sorbitol 250 and 500 mm)-induced difference in Plk3 activity to affect the phosphorylation level of serine 139 (Fig. 2C). Plk3 was also detected as a loading control. In addition, a constitutively active Plk3 was found to be able to catalyze phosphorylation of the H2AXwt fusion protein, but it failed to induce phosphorylation of the Flag-H2AXS139A mutant (Fig. 2D). These results indicate that active Plk3 in hyperosmotic stress-induced corneal epithelial cells is able to phosphorylate H2AX at serine 139 (S139).
FIGURE 2.
Hyperosmotic stress-activated Plk3 phosphorylated H2AX. A, phosphorylation of H2AX by activated Plk3 from HCE cells stimulated with hyperosmotic stress and UV irradiation in HCE cells. B, phosphorylation of H2AX fusion protein by hyperosmotic stress-activated Plk3 following a time course in HCE cells. C, hyperosmotic stress-activated Plk3 failed to phosphorylate the H2AX mutant (H2AXS139A) in which Ser-139 was substituted by an alanine residue. The Plk3 level was detected by Western blot and served as the loading control in HCE cells. D, effect of constitutively activated Plk3 upon phosphorylation of wild type H2AX and H2AXS139A mutant fusion proteins in HCE cells. The asterisk represents a significant difference between untreated control and hyperosmotic stress-induced HCE cells (p < 0.05, n = 3).
Interactions of Plk3 and γH2AX in Hyperosmotic Stress-induced Cells
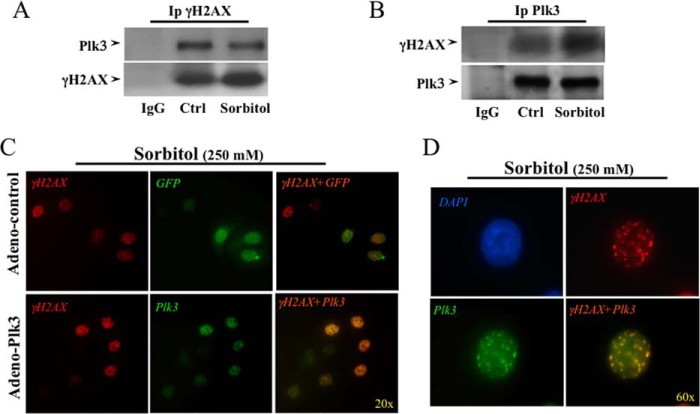
As shown in Fig. 2, Plk3 activated in hyperosmotic stress-stimulated HCE cells was able to directly phosphorylate H2AX fusion proteins. We performed further experiments to substantiate the evidence that Plk3 indeed mediates the phosphorylation process of γH2AX by demonstrating that there are interactions between Plk3 and H2AX proteins in hyperosmotic stress-induced cells. A bidirectional interaction between Plk3 and H2AX was detected by immunocoprecipitation and Western analysis. An anti-phospho-γH2AX antibody was used to pull down Plk3 protein, and in turn, the other anti-Plk3 antibody was applied to pull down phospho-γH2AX in hyperosmotic stress-induced corneal epithelial cells (Fig. 3, A and B). Activations of Plk3 and γH2AX were found and colocalized in the nuclei of hyperosmotic stress-induced corneal epithelial cells detected by immunostaining and microscopy imaging. Both Plk3 and γH2AX were activated to form immune active clusters within 45 min upon 250 mm sorbitol stimulation, and both active Plk3 and γH2AX were colocalized in cell nuclei that were indicated by DAPI nuclear staining under a Nikon fluorescent microscope (20×) (Fig. 3C). In addition, the hyperosmotic stress-induced interaction between Plk3 and γH2AX in the nuclei was more clearly demonstrated by increasing the microscopic power to a higher magnification (60×) in immunostaining experiments (Fig. 3D). In control immunostaining experiments using Plk3 knock-down cells, Plk3 expression was not detected (data not shown). Results of immunocoprecipitation and immunocolocalization indicate that there is indeed a protein-protein interaction between Plk3 and γH2AX upon hyperosmotic stress stimulation in corneal epithelial cells.
FIGURE 3.
