Background: HIV-1 gp41 MPER is a target for inducing broadly neutralizing antibodies.
Results: Gp41int folds into a compact elongated structure that induces neutralizing antibodies upon immunization.
Conclusion: Presentation of gp41int in a lipid environment is beneficial to induce neutralizing antibodies.
Significance: Membrane-anchored gp41int is a promising antigen to improve breadth and potency of anti-gp41 antibody responses.
Keywords: AIDS, Membrane Fusion, Protein Conformation, Vaccine Development, X-ray Scattering, HIV-1, HIV-1 gp41
Abstract
The membrane-proximal external region (MPER) of the human immunodeficiency virus, type 1 (HIV-1) envelope glycoprotein subunit gp41 is targeted by potent broadly neutralizing antibodies 2F5, 4E10, and 10E8. These antibodies recognize linear epitopes and have been suggested to target the fusion intermediate conformation of gp41 that bridges viral and cellular membranes. Anti-MPER antibodies exert different degrees of membrane interaction, which is considered to be the limiting factor for the generation of such antibodies by immunization. Here we characterize a fusion intermediate conformation of gp41 (gp41int-Cys) and show that it folds into an elongated ∼12-nm-long extended structure based on small angle x-ray scattering data. Gp41int-Cys was covalently linked to liposomes via its C-terminal cysteine and used as immunogen. The gp41int-Cys proteoliposomes were administered alone or in prime-boost regimen with trimeric envelope gp140CA018 in guinea pigs and elicited high anti-gp41 IgG titers. The sera interacted with a peptide spanning the MPER region, demonstrated competition with broadly neutralizing antibodies 2F5 and 4E10, and exerted modest lipid binding, indicating the presence of MPER-specific antibodies. Although the neutralization potency generated solely by gp140CA018 was higher than that induced by gp41int-Cys, the majority of animals immunized with gp41int-Cys proteoliposomes induced modest breadth and potency in neutralizing tier 1 pseudoviruses and replication-competent simian/human immunodeficiency viruses in the TZM-bl assay as well as responses against tier 2 HIV-1 in the A3R5 neutralization assay. Our data thus demonstrate that liposomal gp41 MPER formulation can induce neutralization activity, and the strategy serves to improve breadth and potency of such antibodies by improved vaccination protocols.
Introduction
The initial antibody response following acute HIV-110 infection arises ∼13 days following the onset of viremia and is targeted to the gp41 region of the envelope glycoprotein (Env) (1). Although these antibodies are highly mutated and polyreactive (2), they are non-neutralizing and do not select for escape variants (3). Neutralizing antibodies (nAbs) appear only months after infection when the antigen-specific B cells have undergone multiple rounds of somatic hypermutation. The quest for new broadly neutralizing antibodies (bnAbs) over the last few years revealed that a substantial percentage of HIV-1-infected individuals eventually develop broad and potent neutralizing antibodies that act against a large variety of strains. These bnAbs map to four major antigenic sites on Env, including the CD4 binding site (4–9), the V1-V2 loop-specific antibodies that recognize glycopeptides (10–13), glycan V3-directed antibodies (11), and the membrane-proximal external region (MPER) of gp41 (14). A common feature of these bnAbs is their high extent of somatic mutations, a prerequisite to achieve broad neutralization and high potency (15).
The MPER is an attractive target because it is a highly conserved, tryptophan-rich region that plays a critical role in membrane fusion and virus infectivity (16–18). Anti-MPER antibodies 2F5, Z13e1, 4E10, and 10E8 target overlapping linear epitopes within MPER (14, 17, 19–24). The nAbs 2F5 and 4E10 contain hydrophobic heavy chain CDR3 residues that mediate lipid binding (25, 26) and are required for neutralization (27–30). This potential polyreactivity, which may be distinct from pathogenic autoantibodies (31), was suggested to be a major hindrance to induce such antibodies because of their natural elimination through B cell tolerance mechanisms (32–34). The most potent anti-MPER antibody, 10E8, was initially reported to lack autoreactivity (14), but a recent study challenges this and demonstrates modest 10E8 membrane binding activity (35). However, 10E8-like activity is found in a large number of patient sera with broad neutralizing activity, demonstrating that not all anti-MPER antibodies undergo clonal deletion (14), which is in contrast to other studies suggesting that bnAbs to the MPER are rare in infected patients or vaccinated naïve individuals (36–39). Further underlining the importance of anti-MPER antibodies, passive immunization strategies containing nAbs 2F5 and 4E10 protected animals from SHIV infection (40).
A main target of anti-MPER antibodies is a fusion intermediate conformation of gp41 (35, 41, 42) that forms during the receptor binding-induced transitions from native Env (43–46) to the postfusion conformation (47–50). The interaction with a transitional state of Env is further supported by the interaction of nAb Z13e1 with the open CD4-induced Env conformation (51). MPER may become membrane-embedded during entry, and it has been suggested that anti-MPER antibodies extract the epitopes from the bilayer (52, 53).
A number of vaccine approaches targeting the gp41 MPER have been attempted to elicit bnAbs (for a review, see Ref. 54). These include the generation of structurally stabilized peptides, scaffolds, or chimeras (55–64); chimeric virus or virus-like particles by engrafting the MPER antibody epitopes onto other viruses (65–78); gp41 fusion proteins (79–84); and liposomes containing MPER peptides (85). Different prime-boost strategies with peptides, DNA, and whole Env protein have also been explored in these studies. Although the described immunogens induced antibody titers to gp41, only a few studies demonstrated modest neutralization activity depending on the neutralization assay used. Notably, guinea pigs immunized with virus-like particles carrying chimeras of gp41 and hemagglutinin produced sera with modest neutralization of selected tier 1 and tier 2 pseudoviruses using the stringent TZM-bl assay (65). Furthermore, immunization of llamas with gp41 proteoliposomes led to the isolation of an anti-MPER nanobody that neutralized a small number of tier 1 and tier 2 viruses as a bihead (86). Other studies indicate that the postfusion conformation presenting MPER is capable of inducing neutralizing antibodies (87, 88), consistent with the finding that mAb 10E8 interacts with this conformation in vitro (35).
Here we structurally characterized the intermediate conformation of gp41 (gp41int) and coupled it covalently to liposomes, which were then administered alone or in combination with soluble gp140 in guinea pigs. Immunization was performed with a mixture of two adjuvants, Carbopol-971P and MF59, that preserved the liposomal structure prior to immunization. Analyses of the postimmune sera demonstrated strong gp41-specific IgG responses, the presence of antibodies targeting MPER, and neutralizing activity against a panel of tier 1 and tier 2 HIV-1 viruses. Our study thus indicates the benefit of a membrane environment in the induction of neutralizing antibodies by gp41int.
EXPERIMENTAL PROCEDURES
Ethics Statement
The animal study was carried out in strict accordance with the United Kingdom Animals (Scientific Procedure) Act 1986, and the protocol was approved by the local Ethical and Welfare Committee of the University of Cambridge and the United Kingdom Home Office (Project license number 80/2238).
Recombinant Gp140 Purification
HIV-1 gp140CA018 is an A/G recombinant subtype Env derived from a Cameroon patient. The gp140 glycoprotein was purified using a published protocol with Galanthus nivalis agarose affinity column followed by diethylaminoethyl-Sepharose and ceramic hydroxyapatite columns to remove all contaminants (89). The purified glycoprotein was concentrated using an Amicon YM-30 (30-kDa-cutoff) ultrafiltration disc (Millipore) and stored at −80 °C until use.
Gp41 Protein Expression and Purification
The gp41 constructs are based on the HXB2 group M subtype B sequence. Gp41int-Cys and gp41int-fd contain gp41 residues 584–684. Part of gp41 heptad repeat region 1 (HR1) is N-terminally fused in-frame with the GCN4 trimerization domain (90). Gp41int-fd contains a C-terminal fold-on trimerization domain rendering gp41int-fd similar to the reported GCN-gp41-inter construct (91). Both cysteines at positions 598 and 604 have been mutated to serine. Gp41int-fd has the following sequence: MAQIEDKIEEILSKIYHIENEIARIKKLIGEAERYLKDQQLLGIWGSSGKLISTTAVPWNASWSNKSLEQIWNNMTWMEWDREINNYTSLIHSLIEESQNQQEKNEQELLELDKWASLWNWFNITNWLWYIKGYIPEAPRDGQAYVRKDGEWVLLSTFL (underlined amino acids correspond to pIIGCN4, italicized amino acids correspond to gp41 HR1, and underlined S corresponds to cystein residues changed to serine). The cDNA was cloned into the expression vector pETM-13.
Gp41int-Cys is similar to gp41int-fd but has the C-terminal fold-on domain replaced by a single cysteine. The sequence of the construct is as follows: MAQIEDKIEEILSKIYHIENEIARIKKLIGEAERYLKDQQLLGIWGSSGKLISTTAVPWNASWSNKSLEQIWNNMTWMEWDREINNYTSLIHSLIEESQNQQEKNEQELLELDKWASLWNWFNITNWLWYIKSC. The cDNA encoding the fusion protein was cloned into the expression vector pETM-13 (European Molecular Biology Laboratory Heidelberg). For small angle x-ray scattering (SAXS) analysis gp41int-Cys contained an extra tobacco etch virus protease site and a FLAG tag at the C terminus. This construct shows a slightly higher solubility.
Gp41int-fd was expressed in Escherichia coli strain Rosetta 2 (DE3) (Novagen). Cells were grown to an A600 nm of 0.6 and induced with 1 mm isopropyl 1-thio-β-d-galactopyranoside at 37 °C. After 4 h, cells were harvested by centrifugation and lysed in Tris buffer (20 mm Tris, pH 8.5, 0.1 m NaCl). The soluble fraction was discarded, and the pellet was resuspended in Tris buffer supplemented with 0.1% Triton X-100 (Sigma) overnight at 4 °C. Solubilized gp41int-fd in Tris, 0.1% Triton X-100 buffer was bound to a Q-Sepharose column, and Triton X-100 was removed by excessive washing with Tris buffer. Gp41int-fd was eluted by applying a 0.05–0.5 m NaCl gradient in Tris buffer. A final purification step included size exclusion chromatography on a Superose 6 column in 20 mm Bicine, pH 9.3, 100 mm NaCl. Gp41int-Cys was expressed and purified by following the same protocol as for gp41int-fd with the addition of 10 mm DTT in all buffers. A final purification step included size exclusion chromatography on a Superose 6 column in 20 mm Tris, pH 8.5, 100 mm NaCl, 10 mm DTT. Final gp41int-Cys yields were 10 mg/liter of E. coli culture, indicating that production can be scaled up and is suitable for good manufacturing practice production for further immunization trials.
SAXS Analysis of Gp41int-Cys
X-ray scattering data were collected on beam line BM-29 (European Synchrotron Radiation Facility, Grenoble, France) at 20 °C, a wavelength of 0.9919 Å, and a sample-to-detector (PILATUS 1M, DECTRIS) distance of 2.849 m. The scattering intensities of the gp41int-Cys were measured at concentrations of 0.75 and 3 mg/ml in the gel filtration buffer. The data were normalized to the intensity of the incident beam, the scattering of the buffer was subtracted, and the resulting intensities were scaled for concentration. Data processing and analysis were performed using the ATSAS package (92), and molecular weights were estimated based on the method of Putnam et al. (93). The final merged scattering data were further evaluated using PRIMUS (94). The isotropic scattering intensity I(q) was transformed to the distance distribution function P(r) using the program GNOM, which was also used to calculate the particle maximum dimension Dmax (95). Dmax was considered to be optimal when the radius of gyration (Rg) obtained from the P(r) plot was equal to that obtained from the Guinier analysis. For ab initio modeling of the SAXS data, 10 sets of independent models were calculated using Dammin (96).
Circular Dichroism Spectroscopy
All spectra were recorded on a Jasco J-810 spectropolarimeter at 20 °C. Prior to analysis, proteins were dialyzed in 10 mm potassium phosphate, pH 8.0, and the secondary structure content was calculated with the Jasco Spectra Manager 2 software package.
Surface Plasmon Resonance
Surface plasmon resonance analysis was performed with a Biacore X3000 (GE Healthcare). As a flow buffer, 10 mm HEPES, 150 mm NaCl, pH 7.4 with 0.005% P-20 was used. Gp41int-Cys was immobilized to 1000 response units using 50 μg/ml protein in flow buffer on an activated CM-5 sensor chip (GE Healthcare, BR-1000-50) according to the manufacturer's instructions. Specific binding to the target protein was corrected for nonspecific binding to the deactivated control channel. The flow rate was 50 μl/min. Regeneration of the sensor chip was achieved with 100 mm glycine, pH 3.3, 20 mm NaOH for 60 s at 50 μl/min. mAb 2F5 was injected at concentrations of 50, 100, and 250 nm, and bnAb 10E8 was injected at concentrations of 1.3 and 6 μm. Data were analyzed with BIAevalution software version 4.1. The curves were fitted with separate association and dissociation to obtain reasonable χ2 scores and residuals.
