Abstract
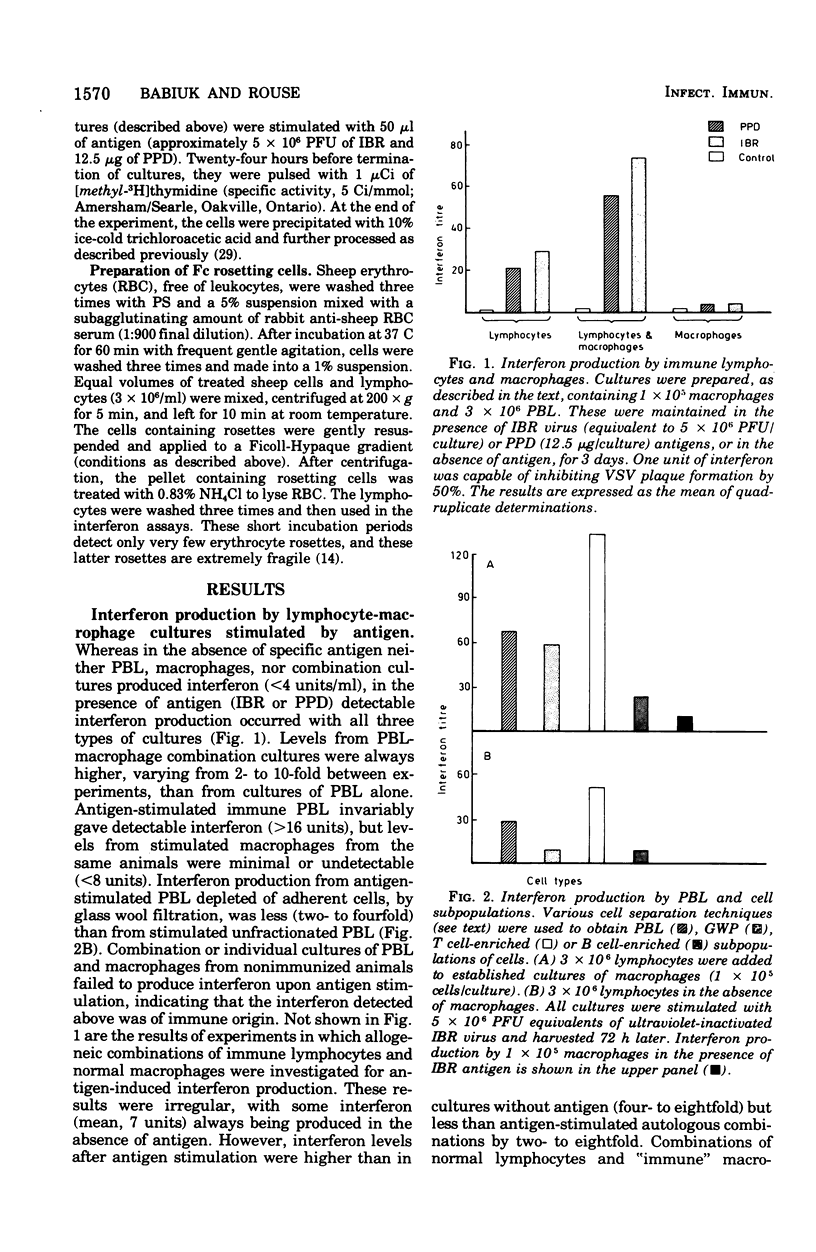
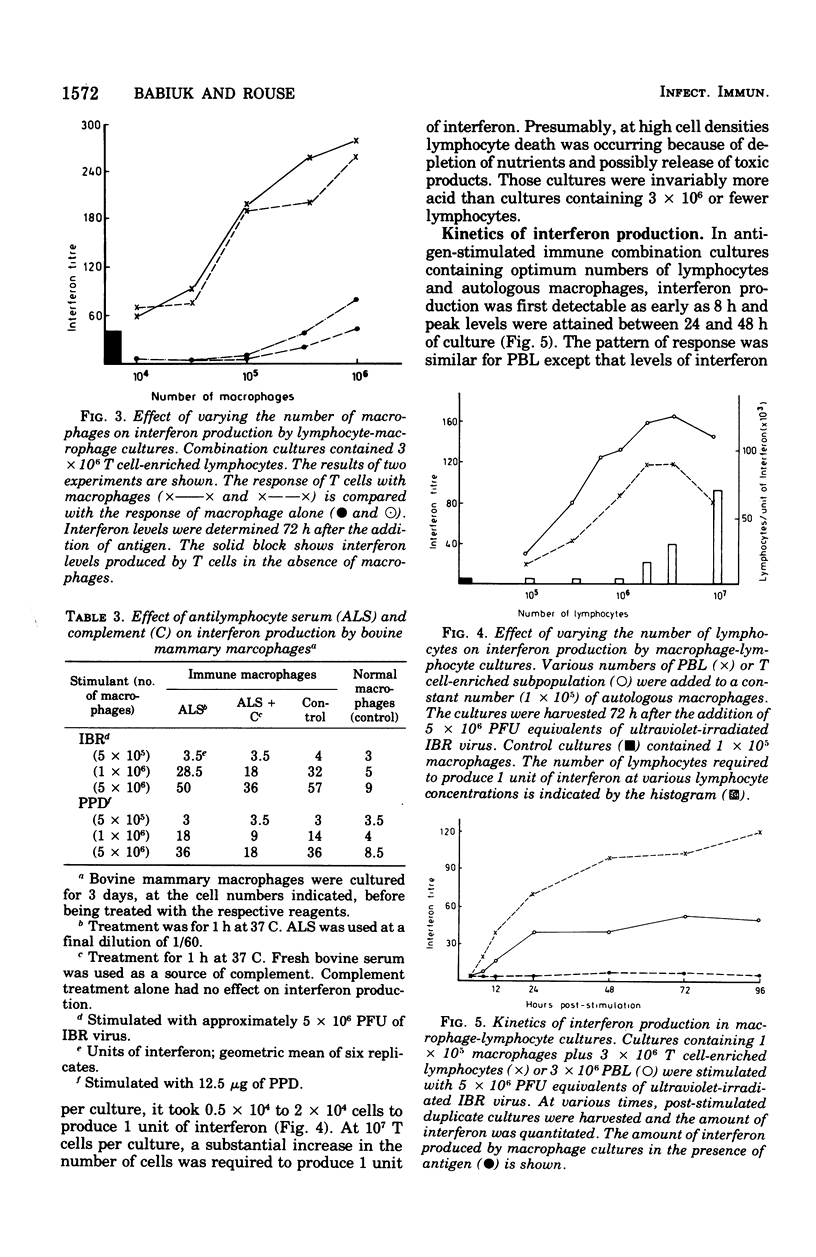
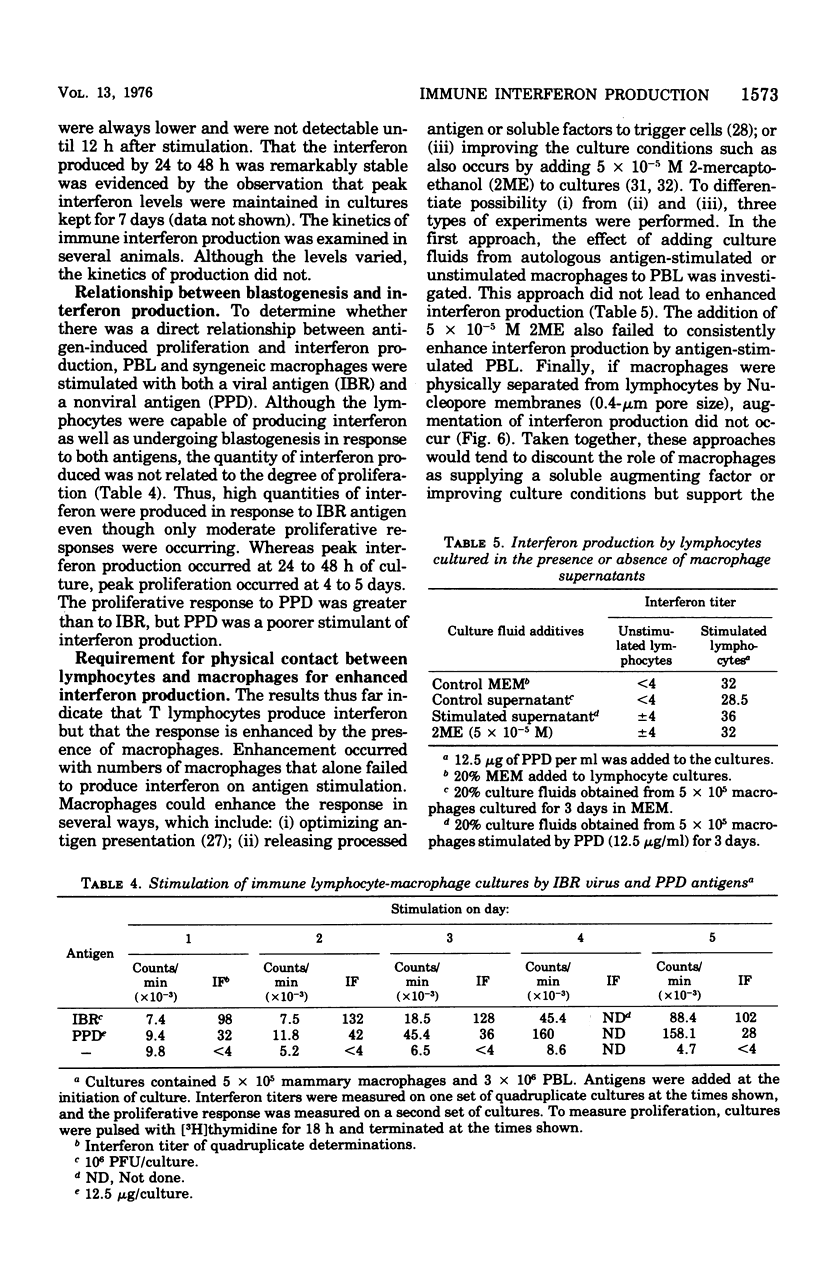
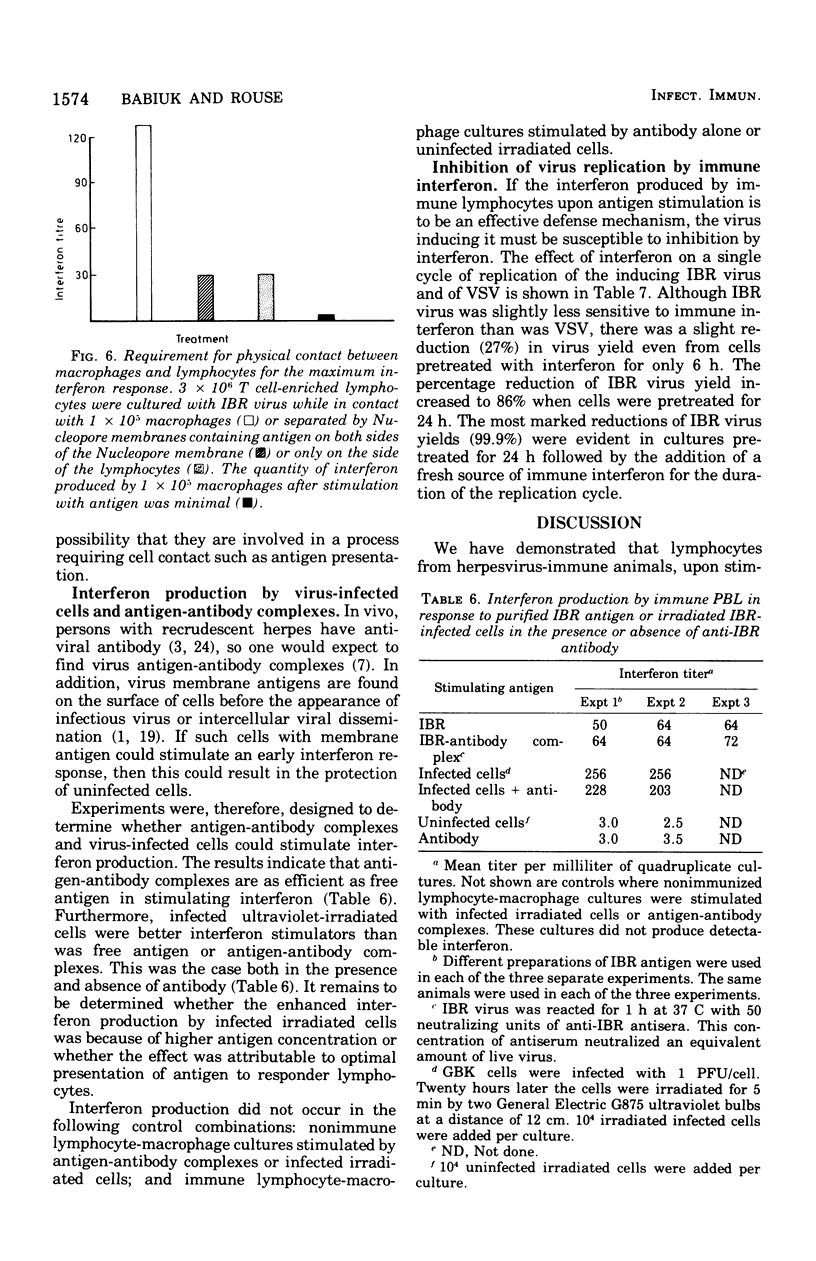
Bovine peripheral blood lymphocytes (PBL) obtained from infectious bovine rhinotracheitis (IBR) virus- and tuberculin-immunized animals produced large quantities of interferon within 24 h of in vitro stimulation by IBR and purified protein derivative antigens. Separation of PBL into populations enriched in T lymphocytes or B lymphocyte provided the antigen-specific step for immune interferon production. A 2- to 10-fold increase in interferon occurred when lymphocytes were combined with autologous macrophages. Although macrophages, even if treated with antilymphocyte serum to remove any contaminating lymphocytes, could produce some interferon, the augmented interferon produced by macrophage-lypmhocyte cultures was not dmpocytes. Direct physical contact between macrophages and lymphocytes was required for the production of enhanced levels of interferon. Antigen-antibody complexes of irradiated virus-infected cells in the presence of antibody were as efficient or better at stimulating interferon than was free antigen. Because IBR virus was inhibited by interferon levels stimulated in cultures by IBR antigen, it was suggested that the local production of interferon by immune cells might play a similar role in curtailing virus dissemination in vivo, thus leading to recovery from disease.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Wardley R. C., Rouse B. T. Defense mechanisms against bovine herpesvirus: relationship of virus-host cell events to susceptibility to antibody-complement cell lysis. Infect Immun. 1975 Nov;12(5):958–963. doi: 10.1128/iai.12.5.958-963.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur B. R., Merigan T. C. Mechanism of the suppressive effect of interferon on antibody synthesis in vivo. J Immunol. 1975 Apr;114(4):1323–1328. [PubMed] [Google Scholar]
