Abstract
Iron (Fe) deficiency can represent a serious constraint on crop growth and productivity. A number of members of the bHLH transcription factor family are known to be involved in the plant Fe deficiency response. Plants have evolved two distinct uptake strategies when challenged by Fe deficiency: dicotyledonous and non-graminaceous species rely mostly on a reduction strategy regulated by bHLH transcription factors, whereas rice relies on a chelation strategy, also regulated by bHLH transcription factors. CmbHLH1, a bHLH transcription factor which is localized within the nucleus, was isolated from chrysanthemum. Its transcription was up-regulated both by Fe deficiency and by the exogenous application of abscisic acid. The roots of transgenic chrysanthemum plants in which CmbHLH1 was up-regulated were better able than those of the wild type chrysanthemum cultivar to acidify their immediate external environment by enhancing the transcription of the H+-ATPase encoding gene CmHA. However, there was no effect of the transgene on the efficiency of uptake of either manganese or zinc. Here, Chrysanthemum CmbHLH1 contributed to Fe uptake via H+-ATPase mediated acidification of the rhizosphere. ABA may be positively involved in the process.
Iron (Fe), as an essential element for plant growth and development, is involved in both the respiratory electron transport chain and chlorophyll synthesis. Fe deficiency, especially where the soil pH is non-acidic, is well recognized to represent a constraint on crop growth and productivity. The plant response to Fe deficiency and the regulation of cellular Fe homeostasis have been intensively studied.
Plants have evolved two distinct strategies to cope with Fe deficiency. In the reduction strategy, root plasma membrane H+-ATPases act to acidify the rhizosphere, thereby increasing the local solubility of Fe1. Fe3+ is then reduced to Fe2+ by a membrane-bound ferric-chelate reductase enzyme such as AtFRO2, and this appears to be a major rate-limiting step in Fe uptake2. Subsequently, Fe2+ is transported into the root by plasma-membrane divalent cation transporters such as IRT1, a plasma-membrane divalent cation transporter regulated by the bHLH (basic helix-loop-helix) transcription factor FIT3,4,5. The alternative strategy involves chelation, here, the mechanism operates through the release of phytosiderophores, which avidly bind Fe3+ 6,7. The chelated complex is transported into the roots via YSL transporters such as the product of OsYSL15, a rice gene which is strongly induced by Fe deficiency throughout the root8.
Certain bHLH-related transcription factors, for example OsIRO2, are Fe deficiency-inducible9. The promoter sequence of OsIRO2 harbors IDE Fe deficiency-responsive pathway cis-acting elements10. OsbHLH133 regulates the transport of Fe between the root and the shoot11. A number of bHLH transcription factors are also thought to be involved in the regulation of Fe uptake in A. thaliana roots exposed to Fe deficient growing conditions5,12. These include PYE, an Fe deficiency inducible transcription factor which is especially strongly expressed in the root pericycle. The targets of PYE are genes implicated in metal homeostasis12. PYE is not only a regulator of the chelation strategy, but may also play a role in the reduction strategy12.
Dimerization of bHLH plays a key role in its function. PYE dimerizes with certain bHLH homologs, notably ILR3 and bHLH115; the former is a component of metal ion-mediated auxin sensing in the A. thaliana root13. ILR3 may control metal homeostasis through the regulation of genes involved in Fe transport12. Peiffer et al. have demonstrated that the FIT/bHLH038 heterodimer induces Fe acquisition genes in soybean14. Interactions between the bHLH transcription factor FIT and EIN3/EIL1 also promote Fe acquisition, revealing a molecular connection between the regulation of Fe acquisition and ethylene signaling in A. thaliana.15.
Chrysanthemum is one of the most popular of ornamental species16. As for many plants, Fe is essential for its normal growth and development. A visible symptom of Fe deficiency in chrysanthemum is interveinal chlorosis on the leaf. As yet there is little information available regarding the regulation of Fe uptake in chrysanthemum. Here, we have identified CmbHLH1, a member of the bHLH family of transcription factors. Its sequence proved to be highly similar to that of AtILR3, and under Fe deficiency, its transgenic up-regulation improved the plant's ability to absorb Fe. The present paper represents the first report to show how a bHLH1 transcription factor regulates the Fe deficiency response of chrysanthemum.