Liposome Production and Characterization
Liposomes were prepared with an HIV-1 virus-like lipid composition (97). The following lipids, phosphatidylserine, phosphatidylcholine, phosphatidylethanolamine, sphingomyelin, 1,2-dipalmitoyl-sn-glycero-3-phosphothioethanol (Avanti Polar Lipids, Inc.), and cholesterol were dissolved in chloroform and mixed in a 1:1:1:2:0.5:4:5 ratio at a final concentration of 5 mg/ml. After solvent evaporation, the lipid film was dried under vacuum. Multilamellar vesicles were obtained by resuspending the lipid film in PBS buffer to a final lipid concentration of 5 mg/ml. The liposome suspension was extruded through a 100-nm membrane (Whatman). Gp41int-Cys was extensively dialyzed against Tris buffer without DTT and then mixed with the liposomes at a 2:1 ratio (w/w). Binding of gp41int-Cys to the liposomes was confirmed by sucrose gradient analysis (0–40% sucrose in PBS; 12 h at 40,000 rpm) and SDS-PAGE. Priming was performed with proteoliposomes present in the 10 and 20% sucrose fractions, and boosting was performed using the complete mixture of gp41int-Cys and liposomes with an estimated 30% of gp41int-Cys cross-linked to liposomes.
For immuno-electron microscopy (EM), gp41int-Cys proteoliposomes were incubated with bnAb 10E8, and binding was confirmed by sucrose gradient centrifugation. Fractions containing 10E8, gp41int-Cys, and liposomes were used for immunogold labeling using an adapted protocol.11 A thin film of carbon was floated in water, picked up with a bare copper grid, and allowed to dry. 4 μl of proteoliposome (gp41int-Cys and mAb 10E8) solution was adsorbed for 1 min on the carbon-coated grid. Excess liquid was blotted away, and the grid was floated on top of a 50-μl drop of 0.1% BSA in PBS for 1 min. After a mild blotting, the grid was floated on a 20-μl drop of protein A-gold diluted 1:10 or 1:20 in 0.1% BSA in PBS for 20 min and then washed in two successive 100-μl drops of water. The grid was stained for 20 s in a 20-μl drop of 2% uranyl acetate, blotted, and allow to dry before EM observation on a JEOL 1200EX. The experiments in Carbopol/MF59 were identical except that before staining with uranyl acetate the grid was floated on top of a 20-μl drop of Carbopol/MF59 for 5 min and then washed in five consecutive 100-μl drops of water. Images were acquired at a nominal magnification of 20,000. The grid squares were scanned for the presence of gold on the liposomes and in the background. It was found that only liposomes displayed more than three to four gold particles; concentration of gold particles in one place was only observed on proteoliposomes and never in the background. This observation was repeated on several EM grids for proteoliposomes in sucrose or after dialysis in PBS and for two different concentrations of protein A-gold. A control with liposomes only was performed, and it was found that they did not cluster the protein A-gold as did the gp41int-Cys proteoliposomes.
Immunization and Serum Collection
In the pilot study, two groups of four Hartley white female guinea pigs were intramuscularly immunized with gp41int-Cys (50 μg) with or without adjuvants at weeks 0 and 4. The adjuvant used was a combination of Carbopol-971P (Lubrizol) and MF59 (Novartis Vaccines and Diagnostics), formulated according to previous protocols (98). Carbopol-971P was first prepared as a 2% (w/v) suspension in PBS before mixing 1:1 (v/v) with gp140CA018 or gp41int-Cys. The mixture was then added 1:1 (v/v) with 50 μl of MF59 and incubated for another 10 min before injection. Serum samples were collected at weeks −2, 2, and 6, and terminal samples were collected at week 8.
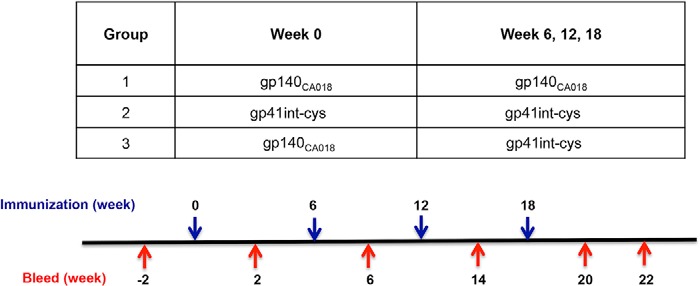
In the immunogenicity study, three groups of six Hartley white female guinea pigs each received four intramuscular immunizations at weeks 0, 6, 12, and 18 (see Table 1). Animals received gp140CA018 (50 μg) (group 1) and gp41int-Cys (50 μg) (group 2), respectively, for all four immunizations. Animals in group 3 were primed once at week 0 with gp140CA018 followed by three boosts with gp41int-Cys at weeks 6, 12, and 18. Serum samples were collected 2 weeks before and 2 weeks after each immunization at weeks −2, 2, 8, 14, and 20, and a terminal sample was collected at week 22. All antigens were formulated in Carbopol-971P and MF59 as described previously (98).
TABLE 1.
Guinea pig immunization regimen and schedule
Animals (six per group) were immunized with different prime-boost regimens as noted in the table. Each animal received four intramuscular injections of 50 μg of recombinant gp140CA018 and/or gp41int-Cys liposomes. Serum samples were taken 2 weeks prior to the immunization study and 2 weeks after each immunization. A terminal sample was collected 4 weeks after the final immunization.
End Point Titer and Avidity Index ELISA
The end point titers of Env-specific antibodies in sera were measured by standard ELISA. Briefly, Maxisorb 96-microwell plates (Nunc) were coated overnight with purified gp41int-fd protein (50 ng/well) diluted in 100 mm carbonate-bicarbonate coating buffer, pH 9.6 and blocked with 5% nonfat milk in PBS containing 1% Tween 20 (PBST). Serum samples were serially diluted 3-fold with sample buffer (2% BSA in PBST) and added to triplicate wells. A guinea pig-specific IgG conjugated to HRP (Jackson ImmunoResearch Laboratories) was used as secondary antibody (diluted 1:15,000 in sample buffer). Between each step, the plates were washed six times with wash buffer (PBST). The plate was developed with one-step 3,3,5,5-tetramethylbenzidine (Pierce Thermo Scientific) before being stopped with 2 m H2SO4. Absorbance values were detected at 450 nm using a Bio-Rad iMark microplate reader.
The avidity indices of vaccinated sera were measured by their resistance to 8 m urea in binding to gp41int-fd antigen. Briefly, microwell plates were prepared and blocked as described above. Serum samples were diluted to give an A450 nm readout between 1.0 and 1.5 in end point ELISA and were added to two sets of triplicate wells. The wells were then washed three times with either PBS-Tween 20 or 8 m urea in PBS-Tween 20 before incubation with an anti-guinea pig secondary antibody. The plates were washed and developed with 3,3,5,5-tetramethylbenzidine as described above. The avidity index was calculated as the percentage of average urea-treated A450 nm/average PBS-Tween 20 A450 nm. Antisera with index values >50% were designated high avidity, those with index values of 30–50% were designated intermediate avidity, and those with index values <30% were designated low avidity.
Anti-lipid ELISA
Antibody response to lipids was measured by ELISA using a protocol described previously (99). Briefly, cholesterol, cardiolipins, phosphatidylcholine, and phosphatidylserine (Sigma-Aldrich) were prepared in a 1:10 (v/v) solution of chloroform and ethanol and coated onto microwell plates (200 ng/well) overnight. The ELISA was then carried out as described above, and the absorbance values were measured at 450 nm using a Bio-Rad iMark microplate reader.
Anti-MPER Peptide ELISA
Anti-MPER ELISAs were performed according to standard procedures. Briefly, MPER peptides having the sequence KKKNEQELLELDKWASLWNWFDITNWLWYIRKKK (italicized amino acids are non-MPER amino acids) were coated at 50 ng/well onto microtiter plates and then blocked with 2% BSA. Serial dilutions of serum were added in triplicate and incubated for 2 h at 16 °C. Detection of antibody binding was carried out as described above.
Real Time Neutralizing Epitope Competition Assay
The real time neutralizing epitope competition assay was performed as described previously (98). Briefly, gp41int-fd protein was coated at 100 ng/well onto microwell plates and then blocked with 2% BSA. The gp41int-fd protein (100 ng/well) was added in triplicate and incubated for 2 h at 16 °C. To determine the approximate epitopes to which the polyclonal sera were raised, antisera from vaccinated animals were diluted according to their end point titer to ∼1 × 104 IgG to compete with Eu3+-labeled monoclonal antibodies (mAbs) (100 ng/well) for binding to antigens. Enhancement solution (PerkinElmer Life Sciences) was added, and relative luciferase units at 620 nm were measured in an Envision luminometer (PerkinElmer Life Sciences). Avidity of the antisera to the epitopes was determined by reduction in the Eu3+ luminescence intensity of the specific mAb with which the serum was competing. The 2F5, 4E10, and 5F3 mAbs were purchased from Polymun (Vienna, Austria), and Z13e1 was kindly provided by D. R. Burton (Scripps Research Institute).
Neutralization Assay
The TZM-bl assay was carried out using a standard protocol with molecularly cloned pseudoviruses (100). Briefly, pseudovirus was incubated with serially diluted guinea pig antisera for 1 h at 37 °C before being placed into wells of 96-well plates seeded with TZM-bl cells. After a 48-h incubation at 37 °C, the cells were lysed, and luciferase signal in the lysate was developed with Britelite Plus substrate (1:1, v/v; PerkinElmer Life Sciences) and read in a luminometer. The A3R5 assay was done with replication-competent HIV-1 inserted with Renilla luciferase reporter gene (101). The inhibition was carried out in the same way described above before being exposed to A3R5 cells and incubated for 4–6 days at 37 °C. The luciferase signal was developed with VivaRen substrate (Promega) and detected with a luminometer.
Statistical Analysis
Comparison of binding, avidity, and neutralization data between groups was performed using a Mann-Whitney U test with GraphPad Prism 6.0 software.
RESULTS
Structural Characterization of Gp41int-Cys
Gp41int-Cys contains the trimeric leucine zipper (pIIGCN4) fused in-frame with part of HR1, which prevents refolding of HR2 onto HR1, leading to the six-helical bundle postfusion conformation. The pIIGCN4 chimera is followed by the Cys-loop region, HR2, MPER, and either the C-terminal trimerization fold-on domain (gp41int-fd) or a C-terminal cysteine (gp41int-Cys). Because cysteines 598 and 604 formed mixed disulfide bonds as observed previously (102), both residues were mutated to serine (Fig. 1A). Gp41int-fd and gp41int-Cys elute in single peaks at 15.9 and 16.4 ml from a Superose 6 size exclusion chromatography column (Fig. 1B). Chemical cross-linking demonstrates that they form trimers in solution. SDS-PAGE shows that the monomer band (migrating at ∼22 kDa) of gp41int-fd cross-links to a band migrating at ∼75 kDa representing the trimer. Likewise, gp41int-Cys cross-linking shifts the monomer band (∼15 kDa) to a new broad band migrating at ∼50 kDa, which corresponds to a trimeric form (Fig. 1B). A small amount of gp41int-fd and gp41int-Cys cross-link to higher molecular weight species under these conditions (Fig. 1B).
FIGURE 1.
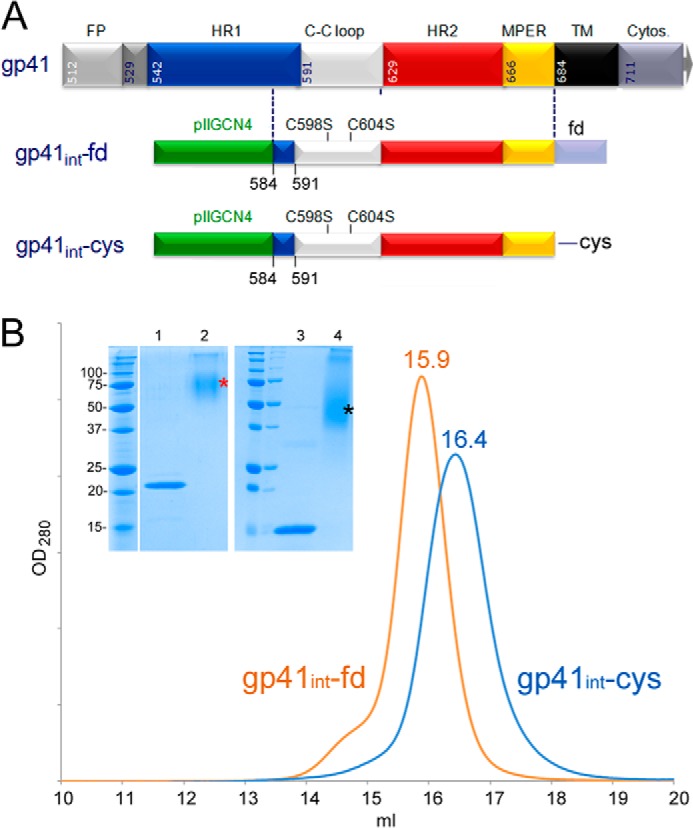
Expression constructs gp41int-fd and gp41int-Cys. A, schematic drawing of gp41. FP, fusion peptide; C-C loop, cysteine loop; TM, transmembrane region; Cytos., cytosolic domain; pIIGCN4, trimeric coiled coil derived from the GCN4 leucine zipper fused to HR1 (90); fd, fold-on domain sequence derived from bacteriophage T4 fibritin. The numbering represents that of full-length HXB2 Env. B, both gp41int-fd and gp41int-Cys were purified on a Superose 6 gel filtration column, and their respective profiles are shown. Notably, gp41int-fd is larger and elutes at 15.9 ml, whereas gp41int-Cys elutes at 16.4 ml. Protein from each peak fraction was cross-linked with 0.5 mm glutaraldehyde and separated by 15% SDS-PAGE. The left panel of the inset shows that gp41int-fd is cross-linked to a band (*) migrating at ∼75 kDa, indicating trimer formation (the monomer band migrates at ∼22 kDa). The right panel of the inset shows the cross-linking results on gp41int-Cys, which cross-links to a new band (*) migrating at ∼50 kDa, representing the trimer (the monomer band migrates at ∼15 kDa).
