Abstract
Due to their excess surface free energy and structural instabilities, nanoparticles exhibit interesting physical and chemical properties. There has been an ever-growing interest in investigating these properties, driven by the desire to further miniaturize electronic devices, develop new functional materials and catalysts. Here, the intriguing question of how diffusion evolves in a single nanoparticle is investigated by measuring the spatial and temporal variations of the diffracted coherent X-ray intensity during copper diffusion into a gold nanocrystal. Dislocation loops formed from the insertion of single layer of extra atoms between neighbouring gold host lattice planes are detected. Au-Cu alloy channels are found to penetrate the nanocrystal due to the differential diffusion rate along different directions. With the advent of higher brilliance sources and free-electron-lasers, Bragg Coherent X-ray Diffraction Imaging can play an important role in unveiling atomic behaviours in three dimensions for nanomaterials during various fundamental processes.
Diffusion is a transport phenomenon whereby the random thermally-activated movement of atoms results in their net transport and intermixing without the bulk motion1. How diffusion happens on the atomic scale in a solid is a fundamental issue and often the crucial step in many processes such as doping silicon wafers to make semiconductor devices2,3, transport of thermal neutrons in nuclear power reactors4 and permeation of atoms through membranes5,6. Rates of numerous chemical reactions are dependent on how fast diffusion can bring reactants together or deliver them to reaction sites on enzymes or other catalysts7,8,9. Accurate models and understanding of these diffusion mechanisms will aid the ability to control the synthesis of materials with desired properties, and to design and fabricate devices with improved performance10,11,12,13. In particular, as functional materials and devices with reduced dimensions have been receiving great attention in recent years, it is imperative to develop techniques suited for performing diffusion characterization at the nano- and micro-scale level14,15,16,17.
Conventional methods such as mechanical and sputter profiling18, secondary ion mass spectrometry19,20 and electron microprobe analysis21,22, investigate diffusion inside solids by measuring the profile of the diffusing element in a solid material and yield a macroscopic quantity-the diffusion coefficient. On the microscopic level, however, it is difficult to directly observe the relatively slow process of the diffusion of atoms in solids because the timescales are too long for experimental techniques that depend on high energy resolution, such as inelastic scattering23. Quasi-elastic neutron scattering24,25 and Mössbauer spectroscopy26,27 can address this issue only for a small number of isotopes and very fast diffusivities. These conventional methods have been applied to investigate diffusion behaviours for samples of nanoparticle ensembles, to obtain the diffusion coefficient17,28,29,30. X-ray photon correlation spectroscopy31,32 based on the diffuse scattering of coherent X-rays has recently been used to elucidate the dynamic behaviour of atoms as a function of their neighbourhood. In these work, it is necessary to integrate and average the signals of a number of structures or from a relatively large area, and sample heterogeneity can cause ambiguity in the analysis results. Transmission electron microscopy (TEM) can resolve the behaviour of individual nanoparticle33,34,35. However, for TEM the the destructive thinning used in sample preparation can be a severe limitation. Also for diffusion studies, the illumination of the nano-structured sample by the electron beam can completely alter the diffusion behaviour33. To date, investigating the diffusion properties for individual nanoparticle with a three-dimension capability is still difficult.
Here we demonstrate a successful investigation of the three-dimensional diffusion behaviours at the atomic level in a single gold nanocrystal with copper as the diffusing species, using Bragg Coherent X-ray Diffraction Imaging36,37,38,39 (BCDI) in an ultrahigh vacuum environment. BCDI recovers the complex electron density of the samples with picometre sensitivity and it is able to detect very small lattice distortion within nanocrystals. Indeed, the 'picometre sensitivity' refers to lattice displacement which is much finer than the atomic spacing. The spatial resolution of the retrieved image itself is around 20 nm in these experiments37,39. The ability to achieve picometre sensitivity from a lower resolution measurement is due to the fact that the strain fields are long-ranged, therefore atomic level defects, such as lattice distortion and dislocation loops, can be identified by their strain signatures. BCDI can currently examine nanocrystals as small as a few tens of nanometers, limited by the diffracted signal obtained over a reasonable data collection time. This will be improved with the new radiation source, i.e. free-electron-laser, becoming available and the developments in beamline optics. In BCDI, the recovered electron density comprises the amplitude, corresponding to the crystal morphology, and the phase, which is related to the displacement field of the atoms from the ideal crystal lattice. The choice of Bragg peak determines the direction in which the lattice distortions can be visualised. Results obtained from non-coplanar Bragg peaks can be combined to recover the full strain tensor38. In this study, experiments were performed in an ultrahigh vacuum chamber with a base pressure of 5 × 10−9 mbar, at beamline I07 of the Diamond Light Source. Time dependent diffraction patterns were recorded by rocking the gold nanocrystal around its (111) Bragg diffraction peak, during copper diffusion at 300°C, and collated to form three-dimensional data sets. By performing reconstruction from the diffraction data using a guided approach40 and partial coherent correction41, three-dimensional images of the gold nanocrystal, including both the phase and amplitude, at different times of diffusion, were obtained and analysed to reveal the evolution of the diffusion of copper.