Results
The CmbHLH1 gene in chrysanthemum cultivar ‘Jinba'
The CmbHLH1 full-length cDNA was isolated from chrysanthemum ‘Jinba' using primers derived from the chrysanthemum ESTs described by Chen et al.17, consisting of a 630 bp open reading frame, an 86 bp 5′-UTR and a 271 bp 3′-UTR. The predicted product of CmbHLH1 was a 210 residue polypeptide. The sequence contained a specific DNA binding/dimerization domain in its central region (positions 50–111), and an oligopeptide AAYPAAVAAA region (159–168) of low compositional complexity. A phylogenetic analysis based on established bHLH sequences showed that CmbHLH1 was highly similar to the A. thaliana protein ILR3 (NP_200279.1) (Fig. S1).
Subcellular localization of CmbHLH1
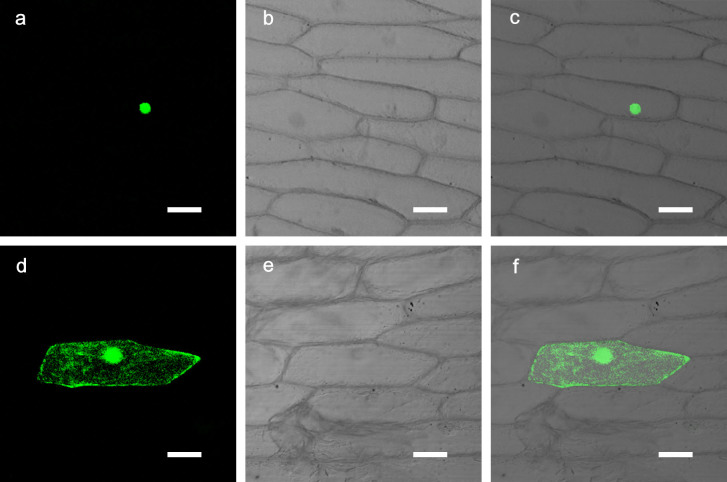
Based on the online software ProtComp v. 9.0 (www.softberry.com), CmbHLH1 was predicted to localize to the nucleus. A transient assay, involving the biolistic introduction of a CmbHLH1-GFP fusion into onion epidermal cells, showed that GFP was expressed largely in the nucleus (Fig. 1a–c). In contrast, in control cells transformed with GFP alone, the GFP signal was dispersed throughout the cell (Fig. 1d–f).
Figure 1. Subcellular localization of CmbHLH1 in onion epidermis cells.
(a–c) Cells transformed with 35S::CmbHLH1-GFP. (d–f) Control cells transformed with 35S::GFP. Images captured (a, d) under bright light to show cell morphology, and (b, e) against a dark field to display GFP fluorescence. The images in (c) and (f) are merged from, respectively, (a) and (b), and (d) and (e). Bars, 50 μm.
The effect of Fe deficiency and ABA (abscisic acid) treatment on the transcription of CmbHLH1
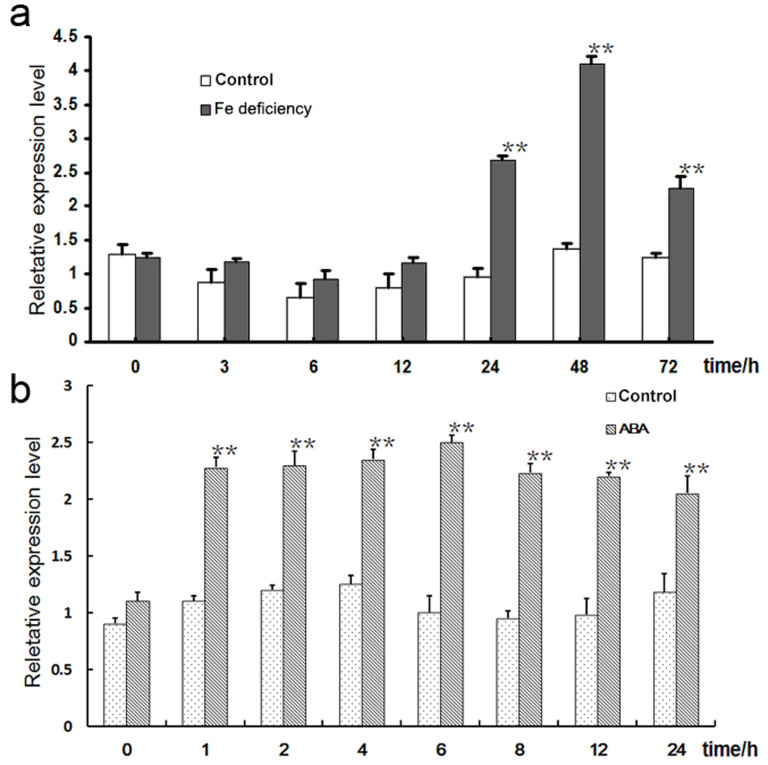
The qPCR analysis suggested that there was little evidence for any Fe deficiency induced up- or down-regulation of CmbHLH1 over the first 12 h, but that transcript abundance peaked by around 48 h (Fig. 2a). This was taken to indicate that CmbHLH1 may be involved in the regulation of genes active in Fe uptake. CmbHLH1 was also induced by ABA treatment (Fig. 2b), which suggested that ABA signaling may be involved in Fe uptake in chrysanthemum.