- Cesario T. C., Poland J. D., Wulff H., Chin T. D., Wenner H. A. Six years experience with herpes simplex virus in a children's home. Am J Epidemiol. 1969 Nov;90(5):416–422. doi: 10.1093/oxfordjournals.aje.a121087. [DOI] [PubMed] [Google Scholar]
- Chen C., Hirsch J. G. The effects of mercaptoethanol and of peritoneal macrophages on the antibody-forming capacity of nonadherent mouse spleen cells in vitro. J Exp Med. 1972 Sep 1;136(3):604–617. doi: 10.1084/jem.136.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N., Mosier D. E. Cell-cell interactions in antibody production. Prog Allergy. 1972;16:40–80. [PubMed] [Google Scholar]
- Cline M. J., Swett V. C. The interaction of human monocytes and lymphocytes. J Exp Med. 1968 Dec 1;128(6):1309–1325. doi: 10.1084/jem.128.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C. A., LeGoff S. G. Shedding of infectious virus/antibody complexes from vesicular lesions of patients with recurrent herpes labialis. Lancet. 1975 Sep 20;2(7934):524–528. doi: 10.1016/s0140-6736(75)90896-x. [DOI] [PubMed] [Google Scholar]
- Docherty J. J., Chopan M. The latent herpes simplex virus. Bacteriol Rev. 1974 Dec;38(4):337–355. doi: 10.1128/br.38.4.337-355.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. B., Cline M. J., Merigan T. C. PPD-stimulated interferon: in vitro macrophage-lymphocyte interaction in the production of a mediator of cellular immunity. Cell Immunol. 1971 Dec;2(6):602–613. doi: 10.1016/0008-8749(71)90008-6. [DOI] [PubMed] [Google Scholar]
- Epstein L. B., Stevens D. A., Merigan T. C. Selective increase in lymphocyte interferon response to vaccinia antigen after revaccination. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2632–2636. doi: 10.1073/pnas.69.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R., Alexander P. Mechanism of immunologically specific killing of tumour cells by macrophages. Nature. 1972 Mar 24;236(5343):168–170. doi: 10.1038/236168a0. [DOI] [PubMed] [Google Scholar]