Determining interaction between Plk3 and H2AX in hyperosmotic stress-induced HCE cells. A, detection of Plk3 and H2AX interaction by immunoprecipitation using an anti-phospho-H2AX (anti-γH2AX) antibody. Plk3 was detected by Western analysis. B, detection of Plk3 and H2AX interaction by immunoprecipitation using an anti-Plk3 antibody. γH2AX was detected by Western analysis using an anti-γH2AX antibody. C, immunocolocalization of Plk3 and γH2AX in adeno-GFP-Plk3-transfected and hyperosmotic stress-induced cells. D, high amplification view (60×) to show colocalization of Plk3 and γH2AX in the nucleus of hyperosmotic stress-induced HCE cells. Immunocolocalization experiments were done by immunostaining of hyperosmotic stress-induced human corneal epithelial cells using antibodies specific to γH2AX. Photo images were taken using a Nikon fluorescent microscope at 20× and 60×, respectively. Data were analyzed with a Nikon software program.
Effect of Altered Plk3 Activity on Hyperosmotic Stress-induced γH2AX
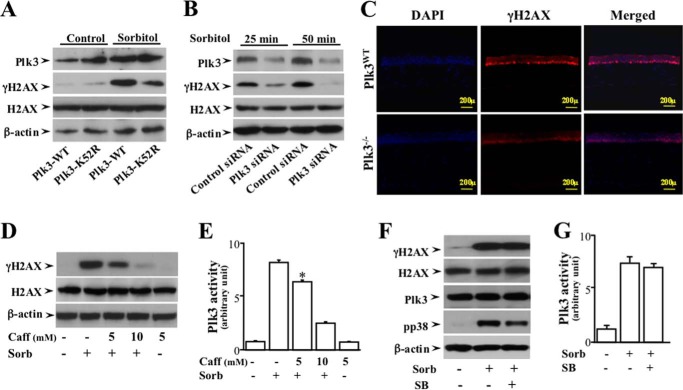
To study functional interaction of Plk3 and γH2AX in response to hyperosmotic stress, Plk3 activities in corneal epithelial cells and mouse cornea were altered by different approaches including: 1) transfection of a kinase-defective Plk3K52R mutant; 2) Plk3 mRNA knock-down with siRNA; and 3) Plk3 knock-out (Plk3−/− transgenic mice). In corneal epithelial cells, transfection of the Plk3K52R mutant resulted in a reduced hyperosmotic stress-induced phosphorylation of H2AX compared with cells transfected with the Plk3 wild type (Plk3WT) construct (Fig. 4A). In Plk3-specific siRNA-transfected corneal epithelial cells, partial knock-down of Plk3 effectively minimized hyperosmotic stress-induced phosphorylation of H2AX at 25 and 50 min (Fig. 4B). Further studies were conducted in mouse corneas obtained from both wild type and Plk3 knock-out (Plk3−/−) mice. In the cornea of wild type mice, localization of γH2AX in the nuclei of hyperosmotic stress-induced basal epithelial layer was detected using immunostaining and microscopy imaging. Active γH2AX was observed to form immune active clusters within 45 min upon hyperosmotic sorbitol stimulation, and γH2AX was colocalized in cell nuclei with DAPI nuclear staining (Fig. 4C). However, there was much less γH2AX found in the nuclei of hyperosmotic stress-induced corneas obtained from Plk3−/− mice. Our results clearly indicate that alterations of Plk3 activity in corneal epithelial cells and the epithelial layer of the mouse cornea markedly interrupted the functional interaction between Plk3 and γH2AX in the nuclei in response to hyperosmotic stress stimulation. It was reported that H2AX is phosphorylated by ATM/ATR that is activated in response to DNA damage, respectively. To study the possible involvement of Plk3 in these pathways, DNA damage-induced activation of ATM/ATR was blocked with 5 to 10 mm caffeine. Blockade of ATM/ATR by caffeine suppressed hyperosmotic stress-induced H2AX phosphorylation and Plk3 activity (Fig. 4, D and E). The relevance of Plk3 in hyperosmotic stress-induced H2AX phosphorylation was further characterized by inhibition of the p38 kinase. Hyperosmotic stress-induced H2AX phosphorylation in the absence or presence of SB202190 (an inhibitor of p38) was also measured in corneal epithelial cells. Inhibition of p38 had no effect on either hyperosmotic stress-induced H2AX phosphorylation or Plk3 activity (Fig. 4, F and G). To measure Plk3 activity without interference by JNK and p38, an immunodepletion (ID) procedure was used to remove JNK and p38 in cell lysates. After the ID procedures, JNK and p38 activities in lysates were markedly diminished to non-detectible levels (data not shown).