Results
Figures 1a–f show the patterns, for the gold (111) diffraction at the copper diffusion time of 0, 2, 4, 6, 8, and 10 hrs, respectively, as a cut-off XZ plane view at the centre position of the three dimensional diffraction rocking curve (All the cut-off images presented in this paper are XZ views unless stated otherwise.). One can see that before copper diffusion the diffraction patterns show the modulated fringes resulting from the coherent illumination and the finite size of the nanocrystal. The fringes present a fairly symmetric pattern, and are most prominent in the facetted directions of the nanocrystal. After 4 hours of diffusion, the diffraction patterns (Fig. 1c–f) become asymmetric with distorted fringes, which is due to the inhomogeneous lattice distortions resulting from the copper atoms diffused into the gold lattice. The phase diagram of Au-Cu system42 predicts that at 300°C, there are three stable Au-Cu alloys, CuAu3, CuAu and Cu3Au. In our experiment, after 10 hours of Cu diffusion, we find clear powder ring traces for Cu3Au, indicating that one of the Au-Cu alloys was formed due to the copper diffusion into the gold host lattice. The scale bars of Fig. 1a–f show that the maximum intensity of the diffraction pattern gradually decreases as the diffusion proceeds. This trend can be seen more clearly in Fig. 1g, which presents the time dependence of the maximum diffraction intensity and the integrated diffraction intensity, and both of them decrease monotonously. The diffraction intensity decrease is due to the diffusion-related decrease of the gold nanocrystal volume, with some gold crystalline parts gradually being turned into Au-Cu alloy.
Figure 1.
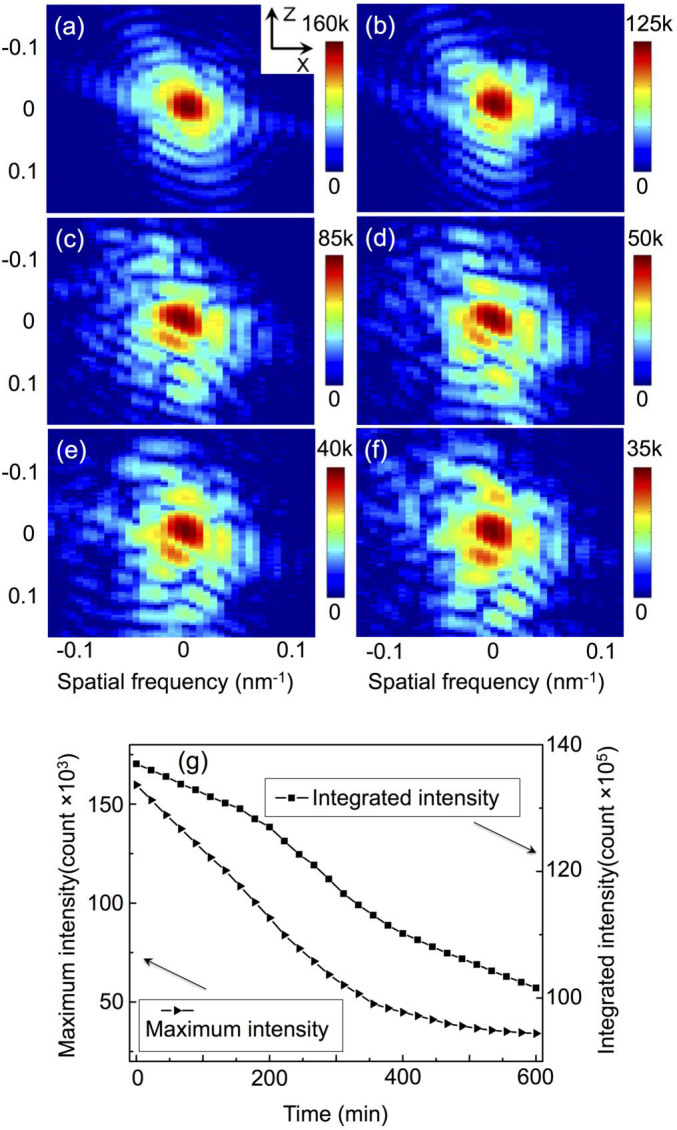
The patterns at the centre position of the three dimensional rocking curve of the gold (111) diffraction at the copper diffusion time of 0 hr (a), 2 hrs (b), 4 hrs (c), 6 hrs (d), 8 hrs (e), and 10 hrs (f), with a XZ plane view; the time dependence of the maximum diffraction intensity and the integrated diffraction intensity from the BCDI measurements (g).