Gp41int-Cys was further analyzed by SAXS (Fig. 2A). Guinier evaluation resulted in an Rg of 3.33 nm and the maximal protein dimension (Dmax) of 117 Å was calculated by the distance distribution function P(r) (Fig. 2B). The shape of gp41int-Cys was determined ab initio, and the trimer model fits the experimental data with the discrepancy χ2 of 0.87 (Fig. 2A). Gp41int-Cys has an elongated ∼106-Å-long structure with a maximal diameter of 37 Å at the top end, a ∼60-Å diameter of the middle part, and a 25-Å diameter of the bottom end (Fig. 2C). We propose that the top end represents the HR1 coiled coil region, which was partly replaced by trimeric pIIGCN4. Docking the pIIGCN4-gp41 hybrid coiled coil into the SAXS envelope corroborates this position for the HR1 part (Fig. 2C). This is also in agreement with the molecular fit of the gp41 coordinates from the SOSIP.664 gp140 crystal structure (43), which fit into the top end and the middle part of the SAXS envelope (Fig. 2C). The SOSIP.664 gp140 crystal structure lacks the Cys-loop region, which could be accommodated by the middle region, and the bottom end would provide space for the missing MPER part (Fig. 2C). The relatively small 25-Å diameter of the bottom end indicates that all three chains are in close proximity prior to entering the membrane, consistent with a trimerization role for the transmembrane region. We conclude that this potential intermediate conformation of gp41 forms a compact and elongated trimer that will bridge the viral and cellular membrane with a distance of 110–120 Å depending on the missing 30 N-terminal amino acids (fusion peptide-proximal region and the fusion peptide).
FIGURE 2.
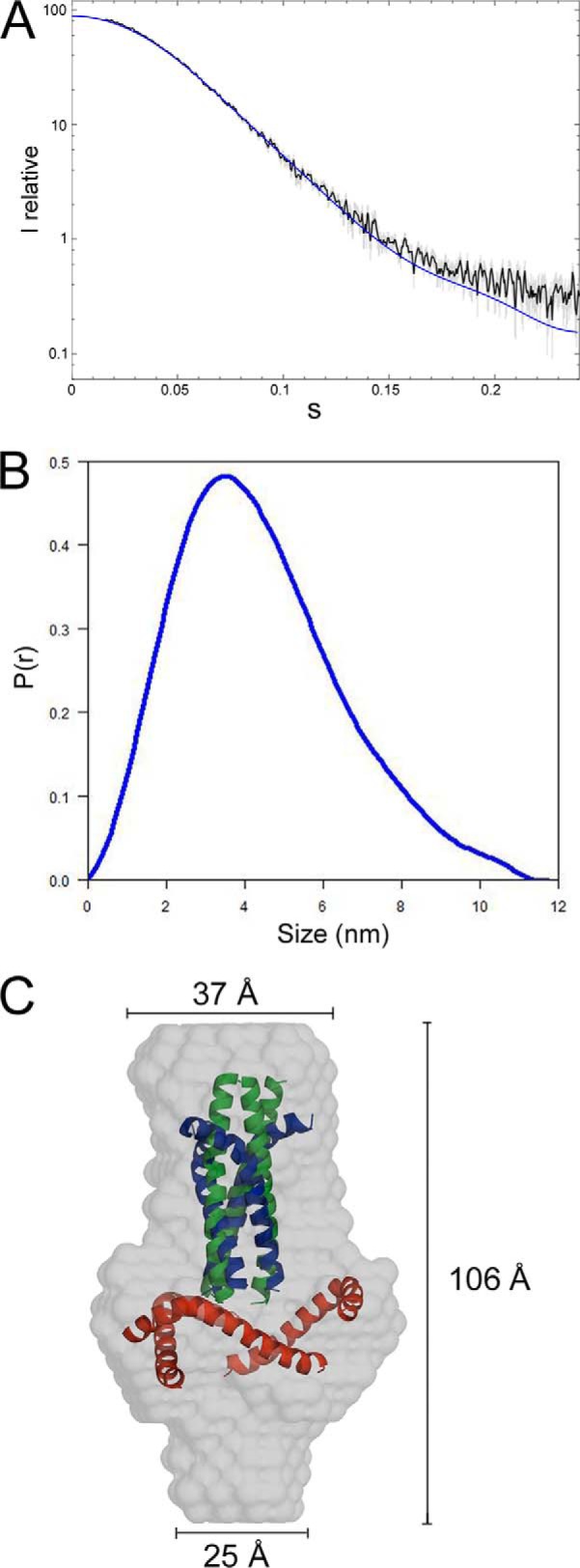
Structural characterization of gp41int-Cys by SAXS. A, experimental scattering intensities obtained for gp41int-Cys are shown as a function of resolution and after averaging and subtraction of solvent scattering. The scattering intensities calculated from the ab initio model (presented in C) with the lowest χ2 values are shown as a blue line. B, the P(r) function of the gp41int-Cys data indicates maximal dimensions (Dmax) of 117 Å. C, molecular envelope of the ab initio-calculated model of gp41int-Cys. The model corresponding to pIIGCN4 (green ribbon) and of the gp41 part derived from the SOSIP.664 gp140 trimer structure (Protein Data Bank code 4NC0; HR1, blue ribbon; HR2, red ribbon) have been docked into the SAXS envelope.
Secondary structure analysis by circular dichroism revealed ∼55% helices, 8% β-strands, 7% turns, and 30% random coil structures. This indicates a relatively low secondary structure content for the rest of gp41 (the Cys-loop region and HR2) because the coiled coil structures of pIIGCN4 and part of HR1 account for 29% helices.
Although the fusion intermediate construct gp41-inter, which is very similar to gp41int-Cys, was extensively tested for binding to neutralizing anti-MPER antibodies (35, 91), we tested gp41int-Cys for binding to bnAbs 10E8 and 2F5. A notable difference between gp41-inter and gp41int-Cys is that the latter lacks the C-terminal fold-on trimerization domain (Fig. 1A). Surface plasmon resonance measurements showed nanomolar affinities for both antibodies. MAb 2F5 interacts with a dissociation constant of 5.3 nm, and mAb 8E10 interacts with a dissociation constant of 57 nm. We conclude that gp41int-Cys has a mostly α-helical, elongated structure that interacts with bnAbs 2F5 and 10E8.
Gp41int-Cys Proteoliposomes
To present the potential fusion intermediate conformation of gp41int-Cys in a lipid environment, we covalently linked gp41int-Cys (Fig. 1) to liposomes containing the lipid composition of HIV-1 (97) and small amounts of 1,2-dipalmitoyl-sn-glycero-3-phosphothioethanol, which allowed disulfide linkage of the C-terminal cysteine and the thiol group of the lipid, thus placing the MPER region close to the membrane. Successful linkage was confirmed by sucrose gradient analysis, and gp41int-Cys is shown to float with the liposomes in the upper fractions of the gradient (Fig. 3A). Dynamic light scattering confirmed a uniform size distribution of liposomes with an average diameter of ∼100 nm. 50 μg per dose was used for each immunization. For immunogold labeling, sucrose gradient-purified gp41int-Cys proteoliposomes (Fig. 3A, 10 and 20% fractions) were incubated with bnAb 10E8 and again purified by sucrose gradient analysis (data not shown). The presence of gold particles at the edge of the gp41int-Cys/10E8 proteoliposomes confirmed that membrane-anchored gp41int-Cys reacted efficiently with bnAb 10E8 (Fig. 3C).
FIGURE 3.
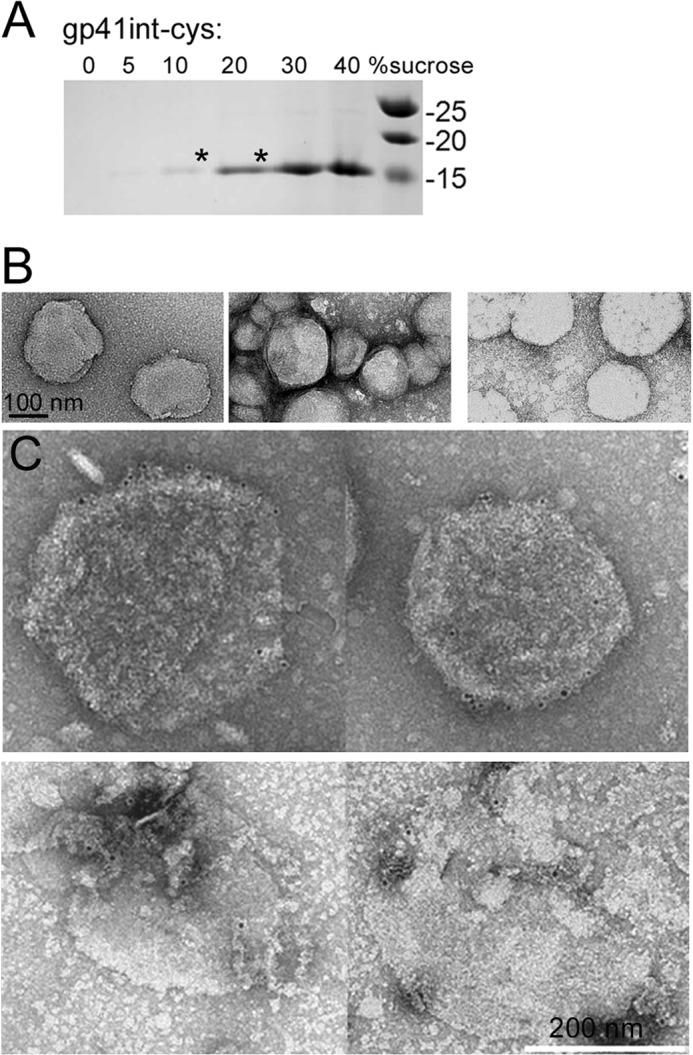
Characterization of gp41int-Cys proteoliposomes. A, gp41int-Cys was covalently linked to liposomes containing a thiol-reactive lipid (1,2-fipalmitoyl-sn-hlycero-3-phosphothioethanol), and proteoliposomes were purified on a sucrose density gradient from 40% sucrose in PBS to 0% sucrose in PBS. Liposome gp41int-Cys mixtures were placed in the bottom of the 40% sucrose fraction, and the gradient was spun at 40,000 rpm for 6 h. Gp41int-Cys associated with liposomes moved together with the liposomes into upper fractions of the gradient (mostly 10 and 20% sucrose; marked by an asterisk). B, liposomes were treated with the Carbopol-971P and MF59 mixture used for immunization and analyzed by negative staining electron microscopy. Left panel, control liposomes; middle panel, gp41int-Cys proteoliposomes; right panel, gp41int-Cys proteoliposomes treated with the Carbopol-971P and MF59 mixture. C, electron microscopy of negatively stained gp41int-Cys proteoliposomes labeled with bnAb 10E8 and protein A-gold (upper panel). Lower panel, same as upper panel except that the gold-labeled gp41int-Cys proteoliposomes were incubated in Carbopol-971P/MF59 before being negatively stained. Scale bar, 200 nm for all panels.