Figure 2. CmbHLH1 transcription in response to (a) Fe deficiency and (b) the application of exogenous ABA. **, difference significant at P < 0.01.
The responsiveness of CmbHLH1 promoter to Fe deficiency and ABA
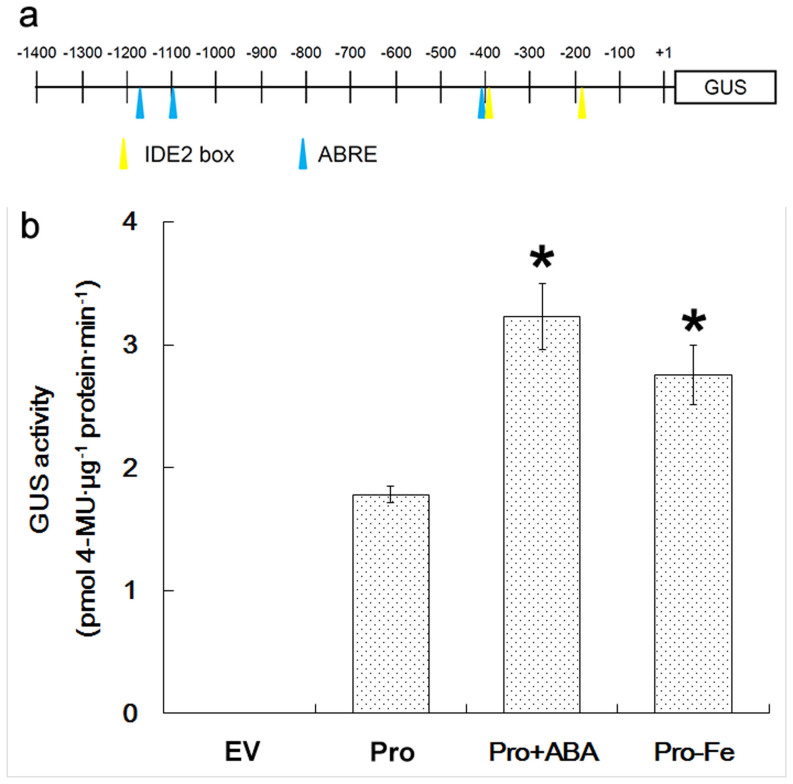
The CmbHLH1 promoter sequence harbored two IDE2 and three ABRE motifs (Fig. 3a). To experimentally confirm the responsiveness of the CmbHLH1 promoter to Fe deficiency and/or ABA, a CmbHLH1pro::GUS fusion was transiently transformed into tobacco leaves. GUS activity was significantly enhanced when the leaves were challenged by either Fe deficiency or ABA treatment (Fig. 3b).
Figure 3. The responsiveness of the CmbHLH1 promoter to Fe deficiency and ABA treatment.
(a) The pORE-CmbHLHpro::GUS construct. The IDE2 box and ABRE elements are highlighted. (b) GUS activity analysis of transgenic tobacco leaves in response to ABA treatment. EV: empty vector control, pro: pORE-CmbHLH1pro::GUS, +ABA: ABA treated plants, -Fe: plants subjected to Fe deficiency. *, difference significant at P < 0.05.