- FISHMAN M., ADLER F. L. Antibody formation initiated in vitro. II. Antibody synthesis in x-irradiated recipients of diffusion chambers containing nucleic acid derived from macrophages incubated with antigen. J Exp Med. 1963 Apr 1;117:595–602. doi: 10.1084/jem.117.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcoff R. Some properties of virus and immune-induced human lymphocyte interferons. J Gen Virol. 1972 Aug;16(2):251–253. doi: 10.1099/0022-1317-16-2-251. [DOI] [PubMed] [Google Scholar]
- HOTCHIN J. E. Use of methyl cellulose gel as a substitute for agar in tissue-culture overlays. Nature. 1955 Feb 19;175(4451):352–352. doi: 10.1038/175352a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The function and interrelationships of T-cell receptors, Ir genes and other histocompatibility gene products. Transplant Rev. 1975;22:175–195. doi: 10.1111/j.1600-065x.1975.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Lodmell D. L., Niwa A., Hayashi K., Notkins A. L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J Exp Med. 1973 Mar 1;137(3):706–720. doi: 10.1084/jem.137.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodmell D. L., Notkins A. L. Cellular immunity to herpes simplex virus mediated by interferon. J Exp Med. 1974 Sep 1;140(3):764–778. doi: 10.1084/jem.140.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias A. J., Roizman B. Infection with herpes-simplex viruses 1 and 2. 1. N Engl J Med. 1973 Sep 27;289(13):667–674. doi: 10.1056/NEJM197309272891305. [DOI] [PubMed] [Google Scholar]
- Pierce C. W., Kapp J. A., Wood D. D., Benacerraf B. Immune responses in vitro. X. Functions of macrophages. J Immunol. 1974 Mar;112(3):1181–1189. [PubMed] [Google Scholar]