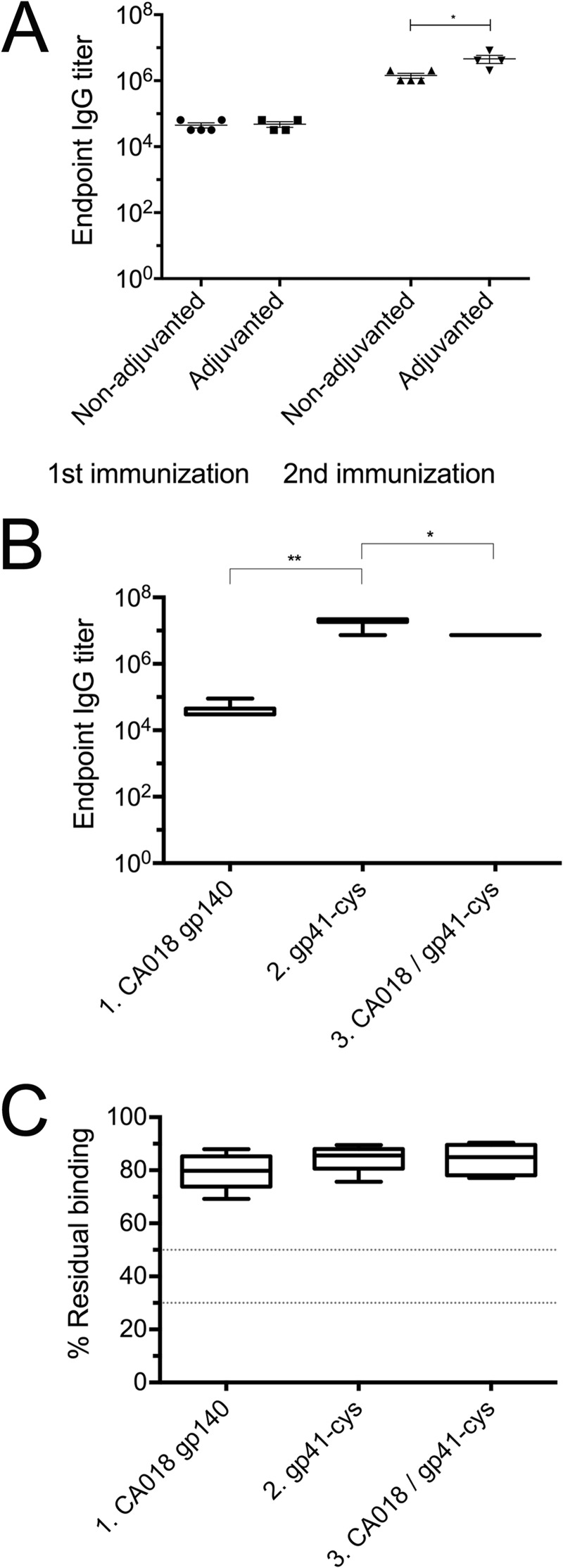
The stability of the proteoliposomes in the presence of the adjuvant mixture of Carbopol-971P and MF59 was assessed by negative staining EM, which demonstrated that this formulation kept the liposomes intact (Fig. 3B) and that bnAb 10E8 could still be detected via gold labeling in the presence of the adjuvant mixture (Fig. 3C). A pilot immunization study was carried out to test the effect of the adjuvants on the antigenicity of the gp41int-Cys proteoliposomes. Importantly, we were able to detect low titers of gp41-specific IgG after a single immunization in both adjuvanted and non-adjuvanted groups (Fig. 4A). The gp41-specific IgG titers increased by a factor of ∼3 in the adjuvanted group compared with the unadjuvanted groups after two immunizations (Fig. 4A; mean = 4.10 × 106 versus 1.35 versus 106, respectively; p < 0.05), indicating that the adjuvant combination was effective in enhancing antigen-specific antibody responses.
FIGURE 4.
IgG titers and relative avidity to gp41int. A, immunogenicity of gp41 proteoliposomes with or without adjuvants was compared. The gp41int-Cys proteoliposomes were capable of eliciting modest titers of gp41-specific IgG response after a single immunization regardless of the use of adjuvants. However, the IgG titer was significantly higher after two immunizations when Carbopol-971P and MF59 adjuvants were used compared with the unadjuvanted group. B, the gp41int-Cys proteoliposome (group 2) elicited significantly higher gp41-specific IgG titers than recombinant gp140CA018 or prime-boost with gp140CA018 and gp41int-Cys. The week −2 prebleed controls have IgG titers below the detection level (data not shown). C, avidity of the antisera was determined by their resistance to 8 m urea in binding to the gp41int-fd antigen. Regardless of the immunization regimen, all antisera tested displayed high avidity, defined as >50% residual binding, as compared with the no-urea controls. Error bars represent S.D.
Gp41int-Cys Proteoliposomes Elicited Anti-MPER IgG Responses
The immunogenicity of gp41int-Cys proteoliposomes was evaluated in vivo by immunization of guinea pigs. Three groups, six guinea pigs each, were immunized four times either with gp41int-Cys proteoliposomes alone, with trimeric Env gp140CA018 alone, or with Env gp140CA018 boosted three times with gp41int-Cys proteoliposomes (Table 1). All immunizations were done with the combined Carbopol-971P and MF59 adjuvants.
Serum antigen-specific IgG was measured by ELISA at the terminal bleed, which is 4 weeks after the final (fourth) immunization. Although animals immunized with gp140CA018 alone (group 1) have high binding IgG titers to whole Env glycoprotein (data not shown), little of these antibody responses was directed toward gp41 (mean = 3.6 × 104; Fig. 4B). In contrast, animals in group 2 (gp41int-Cys proteoliposomes) and group 3 (gp140CA018 plus gp41int-Cys proteoliposomes) developed strong, gp41-specific antibody responses that were 2–3 logs higher in magnitude (group 2 mean, 1.8 × 107; p < 0.01). Priming with gp140CA018 followed by boosting with gp41int-Cys proteoliposomes decreased the overall gp41-specific IgG titers (mean, 7.3 × 106; p < 0.05). To estimate antibody maturation, avidity of the antisera was measured by the 8 m urea displacement method as described previously (103). Regardless of the immunization regime, all antisera were found to have high relative avidity indices, defined as >50% residual binding to gp41int-fd (Fig. 4C).
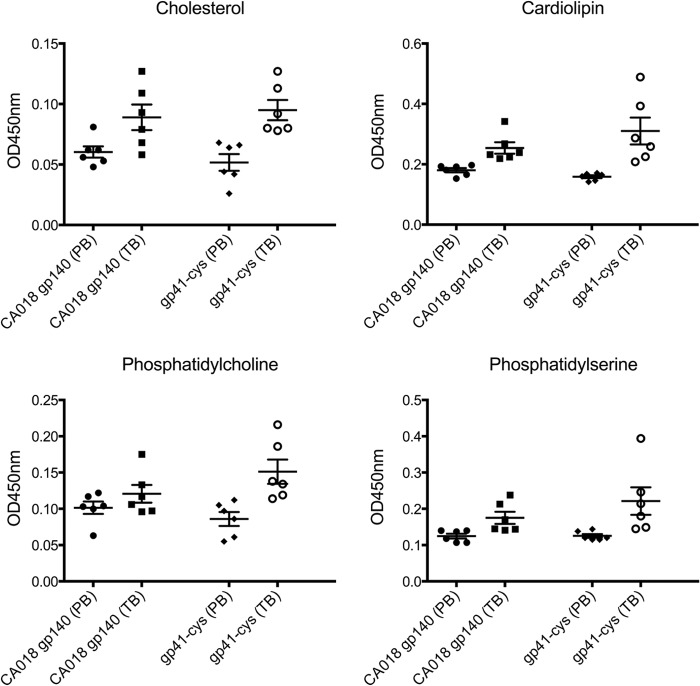
Because anti-MPER nAbs 2F5 and 4E10 have been reported to be polyreactive, we tested the serum IgG responses before and after immunization toward cardiolipin, phosphatidylcholine, phosphatidylserine, and cholesterol. Although no significant binding to the three lipids and to cholesterol was observed in the sera from group 1 (gp140CA018), some animals from group 2 (gp41int-Cys proteoliposomes) showed binding to cardiolipin (two of six), phosphatidylcholine (two of six), and phosphatidylserine (one of six) (Fig. 5). We conclude that the immunization scheme did not induce significant polyreactivity.
FIGURE 5.
Polyreactivity of the antisera. Non-antigen-specific binding to cholesterol, cardiolipin, phosphatidylcholine, and phosphatidylserine was measured by ELISA. The prebleed (PB) sera of gp140 group 1 and gp41int-Cys group 2 were compared with the respective group sera after immunization (terminal bleed (TB)). Error bars represent S.D.
Gp41int-Cys Proteoliposomes Elicited Antibodies against MPER
To determine whether the antibodies elicited by the gp41int-Cys proteoliposomes recognized the neutralization epitopes on the fusion intermediate conformation, the polyclonal antisera were placed to compete with nAbs 2F5, 4E10, and Z13e1 and the non-neutralizing antibody 5F3 for binding to gp41int-fd. The result demonstrated that the sera from the gp41int-Cys liposome immunization (group 2) elicited both 2F5- and 4E10-like antibodies as four of six animal antisera could compete off at least 30% of mAb 2F5 (shown as <70% residual binding), six of six could displace ∼55% of the 4E10 mAb, whereas none could efficiently compete with Z13e1. Group 3 (gp140CA018 plus gp41int-Cys proteoliposomes) revealed a lower activity against 2F5 (three of six) and 4E10 (four of six), and sera from all animals showed cross-reactivity against mAb 5F3. In contrast, group 1 (gp140CA018) demonstrated no significant competition against all four antibodies (Fig. 6A). Consistent with the competition assay, group 1 (gp140CA018) showed no reactivity against an MPER peptide, whereas all six sera from group 2 (gp41int-Cys proteoliposomes) revealed significant binding to the MPER peptide up to a 1:1000 serum dilution (Fig. 6B). Together these results indicate that gp41int-Cys proteoliposomes but not gp140CA018 elicited anti-MPER antibodies.
FIGURE 6.
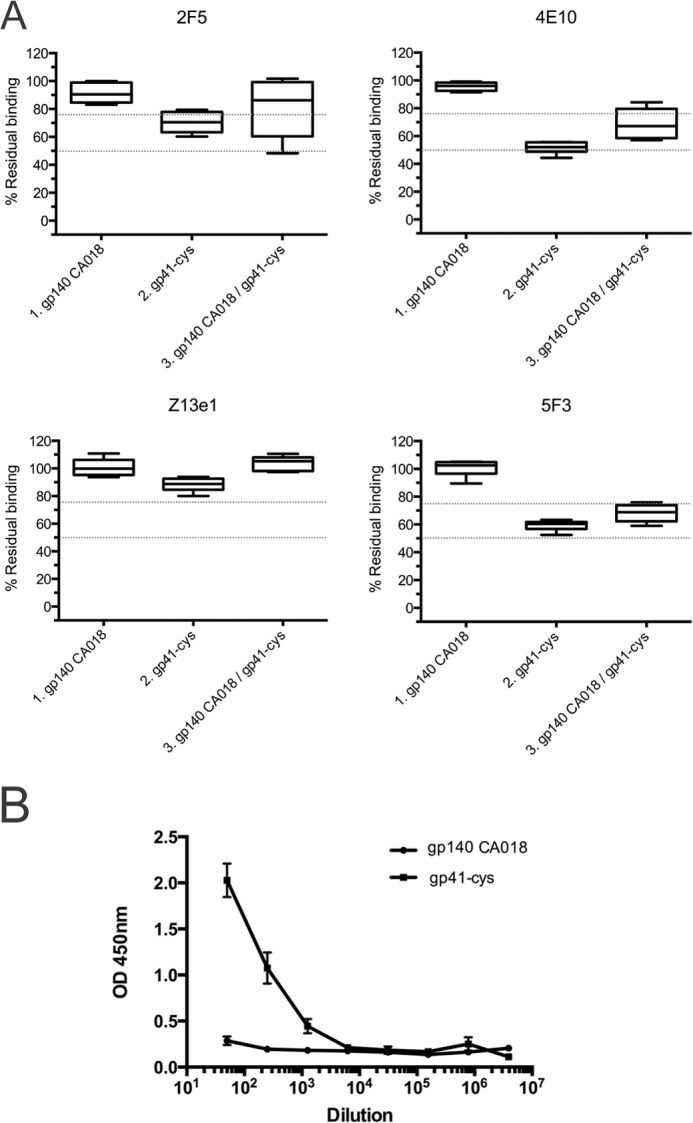
Interaction with the gp41 MPER sequence. A, competition of serum IgG with anti-MPER antibodies upon binding to gp41int-fd. In contrast to those immunized with gp140CA018, antisera from animals that received gp41int-Cys were able to weakly out-compete broadly neutralizing mAb 2F5 for binding to gp41int-fd antigen. Immunization with gp41int-Cys alone or in prime-boost combination with gp140CA018 also elicited antibodies that could compete with broadly neutralizing mAb 4E10 and the non-neutralizing 5F3 antibody. Although the epitope of Z13e1 overlaps those of 2F5 and 4E10, none of the antisera displayed were able to out-compete this mAb. B, serial serum dilutions were tested for binding to an MPER peptide. Each serum was tested in triplicate. Each curve represents the average reactions of the sera from group 1 (gp140CA018) and group 2 (gp41int-Cys proteoliposome) (the sera from 6 animals were pooled and tested 3 times). Error bars represent S.D.
Gp41int-Cys Proteoliposomes Induced Moderate Neutralizing Antibody Responses
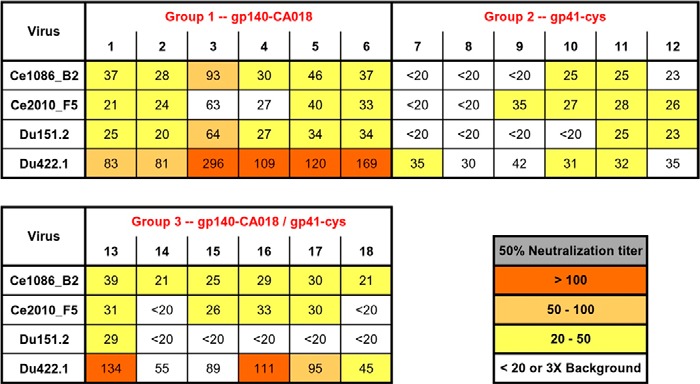
To analyze the breadth and potency of the immune responses elicited by the two gp41 constructs, unfractionated sera from immunized guinea pigs were tested in the TZM-bl neutralization assay with a panel of HIV and SHIV pseudoviruses (Table 2). Gp41int-Cys proteoliposomes (group 2) induced moderate neutralization responses to DJ263.8 and MW965.26 with a mean 50% titer ranging from 58 to 276 (Table 2); however, the sera failed to neutralize SF162.LS even though all antisera could modestly neutralize BaL.P4, SF162.P3, and SF162.P4 SHIVs (Table 2), all of which are subtype B HIV-1 Env chimeric viruses. We speculate that serial passages of these replication-competent SHIVs have induced site mutations in gp41, rendering them more sensitive to neutralization by gp41-specific antibodies; however, this would require confirmation by sequencing.