FIGURE 4.
Effects of altered Plk3 activity on hyperosmotic stress-induced γH2AX in HCE cells. A, effect of overexpressing wild type Plk3 and kinase-silencing Plk3k52R mutant on hyperosmotic stress-induced γH2AX in HCE cells. B, effect of knocking down Plk3 mRNAs on hyperosmotic stress-induced Plk3 γH2AX in HCE cells. C, suppression of hyperosmotic stress-induced γH2AX in the epithelial layer of the Plk3-deficient (Plk3−/−) mouse corneas. Immunostaining experiments were performed to detect hyperosmotic stress-induced activation of γH2AX using a γH2AX specific antibody, and cell nuclei were stained by DAPI in corneas of wild type and Plk3−/− knock-out mice. Photo images were taken using a Nikon fluorescent microscope at 10×. D, effect of inhibiting ATM/ATR with Caff (caffeine) on hyperosmotic stress-induced H2AX phosphorylation. E, effect of inhibiting ATM/ATR with Caff on hyperosmotic stress-induced Plk3 activity. F, effects of inhibiting p38 in the absence or presence of 500 μm SB (SB202190, an inhibitor of p38) on hyperosmotic stress-induced ATF-2 phosphorylation. G, effect of hyperosmotic stress on Plk3 activity. Plk3 activity was determined by immunocomplex kinase assay, and the H2AX fusion protein was used as the substrate. The asterisk symbol indicates a significant difference (p < 0.05, n = 3).
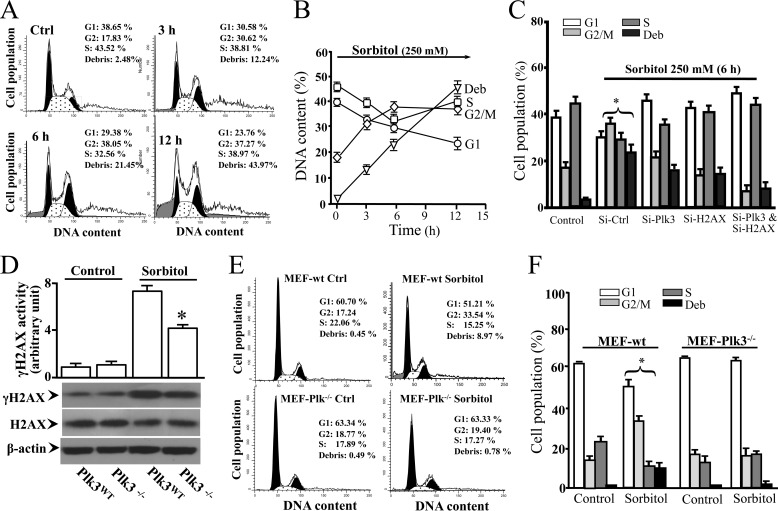
Effects of Hyperosmotic Stress-induced Plk3 Activity on Cell Cycle Progression
It has also been shown that Plk3 acts downstream of both ATM and Chk2 in the DNA damage-dependent signaling pathway to regulate progression of the cell cycle. In the present study, the effect of Plk3 activity on mediating hyperosmotic stress-induced changes in cell cycle progression was analyzed by flow cytometry. In hyperosmotic stress-induced corneal epithelial cells, significantly accumulated population of the cells in the G2/M phase, and apoptotic cells were detected following a 12 h time course (Fig. 5, A and B). In contrast, knock-down of Plk3 mRNA effectively blocked hyperosmotic stress-induced changes in the cell cycle distribution (Fig. 5C). Further study was conducted in mouse embryonic fibroblasts (MEFs) obtained from primary cultures of wild type and Plk3−/− knock-out mice. Consistent with HCE cells, hyperosmotic stress-induced γH2AX activity in Plk3−/− MEFs was significantly suppressed compared with wild type MEFs (Fig. 5D). Cell cycle analysis of wild type MEFs demonstrated that hyperosmotic stress induced significant increases in late G2/M phase populations in apoptotic cells. However, effects of hyperosmotic stress on the changes of the G2/M phase and apoptosis were significantly suppressed in Plk3 knock-out Plk3−/− MEFs (Fig. 5, E and F). The results of hyperosmotic stress-induced cell cycle alterations in Plk3 mRNA knock-down HCE cells and Plk3 knock-out Plk3−/− MEFs were consistent, suggesting that the role of Plk3 in interacting with γH2AX is associated with DNA replication in response to hyperosmotic stress-induced double-strand breaks in S- and G2-phase cells.