The ionic content of the root and its influence over the local rhizosphere pH
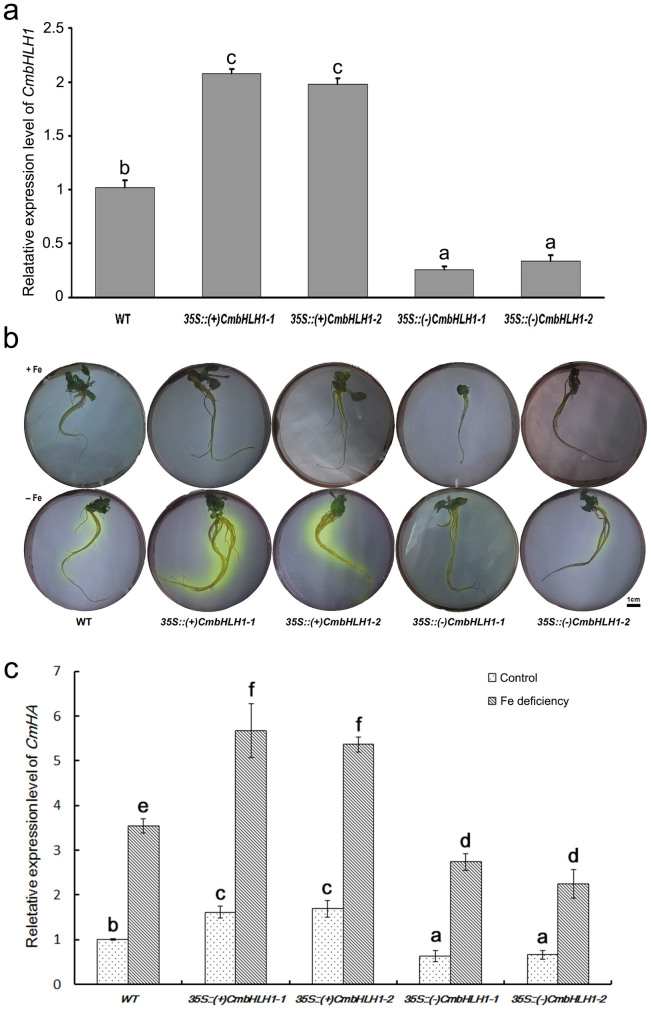
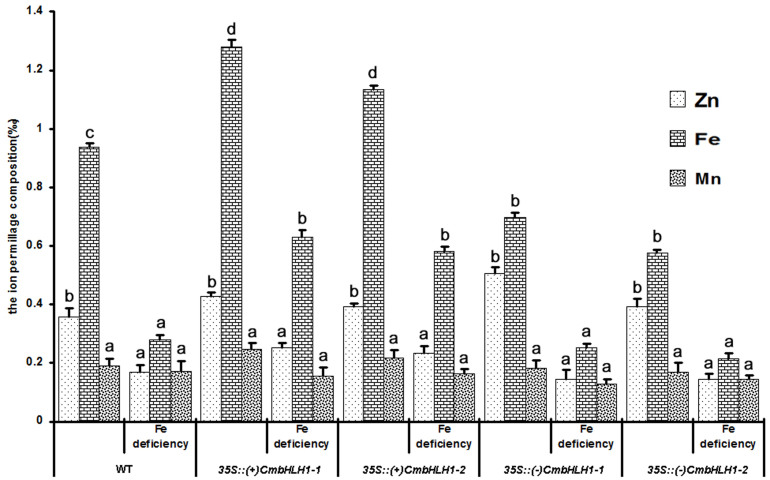
Two independent sense transgenic lines showing a high level of CmbHLH1 transcription, and similarly two independent antisense low level CmbHLH1 transcription transgenic lines, were selected (Fig. 4a). However no additional morphological alterations have been observed between sense, antisense transgenic plants and wild type (Fig. S2). After a three day exposure to Fe deficiency, a bromocresol purple based test showed that the sense transgenics were more capable of acidifying the external medium than were either the control or the antisense transgenics (Fig. 4b). Inspection of the transcript abundance of CmHA, a gene encoding a proton pump ATPase gene, showed that, compared to the control non-transgenic plants, this gene was up-regulated in the sense transgenics, and down-regulated in the antisense ones (Fig. 4c). The former, when exposed to Fe deficiency, accumulated roughly twice as much Fe as the non-transgenic control plants, while the antisense lines accumulated less Fe than the control (Fig. 5). There was no significant difference between the performance of the transgenics and the control plants with respect to either their Zn or their Mn content (Fig. 5). This observation suggested the involvement of CmbHLH1 in the acidification response, which may be the first step employed by chrysanthemum plants to reduce the rhizosphere pH and hence improve the plant's uptake of Fe.
Figure 4. The acidification capacity of CmbHLH1 transgenic plants and expression profiles of CmHA in CmbHLH1 transgenic plants subjected to Fe deficiency.
(a) CmbHLH1 transcription level in sense and antisense transgenic plants was evaluated in whole seedlings at three week old stage. (b) The acidification capacity of CmbHLH1 transgenic plants in Fe deficient growing conditions, as indicated by the bromocresol purple dye. The roots of two independent sense transformants were both able to reduce the local pH to <5.2. (c) The transcription of CmHA in CmbHLH1 transgenic plants subjected to Fe deficiency for 48 h. Values labeled with a different superscript differ from one another at P < 0.05.