TABLE 2.
Serum neutralization measured by the TZM-bl assay
The 50% neutralization titers are colored-coded to reflect their potency range as indicated. Titers below 40 or less than 3-fold of the corresponding prebleed background are considered non-neutralizing and not color-coded.
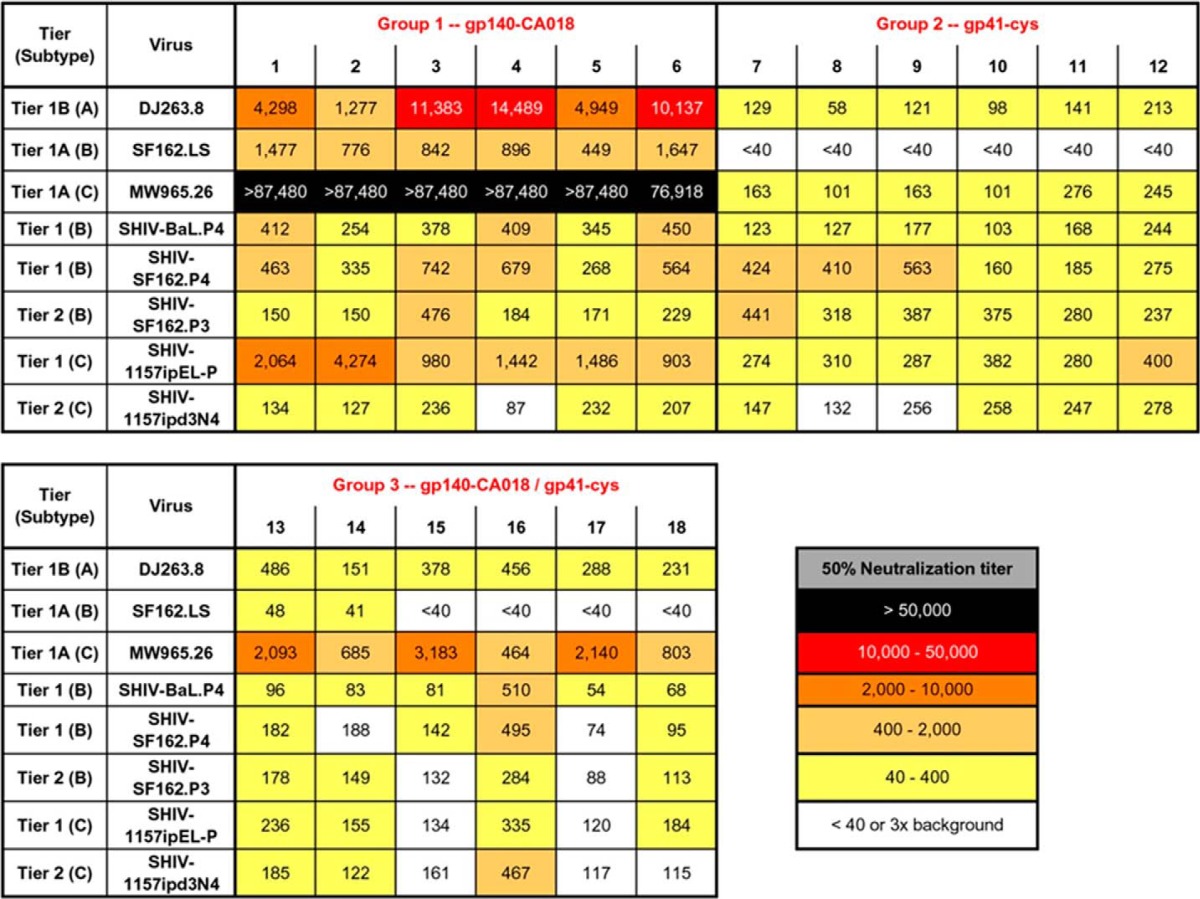
In contrast, gp140CA018 was more immunogenic and elicited a good nAb response. All animals (six of six) immunized with this recombinant glycoprotein (group 1) could neutralize 50% of MW965.26 pseudovirus with titers of 1:76,918 or higher (Table 2). They also potently neutralized DJ263.8 and SHIV-1157ipEL-P and moderately neutralized SF162.LS and SHIVs BaL.P4, SF162.P3/P4, and 1157ipd3N4 (Table 2). Priming with gp140CA018 appeared to have little effect on enhancing the magnitude of nAb responses compared with using gp41int-Cys proteoliposomes alone. Although the 50% neutralization titers against MW965.26 increased by 1 log when animals were primed with gp140CA018 (groups 3 versus groups 2; Table 2), this enhancement effect was not observed with other HIV or SHIV pseudoviruses.
To investigate whether tier 2 viruses could be neutralized, the antisera were tested against four replication-competent subtype C isolates in the A3R5 assay. All animals (six of six) immunized with gp140CA018 (group 1) could neutralize at least three of four tier 2 isolates with low to modest titers (Table 3). Notably, three of six animals receiving gp41int-Cys proteoliposomes (group 2) were also able to neutralize at least one of the four viruses albeit with lower potency (Table 3). In contrast to tier 1 neutralization, priming with gp140CA018 (group 3) enhanced the breadth of the immune response against tier 2 viruses (Table 3). Although overall titers against tier 2 viruses were only modest, the data indicate that the gp41int-Cys proteoliposomes were capable of eliciting nAbs that neutralized tier 2 viruses in the absence of a gp140 prime.
TABLE 3.
Serum neutralization measured by the A3R5 assay
Antisera were assessed for their ability to neutralize replication-competent tier 2 viruses. The 50% neutralization titers are color-coded according to their potency as indicated. Titers >20 and at least 3-fold above the corresponding prebleed background are considered neutralizing. The uncolored block indicates no neutralization.
DISCUSSION
We structurally characterized the conformation of gp41 that was previously suggested to represent the fusion intermediate state of gp41 (41), which assembles during membrane fusion (104). The low resolution envelope of gp41int-Cys reveals an elongated structure that could span a distance of >10 nm between the viral and cellular membranes. Interestingly, the SAXS envelope fits the gp41 coordinates of the native SOSIP.664 gp140 trimer structure reasonably well (43), although the overall helical content of gp41int-Cys argues against a complete helical conformation of HR2 if part of MPER is helical. The potential fitting might indicate either that HR2 adopted the intermediate conformation in the crystal structure due to the absence of MPER or that this region does not change substantially in the absence of gp120. Alternatively, because of the low resolution of the SAXS model, the fit may be by chance, and the Cys-loop region and HR2 may adopt a completely different conformation and/or may accommodate MPER in an extended conformation (105). The bottom end of the SAXS structure is not accounted for by the gp41 coordinates, and we hypothesize that it contains MPER connected to the transmembrane region. This would be in agreement with the small stalk entering the viral membrane identified by EM tomography (106, 107).
Gp41int was suggested to be the main target for anti-MPER nAbs 2F5, 4E10, and 10E8 (35, 41), and the membrane is a crucial factor for anti-MPER antibody neutralization (27–29, 108). We therefore used membrane-coupled gp41int-Cys for immunization of guinea pigs, which may present the membrane-dependent epitopes such that gp41int might induce antibodies that either need to extract the epitope from the membrane (52, 53) or show significant (25, 26, 109) or low membrane binding activity (35) required for neutralization.
Although liposomes permit efficient antigen presentation to antigen-presenting cells and exhibit a depot-forming property at the injection site (110, 111), we improved the immunogenicity of the gp41int-Cys liposomes by the combination of two adjuvants, an anionic polymer (Carbopol-971P) and an oil emulsion (MF59). Carbopol-971P is commonly used for oral or topical application, and MF59 is a licensed adjuvant for influenza vaccine; both can be produced with the good manufacturing practice standard for potential clinical use. This combination has been demonstrated previously to significantly increase binding IgG and neutralization titers in immunization studies with recombinant gp140 as an immunogen (98, 112). Formulation of gp41int-Cys proteoliposomes in this adjuvant combination slightly enlarged the liposome diameter but left them intact, and bnAb 10E8 stayed bound to the gp41 proteoliposomes in the adjuvant mixture. Overall the Carbopol/MF59 mixture improved the immunogenicity of the gp41int-Cys proteoliposomes.
Although gp140int-Cys was based on the peptide sequence of HXB2, we selected gp140CA018 for the immunization study as it displayed superior immunogenicity to other gp140s and elicited robust neutralization responses in guinea pigs12 with the aim that it would enhance the neutralization response in a prime-boost strategy with the gp41int proteoliposomes. The MPER sequences present in gp41int-Cys and CA018 are largely identical with three conservative changes (N675D, T679E, and N680H) and changes at positions 663 (Glu to Ala) and 669 (Ser to Gly). These small differences might have contributed to the fact that priming with gp140 had no beneficial effect on the generation of anti-gp41 antibodies. In addition, it is likely that gp41 present in gp140 adopts a different conformation than gp41 present in gp41int-Cys. Therefore the combination of native-like gp41, presumably present in gp140, and gp41 in an intermediate prefusion state might have no combinatorial beneficial effect. Because the immunogenicity of gp120 within gp140 might be dominant, most of the antibodies are directed to gp120 in this combinatorial approach.
Immunization with gp41int-Cys proteoliposomes produced sera that demonstrated competition with nAbs 2F5 and 4E10 and interacted with an MPER-spanning peptide in a dose-dependent manner, indicating that the sera contained antibodies directed against MPER. Sera from all six animals showed modest neutralization titers against a panel of tier 1 viruses and SHIVs in the stringent TZM-bl assay. Because it has been noted that anti-gp41 antibodies exhibit higher inhibitory activities in peripheral blood mononuclear cell-based assays than the standard TZM-bl cell line-based assay (113) possibly due to a lower surface concentration of CCR5 co-receptors in the former (114), we tested neutralization of a few tier 2 viruses in the A3R5 neutralizing assay (101). This revealed that most animals produced modest nAb activities to replication-competent tier 2 isolates albeit at lower titers. A gp140 prime-gp41int-Cys proteoliposome boost immunization regimen resulted in an increase in neutralization of selected tier 1 and tier 2 viruses. However, gp41-specific antibody activities were significantly lower in the gp140CA018-primed group, and immunization with gp140CA018 alone already resulted in antisera that neutralized tier 1 and tier 2 HIV-1 pseudoviruses with potent to modest titers. Therefore improvement in neutralization in the gp140CA018 prime-gp41 boost group is largely due to the presence of gp120-specific neutralizing antibodies. The potency of anti-gp120 nAbs might be due to an increased half-life, although we did not measure antibody half-life in this study. It has been shown in HIV-1 patients that anti-gp120 antibodies have an average half-life of 81 weeks, whereas anti-gp41 antibodies have an average half-life of 33 weeks (115). Therefore prolonged immunization regimens with repeated doses of gp41 might be required to further improve the neutralization antibody breadth to a level comparable with that induced by gp140.
Although many gp41 antigens have been tested in the past, only a few studies reported tier 1 and tier 2 virus neutralization with animal sera using the standard TZM-bl standard assay (65), enriched total IgGs (116), and isolated antibodies (86, 117). Using a strategy similar to that described here, a gp140 oligomer prime followed by MPER peptide-liposome boost strategy resulted in antibodies that reacted with the gp41 fusion intermediate construct described by Frey et al. (41) and the 2F5 epitope; however, no neutralization was observed, indicating that the complete fusion intermediate conformation coupled to liposomes is a better immunogen than MPER peptides coupled to membranes (85).
Anti-MPER bnAbs bind antigen and membrane components (26, 52, 53). We therefore measured the IgG responses to three lipids, cardiolipin, phosphatidylcholine, phosphatidylserine, and to cholesterol. However, only a few animals that received gp41int-Cys proteoliposomes showed modest interaction with the lipids, consistent with the elimination of polyreactive antibodies by B cell tolerance mechanisms (33, 34). Because of the small sample size, we cannot determine whether the lack of polyreactivity observed in the animal is associated with the modest to weak neutralization of tier 2 isolates. However, the significance of weak polyreactivity is difficult to determine as shown for mAb 10E8 (14, 35). Notably, the immunization-induced llama nanobody 2H10 did not interact with lipids or membranes in vitro but nevertheless required a tryptophan within its CDR3 for neutralization but not for antigen binding (86).
In summary, using the gp41int-Cys proteoliposomes, we were able to obtain promising humoral responses against gp41 that showed neutralization using the stringent TZM-bl assay. This provides an important proof of concept that liposome-based approaches carrying the fusion intermediate conformation of gp41 have the potential to induce neutralizing antibodies in animal models. The generation of antibodies with increased breadth and potency will require additional modifications such as immunofocusing on the MPER region by using a set of different gp41int-Cys sequences, enhanced coupling of gp41int-Cys to liposomes, prolonged immunization schemes, novel nanoparticles combined with proteoliposomes, or combinations thereof.