- Rager-Zisman B., Bloom B. R. Immunological destruction of herpes simplex virus I infected cells. Nature. 1974 Oct 11;251(5475):542–543. doi: 10.1038/251542a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen L. E., Jordan G. W., Stevens D. A., Merigan T. C. Lymphocyte interferon production and transformation after Herpes simplex infections in humans. J Immunol. 1974 Feb;112(2):728–736. [PubMed] [Google Scholar]
- Rosenberg G. L., Snyderman R., Notkins A. L. Production of chemotactic factor and lymphotoxin by human leukocytes stimulated with Herpes simplex virus. Infect Immun. 1974 Jul;10(1):111–115. doi: 10.1128/iai.10.1.111-115.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. S., Lipsky P. E., Shevach E. M. Macrophage-lymphocyte interaction and antigen recognition. Fed Proc. 1975 Jul;34(8):1743–1748. [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Host defense mechanisms against infectious bovine rhinotracheitis virus. II. Inhibition of viral plaque formation by immune peripheral blood lymphocytes. Cell Immunol. 1975 May;17(1):43–56. doi: 10.1016/s0008-8749(75)80005-0. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Host defense mechanisms against infectious bovine rhinotracheitis virus: in vitro stimulation of sensitized lymphocytes by virus antigen. Infect Immun. 1974 Oct;10(4):681–687. doi: 10.1128/iai.10.4.681-687.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Host responses to infectious bovine rhinotracheitis virus. III. Isolation and immunologic activities of bovine T lymphocytes. J Immunol. 1974 Nov;113(5):1391–1398. [PubMed] [Google Scholar]
- Rouse B. T., Wardley R. C., Babiuk L. A. Antibody-dependent cell-mediated cytotoxicity in cows: comparison of effector cell activity against heterologous erthrocyte and herpesvirus-infected bovine target cells. Infect Immun. 1976 May;13(5):1433–1441. doi: 10.1128/iai.13.5.1433-1441.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Wardley R. C., Babiuk L. A. The role of antibody dependent cytotoxicity in recovery from herpesvirus infections. Cell Immunol. 1976 Mar 1;22(1):182–186. doi: 10.1016/0008-8749(76)90019-8. [DOI] [PubMed] [Google Scholar]