Figures 2a–f are the reconstructed phase images, as cut-off views at the centre of the gold nanocrystal at diffusion times of 0, 2, 4, 6, 8, and 10 hrs, respectively. The phase is a projection of the lattice distortions onto the (111) Q vector (shown as the arrow in Fig. 2b). It can be seen that before diffusion, the gold nanocrystal is almost strain free inside, with small phases in the range of ±0.5 rad (here a phase of 2π represents a displacement equal to 0.235 nm, the gold lattice spacing in the {111} direction). These phase features are located at the near surface regions where two facets meet, which we refer to as the crystal ‘edges'. The ‘edges' are curved regions on the equilibrium crystal shape43 where steps tend to be in motion and fluctuating44, and the weak phase features near the ‘edges' are due to strains generally associated with the shape of nanoparticles45. With diffusion proceeding, strain increases significantly inside the nanocrystal. After 6 hrs diffusion (Fig. 2d), there are three characteristic dipole-shaped phase structures, two near the top and one near the left bottom of the crystal. Each of them shows a clear phase wrap where the phase changes abruptly from -π to π over a small volume, indicating atom displacements are equal to the lattice spacing. These are attributed to dislocation loops46,47, consisting of edge dislocations formed from the insertion of a single plane of atoms over a small region between two adjacent lattice planes, and the relaxation of the strain field surrounding it. Dislocation loops have recently been identified in silicon nanowires upon mechanical bending, where the extra plane consists of strained silicon atoms48. In the case here, the extra plane of atoms may consist of either gold or copper, or the mixture of the two. The phase agrees well with model calculations of dislocation loop as illustrated in Supplementary Information 2. It can also be seen from Fig. 2 that the dislocation loops are formed near the crystal ‘edges' where facets meet. These are apparently the locations where defects are likely to be generated, corroborated by the observation noted above that there were already some shape-induced strains at these positions before diffusion. This means it is easier for the diffusion to proceed at positions where strains (defects) are already present.
Figure 2. The reconstructed phase images, as cut-off view at the centre of the gold nanocrystal at diffusion time of 0 hr (a), 2 hrs (b), 4 hrs (c), 6 hrs (d), 8 hrs (e), and 10 hrs (f).
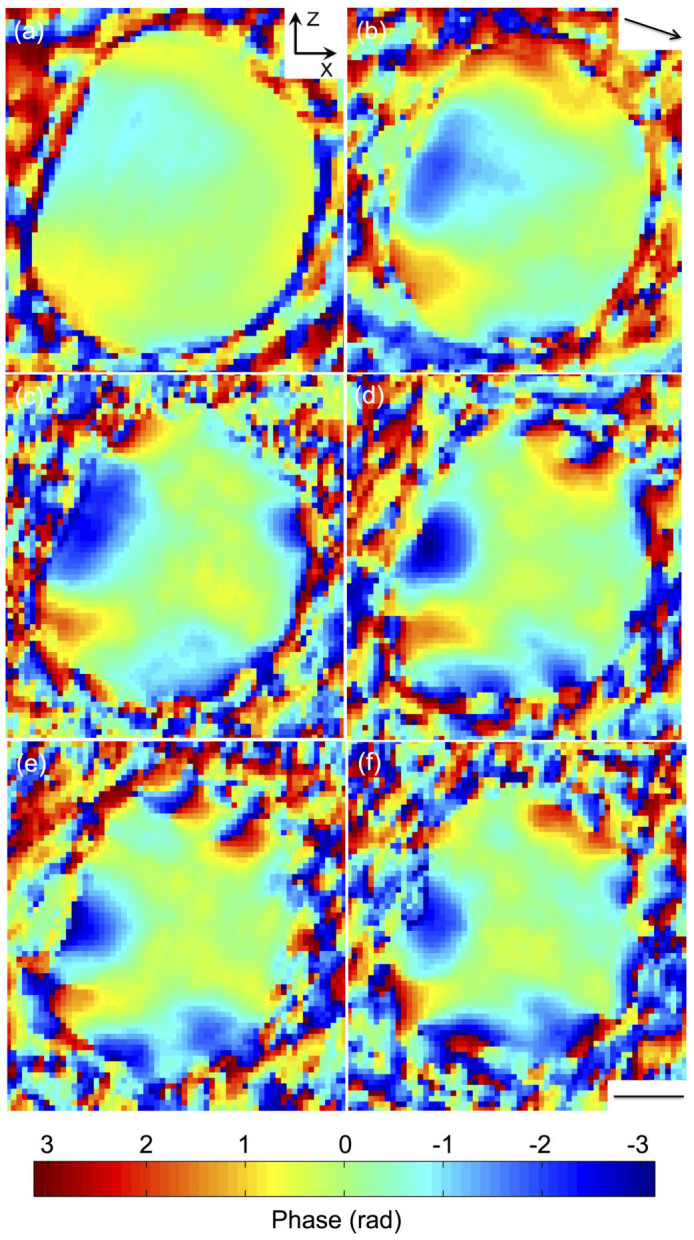
Note that the phase becomes very noisy outside the crystal, where the amplitude of the complex density drops to zero. Arrow in (b) shows the Q-vector direction. Scale bar = 100 nm.