Figure 5. Ion content in the roots of CmbHLH1 sense and antisense transgenic plants.
The sense transgenic lines contained more Fe than either the non-transformed control or the antisense transformant, while the content of Mn and Zn was unaffected by the presence of either transgene. Values labeled with a different superscript differ from one another at P < 0.05.
Discussion
Fe deficiency has been shown to up-regulate a number of A. thaliana bHLH transcription factors, including FIT, bHLH38/39 and PYE, all of which are involved in Fe import5,12,18. A. thaliana also possesses an FIT-independent pathway, in which Fe homeostasis is controlled by bHLH100 and bHLH10119. Here, we have shown that Fe deficiency induced the transcription of a bHLH gene in chrysanthemum. Alignment of its product with homologs from other species revealed a conserved (as yet unnamed) region at the C terminus, which in the A. thaliana protein ILR3 has been implicated as being essential for normal function13. CmbHLH1 activity clearly enhanced the ability of the roots to acidify their external environment (Fig. 4b), perhaps by promoting the release of plasma membrane H+-ATPases1, as implied by the up-regulation of CmHA in the CmbHLH1 sense transgenic chrysanthemum plants (Fig. 4c). A one unit reduction in pH can increase the solubility of Fe ions by 1,000 fold20. Under Fe deficient conditions, the A. thaliana reductase AtFRO2 (and similarly the pea enzyme PsFRO1) both reduce Fe3+ to Fe2+2, and these ions are then transported by plasma-membrane divalent cation transporters such as IRT13,4. The present data have shown that the transcription of CmFRO3 was not disturbed in any of the transgenic lines (Fig. S3a), even though the up-regulated CmbHLH1 transgenic plants were able to accumulate more Fe than the down-regulated ones (Fig. 5). The suggestion is that CmbHLH1, unlike PYE, acts as a positive regulator of Fe intake.
The CmbHLH1 promoter sequence harboured two IDE2 motifs (Fig. 3a). In barley, rice, tobacco and A. thaliana, IDE is associated with the induction of transcription of bHLH under Fe deficient conditions21. IDEF2, a rice transcription factor which binds to IDE2, is thought to regulate a number of genes responsive to Fe deficiency (for example, the two bHLH transcription factors OsIRO2 and OsIRO3), and particularly those implicated in Fe uptake and utilization during the initial response to stress10,21,22. In chrysanthemum, the presence of two IDE2 binding sites in the CmbHLH1 promoter may make a contribution towards the Fe-deficiency induced expression of CmbHLH1 (Fig. 3).
ABA is involved in a wide range of physiological processes in plants. An increase in ABA level was observed in cucumber roots under salt stress conditions, where increased ABA induced the expression of PM-H+-ATPase gene expression23. CmbHLH1 was inducible by exogenously applied ABA (Fig. 2), but the transgenic up-regulation of CmbHLH1 had no effect on the transcription of the ABA marker gene PP2C (Fig. S3b). We found PP2C was also induced by Fe deficiency (Fig. S4), the induction of PP2C was more quickly than CmbHLH1 (Fig. 2a). All these result suggest that CmbHLH1 may not regulate ABA signalling as a feedback, but instead that ABA increases H+-ATPase activity via the induction of CmbHLH1.
bHLH transcription factors regulate Fe uptake through their dimerization with certain other proteins. In A. thaliana, ILR3 interacts with BTS under Fe deficient conditions12, while in soybean, the formation of a FIT/bHLH038 heterodimer induces a set of Fe acquisition genes14. An interaction between FIT and EIN3/EIL1 is thought to promote Fe acquisition15. The possibility that CmbHLH1 needs to interact with other transcription factors or to self-dimerize in order to promote Fe intake is a topic of our ongoing research.
Conclusions
We have described a chrysanthemum bHLH type transcription factor gene which was up-regulated in the root both under conditions of Fe deficiency and by the provision of exogenous ABA. Transgenic chrysanthemum plants in which CmbHLH1 was up-regulated were better able to acidify their immediate root environment, and were more effective in taking up Fe when the medium was Fe deficient. The data suggested that ABA may be involved in the CmbHLH1-regulated absorption of Fe.
Methods
Plant materials and growing conditions
Seedlings of the chrysanthemum cultivar ‘Jinba' were obtained from the Germplasm Resource Preserving Centre, Nanjing Agricultural University, China. Plants were grown in a greenhouse where the day/night temperature was maintained at approximately 25°C/18°C and the relative humidity held at ~70%. The same cultivar was also used for the purpose of transformation by cultivating it on MS medium24.