- Russell A. S. Cell-mediated immunity to herpes simplex virus in man. J Infect Dis. 1974 Feb;129(2):142–146. doi: 10.1093/infdis/129.2.142. [DOI] [PubMed] [Google Scholar]
- Russell A. S., Percy J. S., Kovithavongs T. Cell-mediated immunity to Herpes simplex in humans: lymphocyte cytotoxicity measured by 51-Cr release from infected cells. Infect Immun. 1975 Feb;11(2):355–359. doi: 10.1128/iai.11.2.355-359.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer G. M. Cell-mediated cytotoxicity to trinitrophenyl-modified syngeneic lymphocytes. Eur J Immunol. 1974 Aug;4(8):527–533. doi: 10.1002/eji.1830040802. [DOI] [PubMed] [Google Scholar]
- Shevach E. M., Rosenthal A. S. Function of macrophages in antigen recognition by guinea pig T lymphocytes. II. Role of the macrophage in the regulation of genetic control of the immune response. J Exp Med. 1973 Nov 1;138(5):1213–1229. doi: 10.1084/jem.138.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S. L., Nahmias A. J., Starr S. E., Wood P. A., McFarlin D. E. Detection of cell-dependent cytotoxic antibody to cells infected with herpes simplex virus. Nature. 1974 Sep 27;251(5473):350–352. doi: 10.1038/251350a0. [DOI] [PubMed] [Google Scholar]
- Shortman K., Palmer J. The requirement for macrophages in the in vitro immune response. Cell Immunol. 1971 Oct;2(5):399–410. doi: 10.1016/0008-8749(71)90051-7. [DOI] [PubMed] [Google Scholar]