Figures 3a–f are the images of the reconstructed amplitude, as cut-off view at the centre of the gold nanocrystal, at times of 0, 2, 4, 6, 8, and 10 hrs of copper diffusion, respectively. It can be seen that after 2 hrs diffusion, the ‘edges' have changed significantly, with the top ‘edge' having disappeared and become an almost flat surface (Fig. 3b). As copper diffuses further into the gold nanocrystal (Fig. 3c–f), the crystalline volume appears to shrink. This is because the BCDI image is derived from the signal of the gold nanocrystal part only, through its (111) Bragg peak. The reconstructions indicate that the copper diffusion removes crystalline material by alloy formation: the affected material either has a different lattice constant or is reoriented. Thus the regions where it is easier for copper atoms to diffuse into become transformed and no longer contribute to the Au (111) diffraction signal during the temporal evolution.
Figure 3. The reconstructed amplitude images, as cut-off view at the centre of the gold nanocrystal at diffusion time of 0 hr (a), 2 hrs (b), 4 hrs (c), 6 hrs (d), 8 hrs (e), and 10 hrs (f).
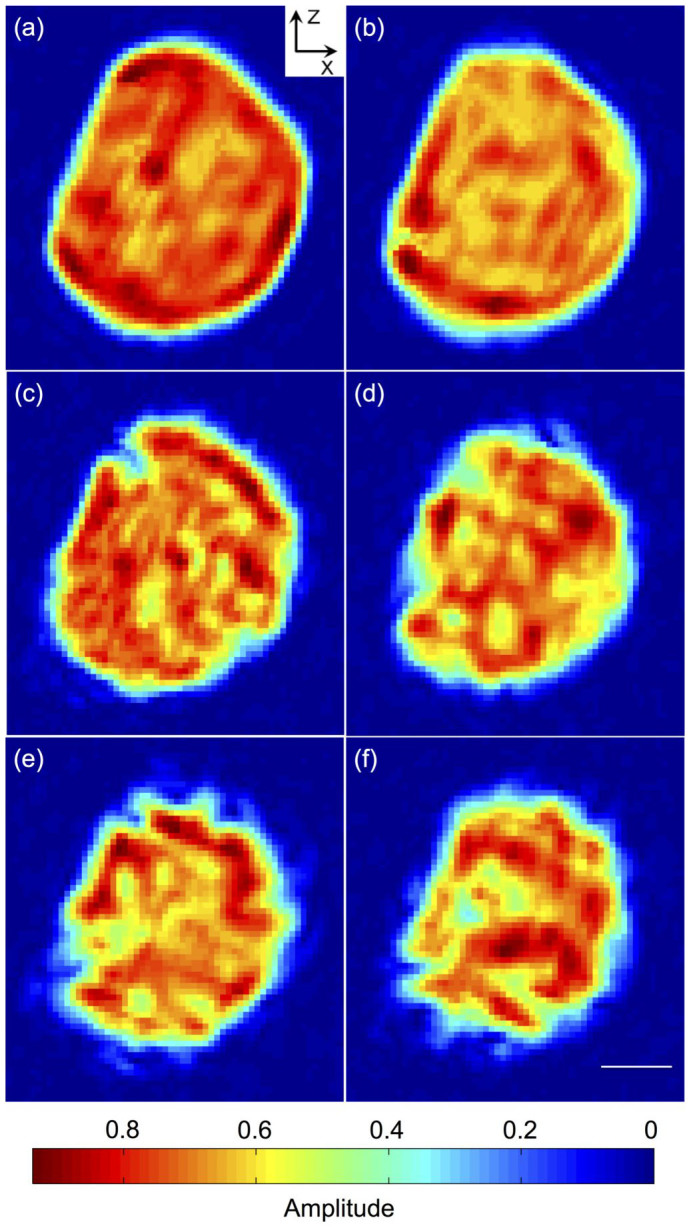
Scale bar = 100 nm.
After 4 hrs diffusion, some ‘channels' started to open in the crystal (Fig. 3c). For the same reasons given above, the channels are not likely to be empty, but contain alloy of mis-oriented crystalline material from the conversion of Au to Au-Cu alloy. The number of channels and their length increases with time, up to 40 nm observed after 6 hrs (Fig. 3d) and longer than 100 nm after 8 hrs diffusion (Fig. 3e). Interestingly Fig. 3c–f shows a surface modulation with the crystal surface becoming rougher following diffusion. All these observations suggest that the diffusion is not an isotropic process and the effective differential diffusion rate along different directions results in the formation of channels and rougher surface. The time dependence of the nanocrystal's volume and surface area are discussed further in Supplementary Information 3 where both show roughly linear relationships.