Acknowledgments
We thank C. Brown and M. Larsen for help on the animal work and A. Hinz for a previous contribution. We thank Kelli Greene and Hongmei Gao for organizing the neutralization assays. We further acknowledge the use of the platforms of the Grenoble Instruct center (Integrated Structural Biology, Grenoble; UMS 3518, CNRS-Commissariat à l'Energie Atomique-Université Joseph Fourier-European Molecular Biology Laboratory), which is supported by French Infrastructure for Integrated Structural Biology Grant ANR-10-INSB-05–02 and Grenoble Alliance for Integrated Structural Cell Biology (GRAL) Grant ANR-10-LABX-49-01.
This work was supported in part by the Bill and Melinda Gates Foundation-funded Consortium of AIDS Vaccine Discovery (Grant 38637), the Comprehensive Antibody-Vaccine Immune Monitoring Consortium (Grant 38619), and Sidaction (to W. W.).
M. Schnos, personal communication.
R. P. J. Lai, P. Tonks, D. J. Seilly, H. Dreja, and J. L. Heeney, unpublished data.
- HIV-1
- human immunodeficiency virus, type 1
- MPER
- membrane-proximal external region
- SAXS
- small angle x-ray scattering
- Env
- envelope glycoprotein
- SHIV
- simian/human immunodeficiency virus
- nAb
- neutralizing antibody
- bnAb
- broadly neutralizing antibody
- gp41int
- intermediate conformation of gp41
- Bicine
- N,N-bis(2-hydroxyethyl)glycine
- HR
- heptad repeat region
- fd
- fold-on domain.
REFERENCES
- 1. Alam S. M., Scearce R. M., Parks R. J., Plonk K., Plonk S. G., Sutherland L. L., Gorny M. K., Zolla-Pazner S., Vanleeuwen S., Moody M. A., Xia S. M., Montefiori D. C., Tomaras G. D., Weinhold K. J., Karim S. A., Hicks C. B., Liao H. X., Robinson J., Shaw G. M., Haynes B. F. (2008) Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection. J. Virol. 82, 115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liao H. X., Chen X., Munshaw S., Zhang R., Marshall D. J., Vandergrift N., Whitesides J. F., Lu X., Yu J. S., Hwang K. K., Gao F., Markowitz M., Heath S. L., Bar K. J., Goepfert P. A., Montefiori D. C., Shaw G. C., Alam S. M., Margolis D. M., Denny T. N., Boyd S. D., Marshal E., Egholm M., Simen B. B., Hanczaruk B., Fire A. Z., Voss G., Kelsoe G., Tomaras G. D., Moody M. A., Kepler T. B., Haynes B. F. (2011) Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J. Exp. Med. 208, 2237–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bar K. J., Tsao C. Y., Iyer S. S., Decker J. M., Yang Y., Bonsignori M., Chen X., Hwang K. K., Montefiori D. C., Liao H. X., Hraber P., Fischer W., Li H., Wang S., Sterrett S., Keele B. F., Ganusov V. V., Perelson A. S., Korber B. T., Georgiev I., McLellan J. S., Pavlicek J. W., Gao F., Haynes B. F., Hahn B. H., Kwong P. D., Shaw G. M. (2012) Early low-titer neutralizing antibodies impede HIV-1 replication and select for virus escape. PLoS Pathog. 8, e1002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corti D., Langedijk J. P., Hinz A., Seaman M. S., Vanzetta F., Fernandez-Rodriguez B. M., Silacci C., Pinna D., Jarrossay D., Balla-Jhagjhoorsingh S., Willems B., Zekveld M. J., Dreja H., O'Sullivan E., Pade C., Orkin C., Jeffs S. A., Montefiori D. C., Davis D., Weissenhorn W., McKnight A., Heeney J. L., Sallusto F., Sattentau Q. J., Weiss R. A., Lanzavecchia A. (2010) Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One 5, e8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheid J. F., Mouquet H., Feldhahn N., Seaman M. S., Velinzon K., Pietzsch J., Ott R. G., Anthony R. M., Zebroski H., Hurley A., Phogat A., Chakrabarti B., Li Y., Connors M., Pereyra F., Walker B. D., Wardemann H., Ho D., Wyatt R. T., Mascola J. R., Ravetch J. V., Nussenzweig M. C. (2009) Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458, 636–640 [DOI] [PubMed] [Google Scholar]
- 6. Wu X., Yang Z. Y., Li Y., Hogerkorp C. M., Schief W. R., Seaman M. S., Zhou T., Schmidt S. D., Wu L., Xu L., Longo N. S., McKee K., O'Dell S., Louder M. K., Wycuff D. L., Feng Y., Nason M., Doria-Rose N., Connors M., Kwong P. D., Roederer M., Wyatt R. T., Nabel G. J., Mascola J. R. (2010) Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329, 856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scheid J. F., Mouquet H., Ueberheide B., Diskin R., Klein F., Oliveira T. Y., Pietzsch J., Fenyo D., Abadir A., Velinzon K., Hurley A., Myung S., Boulad F., Poignard P., Burton D. R., Pereyra F., Ho D. D., Walker B. D., Seaman M. S., Bjorkman P. J., Chait B. T., Nussenzweig M. C. (2011) Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333, 1633–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diskin R., Scheid J. F., Marcovecchio P. M., West A. P., Jr., Klein F., Gao H., Gnanapragasam P. N., Abadir A., Seaman M. S., Nussenzweig M. C., Bjorkman P. J. (2011) Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science 334, 1289–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liao H. X., Lynch R., Zhou T., Gao F., Alam S. M., Boyd S. D., Fire A. Z., Roskin K. M., Schramm C. A., Zhang Z., Zhu J., Shapiro L., NISC Comparative Sequencing Program, Mullikin J. C., Gnanakaran S., Hraber P., Wiehe K., Kelsoe G., Yang G., Xia S. M., Montefiori D. C., Parks R., Lloyd K. E., Scearce R. M., Soderberg K. A., Cohen M., Kamanga G., Louder M. K., Tran L. M., Chen Y., Cai F., Chen S., Moquin S., Du X., Joyce M. G., Srivatsan S., Zhang B., Zheng A., Shaw G. M., Hahn B. H., Kepler T. B., Korber B. T., Kwong P. D., Mascola J. R., Haynes B. F. (2013) Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496, 469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walker L. M., Phogat S. K., Chan-Hui P. Y., Wagner D., Phung P., Goss J. L., Wrin T., Simek M. D., Fling S., Mitcham J. L., Lehrman J. K., Priddy F. H., Olsen O. A., Frey S. M., Hammond P. W., Protocol G Principal Investigators, Kaminsky S., Zamb T., Moyle M., Koff W. C., Poignard P., Burton D. R. (2009) Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326, 285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walker L. M., Huber M., Doores K. J., Falkowska E., Pejchal R., Julien J. P., Wang S. K., Ramos A., Chan-Hui P. Y., Moyle M., Mitcham J. L., Hammond P. W., Olsen O. A., Phung P., Fling S., Wong C. H., Phogat S., Wrin T., Simek M. D., Protocol G Principal Investigators, Koff W. C., Wilson I. A., Burton D. R., Poignard P. (2011) Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477, 466–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McLellan J. S., Pancera M., Carrico C., Gorman J., Julien J. P., Khayat R., Louder R., Pejchal R., Sastry M., Dai K., O'Dell S., Patel N., Shahzad-ul-Hussan S., Yang Y., Zhang B., Zhou T., Zhu J., Boyington J. C., Chuang G. Y., Diwanji D., Georgiev I., Kwon Y. D., Lee D., Louder M. K., Moquin S., Schmidt S. D., Yang Z. Y., Bonsignori M., Crump J. A., Kapiga S. H., Sam N. E., Haynes B. F., Burton D. R., Koff W. C., Walker L. M., Phogat S., Wyatt R., Orwenyo J., Wang L. X., Arthos J., Bewley C. A., Mascola J. R., Nabel G. J., Schief W. R., Ward A. B., Wilson I. A., Kwong P. D. (2011) Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480, 336–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pancera M., Shahzad-Ul-Hussan S., Doria-Rose N. A., McLellan J. S., Bailer R. T., Dai K., Loesgen S., Louder M. K., Staupe R. P., Yang Y., Zhang B., Parks R., Eudailey J., Lloyd K. E., Blinn J., Alam S. M., Haynes B. F., Amin M. N., Wang L. X., Burton D. R., Koff W. C., Nabel G. J., Mascola J. R., Bewley C. A., Kwong P. D. (2013) Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1-V2-directed antibody PG16. Nat. Struct. Mol. Biol. 20, 804–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang J., Ofek G., Laub L., Louder M. K., Doria-Rose N. A., Longo N. S., Imamichi H., Bailer R. T., Chakrabarti B., Sharma S. K., Alam S. M., Wang T., Yang Y., Zhang B., Migueles S. A., Wyatt R., Haynes B. F., Kwong P. D., Mascola J. R., Connors M. (2012) Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491, 406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klein F., Diskin R., Scheid J. F., Gaebler C., Mouquet H., Georgiev I. S., Pancera M., Zhou T., Incesu R. B., Fu B. Z., Gnanapragasam P. N., Oliveira T. Y., Seaman M. S., Kwong P. D., Bjorkman P. J., Nussenzweig M. C. (2013) Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell 153, 126–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muñoz-Barroso I., Salzwedel K., Hunter E., Blumenthal R. (1999) Role of the membrane-proximal domain in the initial stages of human immunodeficiency virus type 1 envelope glycoprotein-mediated membrane fusion. J. Virol. 73, 6089–6092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zwick M. B., Labrijn A. F., Wang M., Spenlehauer C., Saphire E. O., Binley J. M., Moore J. P., Stiegler G., Katinger H., Burton D. R., Parren P. W. (2001) Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75, 10892–10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salzwedel K., West J. T., Hunter E. (1999) A conserved tryptophan-rich motif in the membrane-proximal region of the human immunodeficiency virus type 1 gp41 ectodomain is important for Env-mediated fusion and virus infectivity. J. Virol. 73, 2469–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muster T., Steindl F., Purtscher M., Trkola A., Klima A., Himmler G., Rüker F., Katinger H. (1993) A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67, 6642–6647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stiegler G., Kunert R., Purtscher M., Wolbank S., Voglauer R., Steindl F., Katinger H. (2001) A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 17, 1757–1765 [DOI] [PubMed] [Google Scholar]
- 21. Nelson J. D., Brunel F. M., Jensen R., Crooks E. T., Cardoso R. M., Wang M., Hessell A., Wilson I. A., Binley J. M., Dawson P. E., Burton D. R., Zwick M. B. (2007) An affinity-enhanced neutralizing antibody against the membrane-proximal external region of human immunodeficiency virus type 1 gp41 recognizes an epitope between those of 2F5 and 4E10. J. Virol. 81, 4033–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ofek G., Tang M., Sambor A., Katinger H., Mascola J. R., Wyatt R., Kwong P. D. (2004) Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J. Virol. 78, 10724–10737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cardoso R. M., Zwick M. B., Stanfield R. L., Kunert R., Binley J. M., Katinger H., Burton D. R., Wilson I. A. (2005) Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity 22, 163–173 [DOI] [PubMed] [Google Scholar]
- 24. Pejchal R., Gach J. S., Brunel F. M., Cardoso R. M., Stanfield R. L., Dawson P. E., Burton D. R., Zwick M. B., Wilson I. A. (2009) A conformational switch in human immunodeficiency virus gp41 revealed by the structures of overlapping epitopes recognized by neutralizing antibodies. J. Virol. 83, 8451–8462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haynes B. F., Fleming J., St Clair E. W., Katinger H., Stiegler G., Kunert R., Robinson J., Scearce R. M., Plonk K., Staats H. F., Ortel T. L., Liao H. X., Alam S. M. (2005) Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 308, 1906–1908 [DOI] [PubMed] [Google Scholar]