- Starr S. E., Karatela S. A., Shore S. L., Duffey A., Nahmias A. J. Stimulation of human lymphocytes by Herpes simplex virus antigens. Infect Immun. 1975 Jan;11(1):109–112. doi: 10.1128/iai.11.1.109-112.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R. W., Vincent M. M., Hensen S. A., Fuccillo D. A., Chapa I. A., Canales L. Cellular immune responses to Herpes simplex virus type 1 in recurrent herpes labialis: in vitro blastogenesis and cytotoxicity to infected cell line. J Infect Dis. 1975 May;131(5):528–534. doi: 10.1093/infdis/131.5.528. [DOI] [PubMed] [Google Scholar]
- TERASAKI P. I., MCCLELLAND J. D. MICRODROPLET ASSAY OF HUMAN SERUM CYTOTOXINS. Nature. 1964 Dec 5;204:998–1000. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- Trueblood M. S., Manjara J. Response of bovine viruses to interferon. Cornell Vet. 1972 Jan;62(1):3–12. [PubMed] [Google Scholar]
- Unanue E. R., Calderon J. Evaluation of the role of macrophages in immune induction. Fed Proc. 1975 Jul;34(8):1737–1742. [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]
- Valle M. J., Bobrove A. M., Strober S., Merigan T. C. Immune specific production of interferon by human T cells in combined macrophage-lymphocyte cultures in response to Herpes simplex antigen. J Immunol. 1975 Jan;114(1 Pt 2):435–441. [PubMed] [Google Scholar]
- Valle M. J., Jordan G. W., Haahr S., Merigan T. C. Characteristics of immune interferon produced by human lymphocyte cultures compared to other human interferons. J Immunol. 1975 Jul;115(1):230–233. [PubMed] [Google Scholar]
- Wardley R. C., Rouse B. T., Babiuk L. A. The mammary gland of the ox: a convenient source for the repeated collection of neutrophils and macrophages. J Reticuloendothel Soc. 1976 Jan;19(1):29–36. [PubMed] [Google Scholar]
- Wilton J. M., Ivanyi L., Lehner T. Cell-mediated immunity in Herpesvirus hominis infections. Br Med J. 1972 Mar 18;1(5802):723–726. doi: 10.1136/bmj.1.5802.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Salvin S. B. Production and properties of migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. J Immunol. 1973 Dec;111(6):1914–1922. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]