Isolation of CmbHLH1 and its promoter
‘Jinba' roots were frozen in liquid nitrogen and stored at −70°C. Total RNA was extracted from roots following the protocol provided with the TRIzol reagent (Invitrogen, USA). First strand cDNA was synthesized from DNaseI treated RNA, and served as template for a PCR using CmbHLH1 specific primers (sequences given in Table S1) designed from the bHLH sequence retrieved from the chrysanthemum EST library described by Chen et al.17. Both a 3′ and a 5′ RACE were carried out to obtain the full length cDNA sequence, using primers detailed in Table S1. The full open reading frame was amplified by the primer pair CmbHLH1-Full-F/-R (Table S1) and inserted into the pMD19-T vector (Takara, Japan) for sequencing. The CmbHLH1 promoter sequence was isolated using the thermal asymmetric interlaced PCR (TAIL-PCR) method25, employing the primers detailed in Table S1.
Phylogenetic and promoter sequence analysis
The structural domain of the predicted polypeptide was annotated using SMART 5 software (http://smart.embl.de/)26. An unrooted tree was constructed using the Neighbour Joining Method implemented in the software package MEGA v527. The robustness of each node of the tree was assessed by bootstrap analysis, each comprising 1,000 replications. The promoter sequence was scanned by PLACE software (http://www.dna.affrc.go.jp/PLACE/) to identify plant cis-acting elements28.
Subcellular localization of CmbHLH1
The subcellular localization of CmbHLH1 was identified using a transient assay based on onion epidermal cells. The CmbHLH1 open reading frame, lacking its stop codon, was amplified using a Phusion® High-Fidelity PCR kit (New England Biolabs, lpswich, MA, USA) with the primer pair CmbHLH-Dra-F/-Not-R (Table S1), and the resulting amplicon was cloned into pMD19-T for sequencing. The same fragment was inserted both into the DraI and NotI cloning sites of the pENTRTM1A dual selection vector (Invitrogen) using T4 DNA ligase (Takara), and into pEarleyGate 10329 using LR ClonaseTM II enzyme mix (Invitrogen). The latter plasmid includes GFP as a marker for transgene expression. The construct was bombarded into the onion cells and GFP expression was monitored by confocal laser microscopy30.
Transcription analysis
The transcription analysis of CmbHLH1 in ‘Jinba' seedlings exposed to Fe deficiency was carried out using three week old seedlings grown in a greenhouse under natural light (14 h light/10 h dark, with the light intensity ranging from 50 to 100 μmol m−2 s−1) in a 2:1 mixture of garden soil and vermiculite. The day/night temperature was maintained at 18°C/15°C and the relative humidity at 70–75%. At the end of this period, the plants were transferred to a liquid MS medium, held for three days, and then transferred to a liquid MS medium in which Fe2(SO4)3 was replaced by 300 μM of the iron chelator ferrozine. Control plants were left to grow in liquid MS medium. The plant hormone ABA was added to the hydroponic solution at a concentration of 0.38 mM (the parallel control experiment was run without the addition of ABA). The roots were exposed to this medium for either 0, 3, 6, 12, 24, 48 or 72 h. RNA was extracted from the roots, and the transcript abundance of CmbHLH1, CmHA, CmFRO3 and CmPP2C assayed using quantitative PCR (qPCR), based on primers listed in Table S1. The qPCR assay was conducted using iTaq SYBR Green Supermix (Bio-Rad) and GAPDH (DK941612) was used as a reference gene31. The comparative Ct method (Applied Biosystems bulletin) was used to estimate transcript abundance. Each sample was tested at least in triplicate, and the relative expression abundance was normalized using GAPDH as the internal control. The assay included three biological replication.
Transient expression of CmbHLH1pro::GUS in tobacco
A CmbHLH1pro::GUS plasmid was constructed as follows: first, the CmbHLH1 promoter sequence was amplified with the primer pair Pro-SacII-F/-NheI-R (Table S1), then this amplicon was inserted into the SacII and NheI cloning sites of the pORE-R1 vector32. Transient transformation of tobacco was achieved following Sparkes et al.33. After infiltration, the tobacco plants were held in the dark for two days, then moved to a growth cabinet providing a 14 h photoperiod at a constant temperature of 21°C. The Fe deficiency and ABA treatments followed the methods described above, and the infiltrated leaves were harvested 48 h after the treatment had been imposed. GUS activity was expressed in the form nmol 4-methylumbelliferone generated per min per μg protein34,35. Three independent assays were performed per treatment.