- 26. Alam S. M., McAdams M., Boren D., Rak M., Scearce R. M., Gao F., Camacho Z. T., Gewirth D., Kelsoe G., Chen P., Haynes B. F. (2007) The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J. Immunol. 178, 4424–4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ofek G., McKee K., Yang Y., Yang Z. Y., Skinner J., Guenaga F. J., Wyatt R., Zwick M. B., Nabel G. J., Mascola J. R., Kwong P. D. (2010) Relationship between antibody 2F5 neutralization of HIV-1 and hydrophobicity of its heavy chain third complementarity-determining region. J. Virol. 84, 2955–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scherer E. M., Leaman D. P., Zwick M. B., McMichael A. J., Burton D. R. (2010) Aromatic residues at the edge of the antibody combining site facilitate viral glycoprotein recognition through membrane interactions. Proc. Natl. Acad. Sci. U.S.A. 107, 1529–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alam S. M., Morelli M., Dennison S. M., Liao H. X., Zhang R., Xia S. M., Rits-Volloch S., Sun L., Harrison S. C., Haynes B. F., Chen B. (2009) Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc. Natl. Acad. Sci. U.S.A. 106, 20234–20239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Julien J. P., Huarte N., Maeso R., Taneva S. G., Cunningham A., Nieva J. L., Pai E. F. (2010) Ablation of the complementarity-determining region H3 apex of the anti-HIV-1 broadly neutralizing antibody 2F5 abrogates neutralizing capacity without affecting core epitope binding. J. Virol. 84, 4136–4147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singh H., Henry K. A., Wu S. S., Chruscinski A., Utz P. J., Scott J. K. (2011) Reactivity profiles of broadly neutralizing anti-HIV-1 antibodies are distinct from those of pathogenic autoantibodies. AIDS 25, 1247–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haynes B. F., Nicely N. I., Alam S. M. (2010) HIV-1 autoreactive antibodies: are they good or bad for HIV-1 prevention? Nat. Struct. Mol. Biol. 17, 543–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verkoczy L., Diaz M., Holl T. M., Ouyang Y. B., Bouton-Verville H., Alam S. M., Liao H. X., Kelsoe G., Haynes B. F. (2010) Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc. Natl. Acad. Sci. U.S.A. 107, 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Y., Zhang J., Hwang K. K., Bouton-Verville H., Xia S. M., Newman A., Ouyang Y. B., Haynes B. F., Verkoczy L. (2013) Common tolerance mechanisms, but distinct cross-reactivities associated with gp41 and lipids, limit production of HIV-1 broad neutralizing antibodies 2F5 and 4E10. J. Immunol. 191, 1260–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen J., Frey G., Peng H., Rits-Volloch S., Garrity J., Seaman M. S., Chen B. (2014) Mechanism of HIV-1 neutralization by antibodies targeting a membrane-proximal region of gp41. J. Virol. 88, 1249–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen X., Parks R. J., Montefiori D. C., Kirchherr J. L., Keele B. F., Decker J. M., Blattner W. A., Gao F., Weinhold K. J., Hicks C. B., Greenberg M. L., Hahn B. H., Shaw G. M., Haynes B. F., Tomaras G. D. (2009) In vivo gp41 antibodies targeting the 2F5 monoclonal antibody epitope mediate human immunodeficiency virus type 1 neutralization breadth. J. Virol. 83, 3617–3625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pietzsch J., Scheid J. F., Mouquet H., Seaman M. S., Broder C. C., Nussenzweig M. C. (2010) Anti-gp41 antibodies cloned from HIV-infected patients with broadly neutralizing serologic activity. J. Virol. 84, 5032–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu Z., Qin H. R., Chen W., Zhao Q., Shen X., Schutte R., Wang Y., Ofek G., Streaker E., Prabakaran P., Fouda G. G., Liao H. X., Owens J., Louder M., Yang Y., Klaric K. A., Moody M. A., Mascola J. R., Scott J. K., Kwong P. D., Montefiori D., Haynes B. F., Tomaras G. D., Dimitrov D. S. (2011) Cross-reactive HIV-1-neutralizing human monoclonal antibodies identified from a patient with 2F5-like antibodies. J. Virol. 85, 11401–11408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morris L., Chen X., Alam M., Tomaras G., Zhang R., Marshall D. J., Chen B., Parks R., Foulger A., Jaeger F., Donathan M., Bilska M., Gray E. S., Abdool Karim S. S., Kepler T. B., Whitesides J., Montefiori D., Moody M. A., Liao H. X., Haynes B. F. (2011) Isolation of a human anti-HIV gp41 membrane proximal region neutralizing antibody by antigen-specific single B cell sorting. PLoS One 6, e23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hessell A. J., Rakasz E. G., Tehrani D. M., Huber M., Weisgrau K. L., Landucci G., Forthal D. N., Koff W. C., Poignard P., Watkins D. I., Burton D. R. (2010) Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 84, 1302–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frey G., Peng H., Rits-Volloch S., Morelli M., Cheng Y., Chen B. (2008) A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc. Natl. Acad. Sci. U.S.A. 105, 3739–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dimitrov A. S., Jacobs A., Finnegan C. M., Stiegler G., Katinger H., Blumenthal R. (2007) Exposure of the membrane-proximal external region of HIV-1 gp41 in the course of HIV-1 envelope glycoprotein-mediated fusion. Biochemistry 46, 1398–1401 [DOI] [PubMed] [Google Scholar]
- 43. Julien J. P., Cupo A., Sok D., Stanfield R. L., Lyumkis D., Deller M. C., Klasse P. J., Burton D. R., Sanders R. W., Moore J. P., Ward A. B., Wilson I. A. (2013) Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342, 1477–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lyumkis D., Julien J. P., de Val N., Cupo A., Potter C. S., Klasse P. J., Burton D. R., Sanders R. W., Moore J. P., Carragher B., Wilson I. A., Ward A. B. (2013) Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 342, 1484–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bartesaghi A., Merk A., Borgnia M. J., Milne J. L., Subramaniam S. (2013) Prefusion structure of trimeric HIV-1 envelope glycoprotein determined by cryo-electron microscopy. Nat. Struct. Mol. Biol. 20, 1352–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Merk A., Subramaniam S. (2013) HIV-1 envelope glycoprotein structure. Curr. Opin. Struct. Biol. 23, 268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chan D. C., Fass D., Berger J. M., Kim P. S. (1997) Core structure of gp41 from the HIV envelope glycoprotein. Cell 89, 263–273 [DOI] [PubMed] [Google Scholar]
- 48. Weissenhorn W., Dessen A., Harrison S. C., Skehel J. J., Wiley D. C. (1997) Atomic structure of the ectodomain from HIV-1 gp41. Nature 387, 426–430 [DOI] [PubMed] [Google Scholar]
- 49. Caffrey M., Cai M., Kaufman J., Stahl S. J., Wingfield P. T., Gronenborn A. M., Clore G. M. (1997) Determination of the secondary structure and global topology of the 44 kDa ectodomain of gp41 of the simian immunodeficiency virus by multidimensional nuclear magnetic resonance spectroscopy. J. Mol. Biol. 271, 819–826 [DOI] [PubMed] [Google Scholar]
- 50. Buzon V., Natrajan G., Schibli D., Campelo F., Kozlov M. M., Weissenhorn W. (2010) Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions. PLoS Pathog. 6, e1000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oster A. M., Pieniazek D., Zhang X., Switzer W. M., Ziebell R. A., Mena L. A., Wei X., Johnson K. L., Singh S. K., Thomas P. E., Elmore K. A., Heffelfinger J. D. (2011) Demographic but not geographic insularity in HIV transmission among young black MSM. AIDS 25, 2157–2165 [DOI] [PubMed] [Google Scholar]
- 52. Sun Z. Y., Oh K. J., Kim M., Yu J., Brusic V., Song L., Qiao Z., Wang J. H., Wagner G., Reinherz E. L. (2008) HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity 28, 52–63 [DOI] [PubMed] [Google Scholar]
- 53. Kim M., Sun Z. Y., Rand K. D., Shi X., Song L., Cheng Y., Fahmy A. F., Majumdar S., Ofek G., Yang Y., Kwong P. D., Wang J. H., Engen J. R., Wagner G., Reinherz E. L. (2011) Antibody mechanics on a membrane-bound HIV segment essential for GP41-targeted viral neutralization. Nat. Struct. Mol. Biol. 18, 1235–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Montero M., van Houten N. E., Wang X., Scott J. K. (2008) The membrane-proximal external region of the human immunodeficiency virus type 1 envelope: dominant site of antibody neutralization and target for vaccine design. Microbiol. Mol. Biol. Rev. 72, 54–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Decroix N., Hocini H., Quan C. P., Bellon B., Kazatchkine M. D., Bouvet J. P. (2001) Induction in mucosa of IgG and IgA antibodies against parenterally administered soluble immunogens. Scand. J. Immunol. 53, 401–409 [DOI] [PubMed] [Google Scholar]
- 56. Joyce J. G., Hurni W. M., Bogusky M. J., Garsky V. M., Liang X., Citron M. P., Danzeisen R. C., Miller M. D., Shiver J. W., Keller P. M. (2002) Enhancement of α-helicity in the HIV-1 inhibitory peptide DP178 leads to an increased affinity for human monoclonal antibody 2F5 but does not elicit neutralizing responses in vitro. Implications for vaccine design. J. Biol. Chem. 277, 45811–45820 [DOI] [PubMed] [Google Scholar]
- 57. Liao M., Lu Y., Xiao Y., Dierich M. P., Chen Y. (2000) Induction of high level of specific antibody response to the neutralizing epitope ELDKWA on HIV-1 gp41 by peptide-vaccine. Peptides 21, 463–468 [DOI] [PubMed] [Google Scholar]
- 58. McGaughey G. B., Citron M., Danzeisen R. C., Freidinger R. M., Garsky V. M., Hurni W. M., Joyce J. G., Liang X., Miller M., Shiver J., Bogusky M. J. (2003) HIV-1 vaccine development: constrained peptide immunogens show improved binding to the anti-HIV-1 gp41 MAb. Biochemistry 42, 3214–3223 [DOI] [PubMed] [Google Scholar]
- 59. Ni J., Powell R., Baskakov I. V., DeVico A., Lewis G. K., Wang L. X. (2004) Synthesis, conformation, and immunogenicity of monosaccharide-centered multivalent HIV-1 gp41 peptides containing the sequence of DP178. Bioorg. Med. Chem. 12, 3141–3148 [DOI] [PubMed] [Google Scholar]
- 60. Matoba N., Geyer B. C., Kilbourne J., Alfsen A., Bomsel M., Mor T. S. (2006) Humoral immune responses by prime-boost heterologous route immunizations with CTB-MPR(649–684), a mucosal subunit HIV/AIDS vaccine candidate. Vaccine 24, 5047–5055 [DOI] [PubMed] [Google Scholar]
- 61. Zhou M., Kostoula I., Brill B., Panou E., Sakarellos-Daitsiotis M., Dietrich U. (2012) Prime boost vaccination approaches with different conjugates of a new HIV-1 gp41 epitope encompassing the membrane proximal external region induce neutralizing antibodies in mice. Vaccine 30, 1911–1916 [DOI] [PubMed] [Google Scholar]
- 62. Guenaga J., Dosenovic P., Ofek G., Baker D., Schief W. R., Kwong P. D., Karlsson Hedestam G. B., Wyatt R. T. (2011) Heterologous epitope-scaffold prime:boosting immuno-focuses B cell responses to the HIV-1 gp41 2F5 neutralization determinant. PLoS One 6, e16074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ofek G., Guenaga F. J., Schief W. R., Skinner J., Baker D., Wyatt R., Kwong P. D. (2010) Elicitation of structure-specific antibodies by epitope scaffolds. Proc. Natl. Acad. Sci. U.S.A. 107, 17880–17887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Strasz N., Morozov V. A., Kreutzberger J., Keller M., Eschricht M., Denner J. (2014) Immunization with hybrid proteins containing the membrane proximal external region of HIV-1. AIDS Res. Hum. Retroviruses 30, 498–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ye L., Wen Z., Dong K., Wang X., Bu Z., Zhang H., Compans R. W., Yang C. (2011) Induction of HIV neutralizing antibodies against the MPER of the HIV envelope protein by HA/gp41 chimeric protein-based DNA and VLP vaccines. PLoS One 6, e14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Muster T., Ferko B., Klima A., Purtscher M., Trkola A., Schulz P., Grassauer A., Engelhardt O. G., García-Sástre A., Palese P. (1995) Mucosal model of immunization against human immunodeficiency virus type 1 with a chimeric influenza virus. J. Virol. 69, 6678–6686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Eckhart L., Raffelsberger W., Ferko B., Klima A., Purtscher M., Katinger H., Rüker F. (1996) Immunogenic presentation of a conserved gp41 epitope of human immunodeficiency virus type 1 on recombinant surface antigen of hepatitis B virus. J. Gen. Virol. 77, 2001–2008 [DOI] [PubMed] [Google Scholar]
- 68. Kusov Y. Y., Zamjatina N. A., Poleschuk V. F., Michailov M. I., Morace G., Eberle J., Gauss-Müller V. (2007) Immunogenicity of a chimeric hepatitis A virus (HAV) carrying the HIV gp41 epitope 2F5. Antiviral Res. 73, 101–111 [DOI] [PubMed] [Google Scholar]
- 69. Marusic C., Rizza P., Lattanzi L., Mancini C., Spada M., Belardelli F., Benvenuto E., Capone I. (2001) Chimeric plant virus particles as immunogens for inducing murine and human immune responses against human immunodeficiency virus type 1. J. Virol. 75, 8434–8439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Muster T., Guinea R., Trkola A., Purtscher M., Klima A., Steindl F., Palese P., Katinger H. (1994) Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J. Virol. 68, 4031–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang H., Huang Y., Fayad R., Spear G. T., Qiao L. (2004) Induction of mucosal and systemic neutralizing antibodies against human immunodeficiency virus type 1 (HIV-1) by oral immunization with bovine papillomavirus-HIV-1 gp41 chimeric virus-like particles. J. Virol. 78, 8342–8348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim M., Qiao Z., Yu J., Montefiori D., Reinherz E. L. (2007) Immunogenicity of recombinant human immunodeficiency virus type 1-like particles expressing gp41 derivatives in a pre-fusion state. Vaccine 25, 5102–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Luo M., Yuan F., Liu Y., Jiang S., Song X., Jiang P., Yin X., Ding M., Deng H. (2006) Induction of neutralizing antibody against human immunodeficiency virus type 1 (HIV-1) by immunization with gp41 membrane-proximal external region (MPER) fused with porcine endogenous retrovirus (PERV) p15E fragment. Vaccine 24, 435–442 [DOI] [PubMed] [Google Scholar]