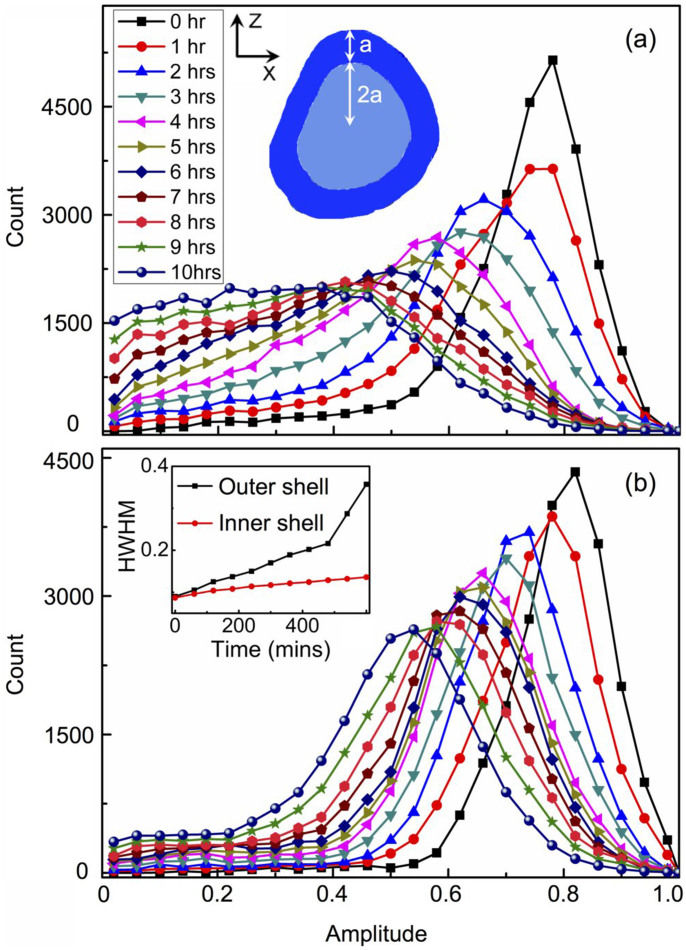
To gain more insight into the diffusion process, the gold nanocrystal is defined as a two-shell structure in three dimensions and Fig. 4a inset shows the schematic XZ view of the shells layout as the cut-off image at the centre of the crystal. The thickness ratio of the outer and inner shell is defined to be 1:2 along the radial direction. To access the fraction of crystal remaining in each shell we examine the distribution of reconstructed amplitude values in each shell. Figure 4a shows the amplitude histogram for the outer shell at different diffusion times. For a perfect crystal the amplitude distribution would be a sharp function, but crystal samples have defects and there are Gaussian-distributed errors in the measurements, so it is reasonable that we observe a Gaussian-like distribution. With diffusion proceeding, the peak value of the amplitude histogram decreases and the distribution become broader, after 6 hrs the histogram no longer resembles a Gaussian shape. This continuous increase in the variation of the density is attributed to the deterioration of crystallinity resulted from the diffusion.
Figure 4. The time dependence of the amplitude histograms for (a) the outer shell and (b) the inner shell of the nanocrystal.
The inset of (a) shows the layout of the shells as a XZ plane view. The time dependence of the HWHM of the amplitude histograms for both shells is inset of (b).
The time series of the amplitude histogram for the inner shell (Fig. 4b) shows that although it follows a similar trend as that of the outer shell, it preserves a Gaussian-like distribution after 10 hrs diffusion. The time dependence of the width (half width at half maximum) of the amplitude histograms is extracted and plotted in the inset of Fig. 4b for both shells. The width increases with diffusion time for both shells, and for the inner shell it increases at a slower pace. These results indicate a higher crystallinity and uniformity in the inner shell. This is due to the diffusion starting from the crystal surface, so the deterioration of crystallinity and broadening of the density variation are more substantial in the outer shell. Further analysis of the amplitude histogram yields a diffusion coefficient D of 8.7 × 10−9 μm2/s, for copper in the gold nanocrystal at 300°C, as described in Supplementary Information 4. It is almost two orders of magnitude larger than that for the bulk sample50.
The enhanced diffusion effects have been observed previously from nanocrystal ensembles29,30,33. In-situ TEM was applied to study copper diffusion in gold nanocrystal ensemble with average size of 10 nm at room temperature, and the diffusion coefficient was estimated to be at least 9 orders of magnitude higher than the bulk sample33. Other examples including silver in gold nanocrystal ensemble34, and gold in semiconductor Ag2S nanocrystal ensemble35 etc., show diffusion coefficients up to 16 orders of magnitude higher than the bulk30. One of the possible reasons for the much higher diffusion coefficient for nanocrystal samples may be due to the higher surface energy. The free energy of activation for atoms to jump from the surface into vacancies inside the nanocrystal is therefore reduced with respect to atoms jumping from lattice sites into vacancies as in bulk diffusion. Here our sample is a single particle with a diameter of 400 nm, the surface to volume ratio is not as high as in the above referenced cases, so the enhancement effect is still significant but not as pronounced. It is worth noting that for the nanocrystal ensembles, the larger free volume in grain boundaries can play a significant role in the diffusion and further complicate the process30. In our case one can see from Fig. 3 that on the left side of the nanocrystal, the evolution of the gold lattice seems to be slower than the other sides. This could be due to the grain boundary effect where the measured nanocrystal is in contact with another crystal or the substrate.