FIGURE 5.
Effect of altered Plk3 activity on cell cycle distribution in hyperosmotic stress-induced HCE cells and MEFs. A, effect of hyperosmotic stimulation on cell cycle progression in HCE cells. B, time course of hyperosmotic stress-induced alteration in cell cycle progression in HCE cells. C, effects of knocking down Plk3 mRNA and/or H2AX mRNA on alterations of cell cycle progress in hyperosmotic stress-induced HCE cells. D, effect of hyperosmotic stress on γH2AX levels in MEFs obtained from wild type (Plk3wt) and Plk3−/− knock-out mice. E, effect of hyperosmotic stimulation on cell cycle progression in Plk3wt and Plk3−/− MEFs. F, comparisons of cell cycle distributions between wild type and Plk3−/− knock-out MEFs in the absence or presence of hyperosmotic stimulation. Asterisk symbols represent a significant difference between hyperosmotic stress-induced control cells and Plk3 and/or H2AX activities suppressed cells (p < 0.05, n = 3).
DISCUSSION
The process of corneal epithelial wound healing can be delayed by various environmental conditions including hyperosmotic stimulation that can result in compromising physiological function of the cornea. It has been shown that cellular responses to hyperosmotic stress include activation of MAP kinase cascades including JNK and p38, and Plk3 signaling pathways to further downstream activation of transcription factors, such as c-Jun and ATF-2 (5, 13, 25, 44, 45). In the present study, we found that extracellular hyperosmotic stresses activated γH2AX phosphorylation as a result of the stress-induced DNA DSBs in corneal epithelial cells, which is consistent with previous reports that hyperosmotic stress can result in DNA damage responses in other types of mammalian cells (34, 46, 47). Interestingly, DNA DSB-induced γH2AX phosphorylation is mediated by Plk3-induced cascade in addition to both ATM and Chk2 in DNA damage-dependent signaling pathways. The effect of hyperosmotic stress on activation of Plk3 subsequently resulting in γH2AX phosphorylation is consistent with previous findings indicating that Plk3 is an important mediator of the cellular response to genotoxic stress in ATM and Chk2 signaling pathways. Furthermore, activity of DNA damage-activated ATM/ATR was inhibited by application of caffeine, an inhibitor of ATM/ATR (25, 48, 49). Inhibition of ATM/ATR by caffeine resulted in suppression of Plk3 activity and H2AX phosphorylation. In addition, inhibition of hyperosmotic stress-induced p38 activation by the SB inhibitor had no effects on H2AX phosphorylation and Plk3 activity (Fig. 4). These results provide further evidence that Plk3 is involved at least partially in the ATM/ATR signaling pathway. The effect of hyperosmotic stress-activated Plk3 on H2AX was also examined by following a time course to show activation processes and the peak time of the Plk3 effect on γH2AX phosphorylation. It has been shown that the ATM-catalyzed phosphorylation site of H2AX is at serine 139 (Ser-139), termed γH2AX. The functional role of γH2AX is known to the response of DNA DSBs, and involves the control of cell cycle progression (50). The effect of Plk3 kinase on phosphorylation of H2AX at Ser-139 is supported by crystallographic structure of the nucleosome indicating that residue serine 139 in H2AX is easily accessible to kinases (51). In the present study, effects of hyperosmotic stress- and UV irradiation-induced Plk3 kinase activation on H2AX phosphorylation were observed at the Ser-139 of H2AX using an antibody specifically against Ser-139 in phosphorylated γH2AX. Further verification was done by demonstrating that the phosphorylation of H2AX (becoming γH2AX) by hyperosmotic stress-induced active Plk3 can be abolished by introducing a mutation to the fusion protein of H2AXS139A substituting Ser-139 with an Ala (alanine) residue (Fig. 2). Thus, we demonstrate for the first time that hyperosmotic stress-activated Plk3 is a functional player in an important event upstream in the DNA DSB-induced pathway to interact with H2AX, resulting in γH2AX activation.