Transgene construction and plant transformation
Both a sense and an antisense version of the CmbHLH1 open reading frame were amplified from ‘Jinba' cDNA using the primer pairs CmbHLH1-Sense-F/-R and CmbHLH1-Antisense-F/-R, respectively (sequences given in Table S1). Each amplicon was inserted separately into the BamHI/SacI cloning site of the pCAMBIA1301 vector, and placed under the control of the CaMV 35S promoter. ‘Jinba' leaf discs were agro-infected as described by Das et al.36. Putative transformants were selected on the basis of hygromycin resistance, and confirmed by both a PCR and an RT-PCR assay.
Ion content of the root and the rhizosphere acidification
A 100 mg sample (three replicates) of the roots exposed to Fe deficiency was rinsed and digested in 5 mL 10 M HNO3, then neutralized with NaOH. The aqueous phase was analyzed for the presence of Fe, zinc (Zn) and manganese (Mn) ions using an inductively coupled plasma-mass spectrometer, following Morrissey et al.37. The pH in the medium immediately surrounding the roots was measured38; for each transgenic line, three plants were transferred to an Fe deficient medium for three days, then plated on 1% agar containing 0.006% w/v bromocresol purple and 0.2 mM CaSO4 (pH 6.5) for a further 24 h. The indicator dye color is pH responsive.
Author Contributions
A.S., M.Z. and P.L. performed the experiments; M.Z. wrote the manuscript; S.C. and J.J. edited the manuscript; A.S., S.C. and J.J. revised the manuscript; J.J. and F.C. designed the experiments.
Supplementary Material
A bHLH transcription factor regulates iron intake under Fe deficiency in chrysanthemum
Acknowledgments
This study is supported by the National Natural Science Foundation of China (Grant No. 31071820, 31071825,31272202), Natural Science Foundation of Jiangsu Province (Grant No. BK2011641, BK2012773), the Fundamental Research Funds for the Central Universities (KYZ201112, KYZ201147), the Program for New Century Excellent Talents in University of Chinese Ministry of Education (Grant No. NCET-10-0492), Youth Science and Technology Innovation Fund (KJ2011009), and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- Santi S., Cesco S., Varanini Z. & Pinton R. Two plasma membrane H+-ATPase genes are differentially expressed in iron-deficient cucumber plants. Plant Physiol. Biochem 43, 287–292 (2005). [DOI] [PubMed] [Google Scholar]
- Robinson N. J., Procter C. M., Connolly E. L. & Guerinot M. L. A ferric-chelate reductase for iron uptake from soils. Nature 397, 694–697 (1999). [DOI] [PubMed] [Google Scholar]
- Eide D., Broderius M., Fett J. & Guerinot M. L. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Nat. Acad. Sci. USA 93, 5624–5628 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot M. L. The ZIP family of metal transporters. BBA-Biomembranes 1465, 190–198 (2000). [DOI] [PubMed] [Google Scholar]
- Colangelo E. P. & Guerinot M. L. The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 16, 3400–3412 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aciksoz S. B., Ozturk L., Gokmen O. O., Römheld V. & Cakmak I. Effect of nitrogen on root release of phytosiderophores and root uptake of Fe (III)-phytosiderophore in Fe-deficient wheat plants. Physiol. Plant. 142, 287–296 (2011). [DOI] [PubMed] [Google Scholar]
- Schaaf G. et al. A putative function for the Arabidopsis Fe–phytosiderophore transporter homolog AtYSL2 in Fe and Zn homeostasis. Plant Cell Physiol. 46, 762–774 (2005). [DOI] [PubMed] [Google Scholar]
- Lee S. et al. Disruption of OsYSL15 leads to iron inefficiency in rice plants. Plant Physiol. 150, 786–800 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogo Y. et al. The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. Plant J. 51, 366–377 (2007). [DOI] [PubMed] [Google Scholar]
- Ogo Y. et al. A novel NAC transcription factor, IDEF2, that recognizes the iron deficiency-responsive element 2 regulates the genes involved in iron homeostasis in plants. J Biol. Chem. 283, 13407–13417 (2008). [DOI] [PubMed] [Google Scholar]
- Wang L. et al. Identification of OsbHLH133 as a regulator of iron distribution between roots and shoots in Oryza sativa. Plant Cell Environ. 36, 224–236 (2013). [DOI] [PubMed] [Google Scholar]