Discussion
Revealing the diffusion processes in a solid on the atomic scale is fundamentally important and difficult. Atomic diffusion in crystalline materials is additionally complicated by the competition of lattice effects which can enhance or impede the process in a directional way. Surface and interface effects can also modify the rates, adding to the complexity of the outcomes for a polycrystalline, heterogeneous or nanostructured material. Our investigation reported here reveals the formation of transient dislocation loops during the diffusion of copper into a single gold nanocrystal, resulted from extra single layer of atoms inserting between the host lattice planes. The results show that it is possible to study the diffusion process on the atomic scale with BCDI. The high spatial resolution of this method allows direct investigation of the size, shape, lattice, surface and interface effects for individual nanocrystal, without having to integrate and average the signals from a large area or ensemble of structures which is necessary in many other methods and can result in ambiguity. Another advantage is that we obtain three-dimensional images for the system under investigation. In the current case, the amplitude images show dynamically how copper invades the gold nanocrystal, starting preferentially along the crystal edges, and the interesting surface modulation due to channel formation along certain directions inside the nanocrystal. With the inherent lattice-distortion sensitivity as demonstrated in this work, and higher brilliance synchrotron sources and free electron lasers planned in the near future, BCDI has the potential to become the method of choice for investigating atomic behaviours in three dimensions for nanomaterials, providing unprecedented insights in many fundamental processes such as diffusion, phase transition, catalysis and imaging phonons (through lattice dynamics39) in semiconductors.
Methods
The sample was prepared by annealing a 20 nm thick gold layer on silicon substrate at 980°C and dewetted to form gold nanocrystals with typical size in the range of 200 nm ~ 1 μm, separated by a few hundred nanometers. The measurements were performed on the Huber diffractometer in experimental hutch 2 of beamline I07, Diamond Light Source. The sample was loaded in the beamline's ultra high vacuum chamber at a base pressure of 5 × 10−9 mbar. The X-ray wavelength of 0.133 nm was selected by a Si-(111) double crystal monochromator. A set of JJ slits was used to select a partial coherent beam with a size of 30 μm in horizontal and 40 μm in vertical. The sample holder was resistively heated to 300°C, and the temperature was stable to 0.1°C. When the temperature reached 300°C and stabilized for another 6 hours, the copper evaporation and BCDI measurements were started. A graphite Knudsen-cell thermal evaporator source was heated to 850°C to evaporate copper onto the gold nanocrystal continuously during the measurements. The vapour pressure was 3.0 × 10−6 mbar, and the evaporator had a 1 mm aperture at 200 mm from the sample. The total deposited copper is estimated to be around 80 nm thick according to the Hertz-Knudsen equation. A Pilatus 100 k area detector (Dectris, 487 × 195 pixels, 172 × 172 μm2 pixel size) was positioned at a distance of 1.0 m from the sample, sufficient for fulfilling the oversampling requirement needed for phasing. In-situ time dependent diffraction patterns were collected by rocking the sample around the gold (111) diffraction, through an angular range of 0.5° in increments of 0.005°, and subsequently stacking the two dimensional patterns to form three dimensional data sets. Three dimensional reconstructions were performed using a guided approach40 and partial coherent correction41. 10 random starts were initiated with each of them being subjected to 10 iterations of error reduction (ER) fitting followed by 170 iterations of hybrid-input output49 (HIO) phasing and then a further 20 iterations of ER. After this first generation, the best fitting was selected and used to generate a further 10 new iterates (Supplementary Information 1). This process was repeated for a total of 6 generations with the final solution averaged from the 2 best fittings.
Author Contributions
G.X., J.C. and I.R. designed the experiments. G.X. and J.C. prepared the samples. G.X., J.C., I.R., C.N. and J.R. carried out the BCDI measurements. G.X., J.C and I.R. performed data analysis and interpretation. G.X. wrote the manuscript, with contributions and comments from all authors.
Supplementary Material
Supplementary Information
Supplementary Video 1_Temporal diffraction pattern - xz view
Supplementary Video 2_Reconstructed amplitude after 10hrs diffusion - xz view
Supplementary Video 3_3D rendering of the Au nanocrystal after 6 hours Cu diffusion
Acknowledgments
This project is supported by the European Research Council as an FP7 Advanced grant ‘Nanosculpture', code 227711.