Functional characterization of hyperosmotic stress-induced γH2AX phosphorylation through activation of Plk3 in the signal pathway was performed by determination of the interaction of Plk3 and γH2AX at protein levels using immunocoprecipitation and colocalization of Plk3 and γH2AX in stress-induced corneal epithelial cells. Results of immunocoprecipitation experiments show that antibodies against each of the Plk3 and γH2AX bi-directionally pulled down both Plk3 and γH2AX proteins, respectively. In addition, both Plk3 and γH2AX proteins can be colocalized in the nuclei of hyperosmotic stress-induced human corneal epithelial cells using immunostaining and fluorescent microscopy (Fig. 3). To further characterize the hyperosmotic stress-induced γH2AX phosphorylation through activation of Plk3 pathway, Plk3 activities were altered by either transfection of cDNA encoding a kinase-domain silencing Plk3 mutant (Plk3K52R) or knock-down of Plk3 mRNA in corneal epithelial cells, and by comparing γH2AX activities in corneal epithelia of wild type and Plk3−/− knock-out mice. The results of transfection experiments showed that both introduction of Plk3K52R and siRNA specific to Plk3 into corneal epithelial cells markedly suppressed hyperosmotic stress-induced γH2AX phosphorylation. There were clearly weakened γH2AX in the epithelial layer of the Plk3−/− mouse cornea when compared with the wild type mouse cornea (Fig. 4). These results provide further evidence that hyperosmotic stress-induced γH2AX phosphorylation indeed, at least in part, are mediated by the Plk3 pathway.
It has been shown that increased γH2AX is an important indicator for radiation stress-induced DNA DSBs in the cell cycle, especially critical in late S/G2 phase. Our studies were conducted to investigate the effect of hyperosmotic stress-induced Plk3 activity on γH2AX and cell cycle progression in human corneal epithelial cells and MEFs. We found that cell distribution in the cell cycle is very different in control human corneal epithelial and wild type MEFs in G1 and S phases, which can be explained by different growth patterns of those cells under culture conditions. However, hyperosmotic stimulation resulted in significant alteration of the cell population in G1 and S phases, and increased cell population in G2/M phase and apoptotic stage in both cell types. Knocking down mRNAs of Plk3, H2AX, and both Plk3 plus H2AX significantly suppressed the effects of hyperosmotic stimulation on cell cycle redistribution. The most interesting observation is that knock-down and knock-out of Plk3 in human corneal epithelial cells and MEFs presented a consistent effect of protection on cell apoptosis in response to hyperosmotic stimulation (Fig. 5). In addition, hyperosmotic stress-induced alterations of cell cycle distributions in G1, G2/M, and S phases were minimized by suppression of Plk3 and H2AX activities. These results provide important evidence suggesting that regulation of γH2AX by Plk3 in hyperosmotic stress-induced human corneal epithelial cells plays significant and functional roles in cell fate.
This study was supported, in whole or in part, by National Institutes of Health Grants EY 022364 (to L. L.) and CA074229 (to W. D.).
- Plk
- polo-like kinase
- ROS
- reactive oxygen species
- MMS
- methylmethane sulfonate
- ATM
- mutated in ataxia telangiectasia
- ATR
- ATM-related
- DSB
- double strand break
- SCGE
- single cell gel electrophoresis assay
- HCE
- human corneal epithelial
- MEF
- mouse embryonic fibroblast.