- Long T. A. et al. The bHLH transcription factor POPEYE regulates response to iron deficiency in Arabidopsis roots. Plant Cell 22, 2219–2236 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampey R. A. et al. An Arabidopsis basic helix-loop-helix leucine zipper protein modulates metal homeostasis and auxin conjugate responsiveness. Genetics 174, 1841–1857 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer G. A. et al. Identification of candidate genes underlying an iron efficiency quantitative trait locus in soybean. Plant Physiol. 158, 1745–1754 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingam S. et al. Interaction between the bHLH transcription factor FIT and ETHYLENE INSENSITIVE3/ETHYLENE INSENSITIVE3-LIKE1 reveals molecular linkage between the regulation of iron acquisition and ethylene signaling in Arabidopsis. Plant Cell 23, 1815–1829 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H. Current status of floriculture–national and international scenario. Commercial floriculture. FAO and DAC, Ministry of Agriculture, Govt. of India, New Delhi, 1–26 (2000). [Google Scholar]
- Chen S. et al. Analysis of expressed sequence tags (ESTs) collected from the inflorescence of chrysanthemum. Plant Mol. Biol. Rep. 27, 503–510 (2009). [Google Scholar]
- Sivitz A., Grinvalds C., Barberon M., Curie C. & Vert G. Proteasome-mediated turnover of the transcriptional activator FIT is required for plant iron-deficiency responses. Plant J. 66, 1044–1052 (2011). [DOI] [PubMed] [Google Scholar]
- Sivitz A. B., Hermand V., Curie C. & Vert G. Arabidopsis bHLH100 and bHLH101 control iron homeostasis via a FIT-independent pathway. PLoS One 7, e44843 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot M. L. & Yi Y. Iron: nutritious, noxious, and not readily available. Plant Physiol. 104, 815 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T. et al. The spatial expression and regulation of transcription factors IDEF1 and IDEF2. Ann. Bot. 105, 1109–1117 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T. et al. The rice transcription factor IDEF1 is essential for the early response to iron deficiency, and induces vegetative expression of late embryogenesis abundant genes. Plant J. 60, 948–961 (2009). [DOI] [PubMed] [Google Scholar]
- Janicka-Russak M. & Kłobus G. Modification of plasma membrane and vacuolar H+-ATPases in response to NaCL and ABA. J. Plant Physiol. 164, 295–302 (2007). [DOI] [PubMed] [Google Scholar]
- Murashige T. & Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15, 473–497 (1962). [Google Scholar]
- Zhao M. et al. PCR primers targeting cis-acting promoter elements in plants. Russ. J. Plant Physiol. 59, 413–418 (2012). [Google Scholar]
- Letunic I. et al. SMART 5: domains in the context of genomes and networks. Nucleic Acids Res. 34, D257–D260 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739, 10.1093/molbev/msr121 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo K., Ugawa Y., Iwamoto M. & Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 27, 297–300 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley K. W. et al. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 45, 616–629 (2006). [DOI] [PubMed] [Google Scholar]
- Song A. et al. Isolation and characterisation of Chrysanthemum crassum SOS1, encoding a putative plasma membrane Na+/H+ antiporter. Plant Biol. (Stuttg) 14, 706–713 (2012). [DOI] [PubMed] [Google Scholar]
- Gu C. et al. Reference gene selection for quantitative real-time PCR in chrysanthemum subjected to biotic and abiotic stress. Mol. Biotechnol. 49, 192–197 (2011). [DOI] [PubMed] [Google Scholar]
- Coutu C. et al. pORE: a modular binary vector series suited for both monocot and dicot plant transformation. Transgenic Res. 16, 771–781 (2007). [DOI] [PubMed] [Google Scholar]
- Sparkes I. A., Runions J., Kearns A. & Hawes C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nature Protoc. 1, 2019–2025 (2006). [DOI] [PubMed] [Google Scholar]
- Jefferson R. A. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol. Biol. Rep. 5, 387–405 (1987). [Google Scholar]
- Gallagher S. R. GUS protocols: using the GUS gene as a reporter of gene expression. (Access Online via Elsevier, 1992). [Google Scholar]
- Das D., Reddy M., Upadhyaya K. & Sopory S. An efficient leaf-disc culture method for the regeneration via somatic embryogenesis and transformation of grape (Vitis vinifera L.). Plant Cell Rep. 20, 999–1005 (2002). [Google Scholar]
- Morrissey J. et al. The ferroportin metal efflux proteins function in iron and cobalt homeostasis in Arabidopsis. Plant Cell 21, 3326–3338 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H., Römheld V. & Ossenberg-Neuhaus H. Rapid method for measuring changes in pH and reducing processes along roots of intact plants. Zeitschrift für Pflanzenphysiologie 105, 407–416 (1982). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A bHLH transcription factor regulates iron intake under Fe deficiency in chrysanthemum