References
- Jean Philibert, One and a half century of diffusion: Fick, Einstein, before and beyond. Diffusion Fundamentals 2, 1.1–1.10 (2005). [Google Scholar]
- Windl W., Bunea M. M., Stumpf R., Dunham S. T. & Masquelier M. P. First-principles study of boron diffusion in silicon. Phys. Rev. Lett. 83, 4345–4348 (1999). [Google Scholar]
- Mirabella S., Salvador D., Napolitani E., Bruno E. & Priolo F. Mechanisms of boron diffusion in silicon and germanium. J. Appl. Phys. 113, 031101 (2013). [Google Scholar]
- Stacey W. M. Nuclear reactor physics. (Wiley., 2007). [Google Scholar]
- Dolan M. D., McLennan K. G. & Way J. D. Diffusion of atomic hydrogen through V-Ni alloy membranes under nondilute conditions. J. Phys. Chem. C 116, 1512–1518 (2011). [Google Scholar]
- Nair R. R., Wu H. A., Jayaram P. N., Grigorieva I. V. & Geim A. K. Unimpeded permeation of water through helium-leak−tight graphene-based membranes. Science 335, 442–444 (2012). [DOI] [PubMed] [Google Scholar]
- Medema M. H., Breitling R., Bovenberg R. & Takano E. Exploiting plug-and-play synthetic biology for drug discovery and production in microorganisms. Nature Rev. Microbiol. 9, 131–137 (2011). [DOI] [PubMed] [Google Scholar]
- Zebda A. et al. Mediatorless high-power glucose biofuel cells based on compressed carbon nanotube-enzyme electrodes. Nat. Commun. 2, 370 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai S. et al. Role of subsurface diffusion and Ostwald ripening in catalyst formation for single-walled carbon nanotube forest growth. J. Am. Chem. Soc. 134, 2148–2153 (2012). [DOI] [PubMed] [Google Scholar]
- Peng Z., Yan Z., Sun Z. & Tour J. M. Direct growth of bilayer graphene on SiO2 substrates by carbon diffusion through nickel. ACS Nano 5, 8241–8247 (2011). [DOI] [PubMed] [Google Scholar]
- Yao J. et al. Contra-diffusion synthesis of ZIF-8 films on a polymer substrate. Chem. Commun. 47, 2559–2561 (2011). [DOI] [PubMed] [Google Scholar]
- Ching C. T. S., Connolly P. A novel diffusion cell ideal for the study of membrane extraction/permeation processes and for device/sensor development. Sensor. Actuat. B-Chem. 129, 30–34 (2008). [Google Scholar]
- Lan X. X. et al. The interface inter-diffusion induced enhancement of the charge-trapping capability in HfO2/Al2O3 multilayered memory devices. Appl. Phys. Lett. 103, 192905 (2013). [Google Scholar]
- Norris D. J., Efros A. L. & Erwin S. C. Doped nanocrystals. Science 319, 1776–1779 (2008). [DOI] [PubMed] [Google Scholar]
- Balke N. et al. Nanoscale mapping of ion diffusion in a lithium-ion battery cathode. Nat. Nanotechnol. 5, 749–754 (2010). [DOI] [PubMed] [Google Scholar]
- Holmberg V. C., Helps J. R., Mkhoyan K. A. & Norris D. J. Imaging impurities in semiconductor nanostructures. Chem. Mater. 25, 1332–1350 (2013). [Google Scholar]
- Vlaskin V. A., Barrows C. J., Erickson C. S. & Gamelin D. R. Nanocrystal diffusion doping. J. Am. Chem. Soc. 135, 14380–14389 (2013). [DOI] [PubMed] [Google Scholar]
- Oswald S. & Baunack S. Comparison of depth profiling techniques using ion sputtering from the practical point of view. Thin Solid Films 425, 9–19 (2003). [Google Scholar]
- Lee J. K. W., Williams I. S. & Ellis D. J. Pb, U and Th diffusion in natural zircon. Nature 390, 159–162 (1997). [Google Scholar]
- McPhail D. S. Applications of secondary ion mass spectrometry (SIMS) in Materials Science. J. Mater. Sci. 41, 873–903 (2006). [Google Scholar]
- Zhao J. C. A combinatorial approach for structural materials. Adv. Eng. Mater. 3, 143–147 (2001). [Google Scholar]
- Spandler C., O'Neill H. C. & Kamenetsky V. S. Survival times of anomalous melt inclusions from element diffusion in olivine and chromite. Nature 447, 303–306 (2007). [DOI] [PubMed] [Google Scholar]
- Kargl F. & Meyer A. Inelastic neutron scattering on sodium aluminosilicate melts: sodium diffusion and intermediate range order. Chem. Geol. 213, 165–172 (2004). [Google Scholar]
- Rosenbach N. et al. Quasi-elastic neutron scattering and molecular dynamics study of methane diffusion in metal organic frameworks MIL-47(V) and MIL-53(Cr). Angew. Chem. Int. Ed. 47, 6611–6615 (2008). [DOI] [PubMed] [Google Scholar]
- Yamada T. et al. Quasi-elastic neutron scattering studies on dynamics of water confined in nanoporous copper rubeanate hydrates. J. Phys. Chem. B 115, 13563–13569 (2011). [DOI] [PubMed] [Google Scholar]