REFERENCES
- 1. Foulks G. N. (2007) The correlation between the tear film lipid layer and dry eye disease. Surv. Ophthalmol. 52, 369–374 [DOI] [PubMed] [Google Scholar]
- 2. Wu S. G., Jeng F. R., Wei S. Y., Su C. Z., Chung T. C., Chang W. J., Chang H. W. (1998) Red blood cell osmotic fragility in chronically hemodialyzed patients. Nephron. 78, 28–32 [DOI] [PubMed] [Google Scholar]
- 3. Mitono H., Endoh H., Okazaki K., Ichinose T., Masuki S., Takamata A., Nose H. (2005) Acute hypoosmolality attenuates the suppression of cutaneous vasodilation with increased exercise intensity. J. Appl. Physiol. 99, 902–908 [DOI] [PubMed] [Google Scholar]
- 4. Ito T., Itoh T., Hayano T., Yamauchi K., Takamata A. (2005) Plasma hyperosmolality augments peripheral vascular response to baroreceptor unloading during heat stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R432–R440 [DOI] [PubMed] [Google Scholar]
- 5. Burg M. B., Ferraris J. D., Dmitrieva N. I. (2007) Cellular response to hyperosmotic stresses. Physiol. Rev. 87, 1441–1474 [DOI] [PubMed] [Google Scholar]
- 6. Lunn J. A., Jacamo R., Rozengurt E. (2007) Preferential phosphorylation of focal adhesion kinase tyrosine 861 is critical for mediating an anti-apoptotic response to hyperosmotic stress. J. Biol. Chem. 282, 10370–10379 [DOI] [PubMed] [Google Scholar]
- 7. Lang F., Busch G. L., Ritter M., Völkl H., Waldegger S., Gulbins E., Häussinger D. (1998) Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 78, 247–306 [DOI] [PubMed] [Google Scholar]
- 8. Yu S. P., Choi D. W. (2000) Ions, cell volume, and apoptosis. Proc. Natl. Acad. Sci. U.S.A. 97, 9360–9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deutsch C., Slater L., Goldstein P. (1982) Volume regulation of human peripheral blood lymphocytes and stimulated proliferation of volume-adapted cells. Biochim. Biophys. Acta 721, 262–267 [DOI] [PubMed] [Google Scholar]
- 10. Westfall P. J., Patterson J. C., Chen R. E., Thorner J. (2008) Stress resistance and signal fidelity independent of nuclear MAPK function. Proc. Natl. Acad. Sci. U.S.A. 105, 12212–12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sheikh-Hamad D., Gustin M. C. (2004) MAP kinases and the adaptive response to hypertonicity: functional preservation from yeast to mammals. Am. J. Physiol. Renal Physiol. 287, F1102–F1110 [DOI] [PubMed] [Google Scholar]
- 12. Lu L., Reinach P. S., Kao W. W. (2001) Corneal epithelial wound healing. Exp. Biol. Med. 226, 653–664 [DOI] [PubMed] [Google Scholar]
- 13. Corrales R. M., Luo L., Chang E. Y., Pflugfelder S. C. (2008) Effects of osmoprotectants on hyperosmolar stress in cultured human corneal epithelial cells. Cornea 27, 574–579 [DOI] [PubMed] [Google Scholar]
- 14. Galcheva-Gargova Z., Dérijard B., Wu I. H., Davis R. J. (1994) An osmosensing signal transduction pathway in mammalian cells. Science 265, 806–808 [DOI] [PubMed] [Google Scholar]
- 15. Kyriakis J. M., Banerjee P., Nikolakaki E., Dai T., Rubie E. A., Ahmad M. F., Avruch J., Woodgett J. R. (1994) The stress-activated protein kinase subfamily of c-Jun kinases. Nature 369, 156–160 [DOI] [PubMed] [Google Scholar]
- 16. Rosette C., Karin M. (1996) Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science 274, 1194–1197 [DOI] [PubMed] [Google Scholar]
- 17. Wang L., Dai W., Lu L. (2007) Stress-induced c-Jun activation mediated by Polo-like kinase 3 in corneal epithelial cells. J. Biol. Chem. 282, 32121–32127 [DOI] [PubMed] [Google Scholar]