- 74. Jain S., Patrick A. J., Rosenthal K. L. (2010) Multiple tandem copies of conserved gp41 epitopes incorporated in gag virus-like particles elicit systemic and mucosal antibodies in an optimized heterologous vector delivery regimen. Vaccine 28, 7070–7080 [DOI] [PubMed] [Google Scholar]
- 75. Kamdem Toukam D., Tenbusch M., Stang A., Temchura V., Storcksdieck Genannt Bonsmann M., Grewe B., Koch S., Meyerhans A., Nchinda G., Kaptue L., Uberla K. (2012) Targeting antibody responses to the membrane proximal external region of the envelope glycoprotein of human immunodeficiency virus. PLoS One 7, e38068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Arnold G. F., Velasco P. K., Holmes A. K., Wrin T., Geisler S. C., Phung P., Tian Y., Resnick D. A., Ma X., Mariano T. M., Petropoulos C. J., Taylor J. W., Katinger H., Arnold E. (2009) Broad neutralization of human immunodeficiency virus type 1 (HIV-1) elicited from human rhinoviruses that display the HIV-1 gp41 ELDKWA epitope. J. Virol. 83, 5087–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bomsel M., Tudor D., Drillet A. S., Alfsen A., Ganor Y., Roger M. G., Mouz N., Amacker M., Chalifour A., Diomede L., Devillier G., Cong Z., Wei Q., Gao H., Qin C., Yang G. B., Zurbriggen R., Lopalco L., Fleury S. (2011) Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity 34, 269–280 [DOI] [PubMed] [Google Scholar]
- 78. Yi G., Lapelosa M., Bradley R., Mariano T. M., Dietz D. E., Hughes S., Wrin T., Petropoulos C., Gallicchio E., Levy R. M., Arnold E., Arnold G. F. (2013) Chimeric rhinoviruses displaying MPER epitopes elicit anti-HIV neutralizing responses. PLoS One 8, e72205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Law M., Cardoso R. M., Wilson I. A., Burton D. R. (2007) Antigenic and immunogenic study of membrane-proximal external region-grafted gp120 antigens by a DNA prime-protein boost immunization strategy. J. Virol. 81, 4272–4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Coëffier E., Clément J. M., Cussac V., Khodaei-Boorane N., Jehanno M., Rojas M., Dridi A., Latour M., El Habib R., Barré-Sinoussi F., Hofnung M., Leclerc C. (2000) Antigenicity and immunogenicity of the HIV-1 gp41 epitope ELDKWA inserted into permissive sites of the MalE protein. Vaccine 19, 684–693 [DOI] [PubMed] [Google Scholar]
- 81. Liang X., Munshi S., Shendure J., Mark G., 3rd, Davies M. E., Freed D. C., Montefiori D. C., Shiver J. W. (1999) Epitope insertion into variable loops of HIV-1 gp120 as a potential means to improve immunogenicity of viral envelope protein. Vaccine 17, 2862–2872 [DOI] [PubMed] [Google Scholar]
- 82. Mantis N. J., Kozlowski P. A., Mielcarz D. W., Weissenhorn W., Neutra M. R. (2001) Immunization of mice with recombinant gp41 in a systemic prime/mucosal boost protocol induces HIV-1-specific serum IgG and secretory IgA antibodies. Vaccine 19, 3990–4001 [DOI] [PubMed] [Google Scholar]
- 83. Hinz A., Schoehn G., Quendler H., Hulsik D. L., Stiegler G., Katinger H., Seaman M. S., Montefiori D., Weissenhorn W. (2009) Characterization of a trimeric MPER containing HIV-1 gp41 antigen. Virology 390, 221–227 [DOI] [PubMed] [Google Scholar]
- 84. Behrendt R., Fiebig U., Kurth R., Denner J. (2012) Induction of antibodies binding to the membrane proximal external region of gp36 of HIV-2. Intervirology 55, 252–256 [DOI] [PubMed] [Google Scholar]
- 85. Dennison S. M., Sutherland L. L., Jaeger F. H., Anasti K. M., Parks R., Stewart S., Bowman C., Xia S. M., Zhang R., Shen X., Scearce R. M., Ofek G., Yang Y., Kwong P. D., Santra S., Liao H. X., Tomaras G., Letvin N. L., Chen B., Alam S. M., Haynes B. F. (2011) Induction of antibodies in rhesus macaques that recognize a fusion-intermediate conformation of HIV-1 gp41. PLoS One 6, e27824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lutje Hulsik D., Liu Y. Y., Strokappe N. M., Battella S., El Khattabi M., McCoy L. E., Sabin C., Hinz A., Hock M., Macheboeuf P., Bonvin A. M., Langedijk J. P., Davis D., Forsman Quigley A., Aasa-Chapman M. M., Seaman M. S., Ramos A., Poignard P., Favier A., Simorre J. P., Weiss R. A., Verrips C. T., Weissenhorn W., Rutten L. (2013) A gp41 MPER-specific llama VHH requires a hydrophobic CDR3 for neutralization but not for antigen recognition. PLoS Pathog. 9, e1003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Samygina V. R., Sokolov A. V., Bourenkov G., Petoukhov M. V., Pulina M. O., Zakharova E. T., Vasilyev V. B., Bartunik H., Svergun D. I. (2013) Ceruloplasmin: macromolecular assemblies with iron-containing acute phase proteins. PLoS One 8, e67145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang J., Tong P., Lu L., Zhou L., Xu L., Jiang S., Chen Y. H. (2011) HIV-1 gp41 core with exposed membrane-proximal external region inducing broad HIV-1 neutralizing antibodies. PLoS One 6, e18233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Srivastava I. K., Stamatatos L., Legg H., Kan E., Fong A., Coates S. R., Leung L., Wininger M., Donnelly J. J., Ulmer J. B., Barnett S. W. (2002) Purification and characterization of oligomeric envelope glycoprotein from a primary R5 subtype B human immunodeficiency virus. J. Virol. 76, 2835–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Weissenhorn W., Calder L. J., Dessen A., Laue T., Skehel J. J., Wiley D. C. (1997) Assembly of a rod-shaped chimera of a trimeric GCN4 zipper and the HIV-1 gp41 ectodomain expressed in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 94, 6065–6069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Frey G., Chen J., Rits-Volloch S., Freeman M. M., Zolla-Pazner S., Chen B. (2010) Distinct conformational states of HIV-1 gp41 are recognized by neutralizing and non-neutralizing antibodies. Nat. Struct. Mol. Biol. 17, 1486–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Konarev P. V., Petoukhov M. V., Volkov V. V., Svergun D. I. (2006) ATSAS 2.1, a program package for small-angle scattering data analysis. J. Appl. Crystallogr. 39, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Putnam C. D., Hammel M., Hura G. L., Tainer J. A. (2007) X-ray solution scattering (SAXS) combined with crystallography and computation: defining accurate macromolecular structures, conformations and assemblies in solution. Q. Rev. Biophys. 40, 191–285 [DOI] [PubMed] [Google Scholar]
- 94. Konarev P. V., Volkov V. V., Sokolova A. V., Koch M. H. J., Svergun D. I. (2003) PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J. Appl. Crystallogr. 36, 1277–1282 [Google Scholar]
- 95. Svergun D. I. (1992) Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 25, 495–503 [Google Scholar]
- 96. Svergun D. I. (1999) Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys. J. 76, 2879–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Brügger B., Glass B., Haberkant P., Leibrecht I., Wieland F. T., Kräusslich H. G. (2006) The HIV lipidome: a raft with an unusual composition. Proc. Natl. Acad. Sci. U.S.A. 103, 2641–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lai R. P., Seaman M. S., Tonks P., Wegmann F., Seilly D. J., Frost S. D., LaBranche C. C., Montefiori D. C., Dey A. K., Srivastava I. K., Sattentau Q., Barnett S. W., Heeney J. L. (2012) Mixed adjuvant formulations reveal a new combination that elicit antibody response comparable to Freund's adjuvants. PLoS One 7, e35083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Freudenberg M. A., Fomsgaard A., Mitov I., Galanos C. (1989) ELISA for antibodies to lipid A, lipopolysaccharides and other hydrophobic antigens. Infection 17, 322–328 [DOI] [PubMed] [Google Scholar]
- 100. Li M., Gao F., Mascola J. R., Stamatatos L., Polonis V. R., Koutsoukos M., Voss G., Goepfert P., Gilbert P., Greene K. M., Bilska M., Kothe D. L., Salazar-Gonzalez J. F., Wei X., Decker J. M., Hahn B. H., Montefiori D. C. (2005) Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79, 10108–10125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Edmonds T. G., Ding H., Yuan X., Wei Q., Smith K. S., Conway J. A., Wieczorek L., Brown B., Polonis V., West J. T., Montefiori D. C., Kappes J. C., Ochsenbauer C. (2010) Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology 408, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Weissenhorn W., Wharton S. A., Calder L. J., Earl P. L., Moss B., Aliprandis E., Skehel J. J., Wiley D. C. (1996) The ectodomain of HIV-1 env subunit gp41 forms a soluble, α-helical, rod-like oligomer in the absence of gp120 and the N-terminal fusion peptide. EMBO J. 15, 1507–1514 [PMC free article] [PubMed] [Google Scholar]
- 103. Cole K. S., Rowles J. L., Jagerski B. A., Murphey-Corb M., Unangst T., Clements J. E., Robinson J., Wyand M. S., Desrosiers R. C., Montelaro R. C. (1997) Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J. Virol. 71, 5069–5079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Weissenhorn W., Hinz A., Gaudin Y. (2007) Virus membrane fusion. FEBS Lett. 581, 2150–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Reardon P. N., Sage H., Dennison S. M., Martin J. W., Donald B. R., Alam S. M., Haynes B. F., Spicer L. D. (2014) Structure of an HIV-1-neutralizing antibody target, the lipid-bound gp41 envelope membrane proximal region trimer. Proc. Natl. Acad. Sci. U.S.A. 111, 1391–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Liu J., Bartesaghi A., Borgnia M. J., Sapiro G., Subramaniam S. (2008) Molecular architecture of native HIV-1 gp120 trimers. Nature 455, 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Meyerson J. R., Tran E. E., Kuybeda O., Chen W., Dimitrov D. S., Gorlani A., Verrips T., Lifson J. D., Subramaniam S. (2013) Molecular structures of trimeric HIV-1 Env in complex with small antibody derivatives. Proc. Natl. Acad. Sci. U.S.A. 110, 513–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Julien J. P., Bryson S., Nieva J. L., Pai E. F. (2008) Structural details of HIV-1 recognition by the broadly neutralizing monoclonal antibody 2F5: epitope conformation, antigen-recognition loop mobility, and anion-binding site. J. Mol. Biol. 384, 377–392 [DOI] [PubMed] [Google Scholar]
- 109. Scherer E. M., Zwick M. B., Teyton L., Burton D. R. (2007) Difficulties in eliciting broadly neutralizing anti-HIV antibodies are not explained by cardiolipin autoreactivity. AIDS 21, 2131–2139 [DOI] [PubMed] [Google Scholar]
- 110. Buiting A. M., van Rooijen N., Claassen E. (1992) Liposomes as antigen carriers and adjuvants in vivo. Res. Immunol. 143, 541–548 [DOI] [PubMed] [Google Scholar]
- 111. Fortin A., Thérien H. M. (1993) Mechanism of liposome adjuvanticity: an in vivo approach. Immunobiology 188, 316–322 [DOI] [PubMed] [Google Scholar]
- 112. Dey A. K., Burke B., Sun Y., Hartog K., Heeney J. L., Montefiori D., Srivastava I. K., Barnett S. W. (2012) Use of a polyanionic carbomer, Carbopol971P, in combination with MF59, improves antibody responses to HIV-1 envelope glycoprotein. Vaccine 30, 2749–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Binley J. M., Wrin T., Korber B., Zwick M. B., Wang M., Chappey C., Stiegler G., Kunert R., Zolla-Pazner S., Katinger H., Petropoulos C. J., Burton D. R. (2004) Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78, 13232–13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Choudhry V., Zhang M. Y., Harris I., Sidorov I. A., Vu B., Dimitrov A. S., Fouts T., Dimitrov D. S. (2006) Increased efficacy of HIV-1 neutralization by antibodies at low CCR5 surface concentration. Biochem. Biophys. Res. Commun. 348, 1107–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bonsignori M., Moody M. A., Parks R. J., Holl T. M., Kelsoe G., Hicks C. B., Vandergrift N., Tomaras G. D., Haynes B. F. (2009) HIV-1 envelope induces memory B cell responses that correlate with plasma antibody levels after envelope gp120 protein vaccination or HIV-1 infection. J. Immunol. 183, 2708–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pastori C., Tudor D., Diomede L., Drillet A. S., Jegerlehner A., Rohn T. A., Bomsel M., Lopalco L. (2012) Virus like particle based strategy to elicit HIV-protective antibodies to the α-helical regions of gp41. Virology 431, 1–11 [DOI] [PubMed] [Google Scholar]
- 117. Dawood R., Benjelloun F., Pin J. J., Kone A., Chanut B., Jospin F., Lucht F., Verrier B., Moog C., Genin C., Paul S. (2013) Generation of HIV-1 potent and broad neutralizing antibodies by immunization with postfusion HR1/HR2 complex. AIDS 27, 717–730 [DOI] [PubMed] [Google Scholar]