- Rennhofer M. et al. Self-diffusion of iron in L10-ordered FePt thin films. Phys. Rev. B 74, 104301 (2006). [Google Scholar]
- Gupta A., Kumar D. & Phatak V. Asymmetric diffusion at the interfaces in Fe/Si multilayers. Phys. Rev. B 81, 155402 (2010). [Google Scholar]
- Brossmann U., Knöner G., Schaefer H. E. & Würschum R. Oxygen diffusion in nanocrystalline ZrO2. Rev. Adv. Mater. Sci. 6, 7–11 (2004). [Google Scholar]
- Chen Y. & Schuh C. A. Contribution of triple junctions to the diffusion anomaly in nanocrystalline materials. Scripta Mater. 57, 253–256 (2007). [Google Scholar]
- Würschum R., Herth S. & Brossmann U. Diffusion in nanocrystalline metals and alloys–a status report. Adv. Eng. Mater. 5, 365–372 (2003). [Google Scholar]
- Leitner M., Sepiol B., Stadler L. M., Pfau B. & Gero Vogl. Atomic diffusion studied with coherent X-rays. Nature Mater. 8, 717–720 (2009). [DOI] [PubMed] [Google Scholar]
- Stana M., Leitner M., Ross M. & Sepiol B. Studies of atomic diffusion in Ni–Pt solid solution by X-ray photon correlation spectroscopy. J. Phys.: Condens. Matter 25, 065401 (2013). [DOI] [PubMed] [Google Scholar]
- Mori H., Komatsu M., Takeda K. & Fujita H. Spontaneous alloying of copper into gold atom clusters. Phil. Mag. Lett. 63, 173–178 (1991). [Google Scholar]
- Shibata T. et al. Size-dependent spontaneous alloying of Au-Ag nanoparticles. J. Am. Chem. Soc. 124, 11989–11996 (2002). [DOI] [PubMed] [Google Scholar]
- Yang J. & Ying J. Y. Diffusion of gold from the inner core to the surface of Ag2S nanocrystals. J. Am. Chem. Soc. 132, 2114–2115 (2010). [DOI] [PubMed] [Google Scholar]
- Pfeifer M. A., Williams G. J., Vartanyants I. A., Harder R. & Robinson I. K. Three-dimensional mapping of a deformation field inside a nanocrystal. Nature 442, 63–66 (2006). [DOI] [PubMed] [Google Scholar]
- Robinson I. & Harder R. Coherent diffraction imaging of strains on the nanoscale. Nature Mater. 8, 291–298 (2009). [DOI] [PubMed] [Google Scholar]
- Newton M., Leake S., Harder R. & Robinson I. K. Three-dimensional imaging of strain in a single ZnO nanorod. Nature Mater. 9, 120–124 (2010). [DOI] [PubMed] [Google Scholar]
- Clark J. et al. Ultrafast three-dimensional imaging of lattice dynamics in individual gold nanocrystals. Science 341, 56–59 (2013). [DOI] [PubMed] [Google Scholar]
- Chen C. C., Miao J., Wang C. W. & Lee T. K. Application of optimization technique to noncrystalline x-ray diffraction microscopy: Guided hybrid input-output method. Phys. Rev. B 76, 064113 (2007). [Google Scholar]
- Clark J. N., Huang X., Harder R. & Robinson I. K. High-resolution three-dimensional partially coherent diffraction imaging. Nature Commun. 3, 993 (2012). [DOI] [PubMed] [Google Scholar]
- Ozolins V., Wolverton C. & Zunger A. Cu-Au, Ag-Au, Cu-Ag, and Ni-Au intermetallics: First-principles study of temperature-composition phase diagrams and structures. Phys. Rev. B 57, 6427–6443 (1998). [Google Scholar]
- Wortis M. Chemistry and physics of solid surfaces. VII [Vanselow, R. & Howe, R. (ed.)] (Springer, Berlin, 1988). [Google Scholar]
- Kuipers L., Hoogeman M. S. & Frenken J. W. M. Step dynamics on Au(110) studied with a high-temperature, high-speed scanning tunneling microscope. Phys. Rev. Lett. 71, 3517–3520 (1993). [DOI] [PubMed] [Google Scholar]
- Robinson I. Nanoparticle Structure by Coherent X-ray Diffraction. J. Phys. Soc. Jpn. 82, 021012 (2013). [Google Scholar]
- Hull D. & Bacon D. J. Introduction to Dislocations. (Butterworth-Heinemann., 2001). [Google Scholar]
- Hytch M. J., Putaux J. L. & Penisson J. M. Measurement of the displacement field of dislocations to 0.03 angstrom by electron microscopy. Nature 423, 270–273 (2003). [DOI] [PubMed] [Google Scholar]
- Shi X. et al. Mechanical breakdown of bent silicon nanowires imaged by coherent x-ray diffraction. New J. Phys. 15,123007 (2013). [Google Scholar]
- Fienup J. R. Phase retrieval algorithms: a comparison. Appl. Opt. 21, 2758–2769 (1982). [DOI] [PubMed] [Google Scholar]
- Gale W. F. & Totemeier T. C. Smithells metals reference book. (Butterworth-Heinemann., 2003). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Video 1_Temporal diffraction pattern - xz view
Supplementary Video 2_Reconstructed amplitude after 10hrs diffusion - xz view
Supplementary Video 3_3D rendering of the Au nanocrystal after 6 hours Cu diffusion