- 18. Wang L., Gao J., Dai W., Lu L. (2008) Activation of Polo-like kinase 3 by hypoxic stresses. J. Biol. Chem. 283, 25928–25935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang N., Wang X., Jhanwar-Uniyal M., Darzynkiewicz Z., Dai W. (2006) Polo box domain of Plk3 functions as a centrosome localization signal, overexpression of which causes mitotic arrest, cytokinesis defects, and apoptosis. J. Biol. Chem. 281, 10577–10582 [DOI] [PubMed] [Google Scholar]
- 20. Xie S., Xie B., Lee M. Y., Dai W. (2005) Regulation of cell cycle checkpoints by polo-like kinases. Oncogene 24, 277–286 [DOI] [PubMed] [Google Scholar]
- 21. Dai W. (2005) Polo-like kinases, an introduction. Oncogene 24, 214–216 [DOI] [PubMed] [Google Scholar]
- 22. Donohue P. J., Alberts G. F., Guo Y., Winkles J. A. (1995) Identification by targeted differential display of an immediate early gene encoding a putative serine/threonine kinase. J. Biol. Chem. 270, 10351–10357 [DOI] [PubMed] [Google Scholar]
- 23. Hamanaka R., Smith M. R., O'Connor P. M., Maloid S., Mihalic K., Spivak J. L., Longo D. L., Ferris D. K. (1995) Polo-like kinase is a cell cycle-regulated kinase activated during mitosis. J. Biol. Chem. 270, 21086–21091 [DOI] [PubMed] [Google Scholar]
- 24. Li B., Ouyang B., Pan H., Reissmann P. T., Slamon D. J., Arceci R., Lu L., Dai W. (1996) Prk, a cytokine-inducible human protein serine/threonine kinase whose expression appears to be down-regulated in lung carcinomas. J. Biol. Chem. 271, 19402–19408 [DOI] [PubMed] [Google Scholar]
- 25. Wang L., Payton R., Dai W., Lu L. (2011) Hyperosmotic stress-induced ATF-2 activation through Polo-like kinase 3 in human corneal epithelial cells. J. Biol. Chem. 286, 1951–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xie S., Wang Q., Wu H., Cogswell J., Lu L., Jhanwar-Uniyal M., Dai W. (2001) Reactive oxygen species-induced phosphorylation of p53 on serine 20 is mediated in part by polo-like kinase-3. J. Biol. Chem. 276, 36194–36199 [DOI] [PubMed] [Google Scholar]
- 27. Xie S., Wu H., Wang Q., Cogswell J. P., Husain I., Conn C., Stambrook P., Jhanwar-Uniyal M., Dai W. (2001) Plk3 functionally links DNA damage to cell cycle arrest and apoptosis at least in part via the p53 pathway. J. Biol. Chem. 276, 43305–43312 [DOI] [PubMed] [Google Scholar]
- 28. Ouyang B., Pan H., Lu L., Li J., Stambrook P., Li B., Dai W. (1997) Human Prk is a conserved protein serine/threonine kinase involved in regulating M phase functions. J. Biol. Chem. 272, 28646–28651 [DOI] [PubMed] [Google Scholar]
- 29. Westphal C. H. (1997) Cell-cycle signaling: Atm displays its many talents. Curr. Biol. 7, R789–R792 [DOI] [PubMed] [Google Scholar]
- 30. Nakamura Y. (1998) ATM: the p53 booster. Nat. Med. 4, 1231–1232 [DOI] [PubMed] [Google Scholar]
- 31. de Klein A., Muijtjens M., van Os R., Verhoeven Y., Smit B., Carr A. M., Lehmann A. R., Hoeijmakers J. H. (2000) Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr. Biol. 10, 479–482 [DOI] [PubMed] [Google Scholar]
- 32. Bahassi el M., Conn C. W., Myer D. L., Hennigan R. F., McGowan C. H., Sanchez Y., Stambrook P. J. (2002) Mammalian Polo-like kinase 3 (Plk3) is a multifunctional protein involved in stress response pathways. Oncogene 21, 6633–6640 [DOI] [PubMed] [Google Scholar]
- 33. Bahassi el M., Myer D. L., McKenney R. J., Hennigan R. F., Stambrook P. J. (2006) Priming phosphorylation of Chk2 by polo-like kinase 3 (Plk3) mediates its full activation by ATM and a downstream checkpoint in response to DNA damage. Mutat. Res. 596, 166–176 [DOI] [PubMed] [Google Scholar]
- 34. Thatcher T. H., Gorovsky M. A. (1994) Phylogenetic analysis of the core histones H2A, H2B, H3, and H4. Nucleic Acids Res. 22, 174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pilch D. R., Sedelnikova O. A., Redon C., Celeste A., Nussenzweig A., Bonner W. M. (2003) Characteristics of gamma-H2AX foci at DNA double-strand breaks sites. Biochem. Cell Biol. 81, 123–129 [DOI] [PubMed] [Google Scholar]
- 36. Rogakou E. P., Pilch D. R., Orr A. H., Ivanova V. S., Bonner W. M. (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868 [DOI] [PubMed] [Google Scholar]
- 37. Kurose A., Tanaka T., Huang X., Halicka H. D., Traganos F., Dai W., Darzynkiewicz Z. (2005) Assessment of ATM phosphorylation on Ser-1981 induced by DNA topoisomerase I and II inhibitors in relation to Ser-139-histone H2AX phosphorylation, cell cycle phase, and apoptosis. Cytometry A 68, 1–9 [DOI] [PubMed] [Google Scholar]
- 38. Furuta T., Takemura H., Liao Z. Y., Aune G. J., Redon C., Sedelnikova O. A., Pilch D. R., Rogakou E. P., Celeste A., Chen H. T., Nussenzweig A., Aladjem M. I., Bonner W. M., Pommier Y. (2003) Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J. Biol. Chem. 278, 20303–20312 [DOI] [PubMed] [Google Scholar]
- 39. Huyen Y., Zgheib O., Ditullio R. A., Jr., Gorgoulis V. G., Zacharatos P., Petty T. J., Sheston E. A., Mellert H. S., Stavridi E. S., Halazonetis T. D. (2004) Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature 432, 406–411 [DOI] [PubMed] [Google Scholar]
- 40. Falck J., Mailand N., Syljuåsen R. G., Bartek J., Lukas J. (2001) The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 410, 842–847 [DOI] [PubMed] [Google Scholar]
- 41. Abraham R. T. (2004) PI 3-kinase related kinases: 'big' players in stress-induced signaling pathways. DNA Repair 3, 883–887 [DOI] [PubMed] [Google Scholar]
- 42. Xu D., Yao Y., Lu L., Costa M., Dai W. (2010) Plk3 functions as an essential component of the hypoxia regulatory pathway by direct phosphorylation of HIF-1α. J. Biol. Chem. 285, 38944–38950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pommier Y., Leo E., Zhang H., Marchand C. (2010) DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 17, 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang Z., Tunnacliffe A. (2004) Response of human cells to desiccation: comparison with hyperosmotic stress response. J. Physiol. 558, 181–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Uhlik M. T., Abell A. N., Johnson N. L., Sun W., Cuevas B. D., Lobel-Rice K. E., Horne E. A., Dell'Acqua M. L., Johnson G. L. (2003) Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat. Cell Biol. 5, 1104–1110 [DOI] [PubMed] [Google Scholar]
- 46. West M. H., Bonner W. M. (1980) Histone 2A, a heteromorphous family of eight protein species. Biochemistry 19, 3238–3245 [DOI] [PubMed] [Google Scholar]
- 47. Pehrson J. R., Fuji R. N. (1998) Evolutionary conservation of histone macroH2A subtypes and domains. Nucleic Acids Res. 26, 2837–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang L., Dai W., Lu L. (2011) Hyperosmotic Stress-Induced Corneal Epithelial Cell Death through Activation of Polo-like Kinase 3 and c-Jun. Invest. Ophthalmol. Vis. Sci. 52, 3200–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang L., Lu L. (2007) Pathway-specific effect of caffeine on protection against UV irradiation-induced apoptosis in corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 48, 652–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fernandez-Capetillo O., Lee A., Nussenzweig M., Nussenzweig A. (2004) H2AX: the histone guardian of the genome. DNA Repair 3, 959–967 [DOI] [PubMed] [Google Scholar]
- 51. Luger K., Mäder A. W., Richmond R. K., Sargent D. F., Richmond T. J. (1997) Crystal structure of the nucleosome core particle at 2.8 Ä resolution. Nature 389, 251–260 [DOI] [PubMed] [Google Scholar]