Abstract
Absolute numbers of lymphocytes are decreased in uninfected infants born to HIV-1-infected women (HIV-1-exposed). Although the exact mechanism is unknown, fetal exposure to maternal HIV-1-infection could prime the immune system and affect T cell trafficking. We compared the expression of chemokine receptors on cord blood CD4+ T cells from HIV-1-exposed children and healthy controls. At baseline CD4+ T cells had a largely naïve phenotype. However, stimulation with cytokines resulted in an upregulation of inflammatory response-related chemokine receptors on CD4+ T cells, with HIV-1-exposed infants having a significantly higher frequency of CD4+ T cells expressing, in particularly Th2 associated chemokine receptors (CCR3 p < 0.01, CCR8 p = 0.03). Numbers of naive CCR7+ CD4+ T cells were reduced (p = 0.01) in HIV-1-exposed infants. We further assessed whether the inflammatory phenotype was associated with susceptibility to HIV-1 and detected higher levels of p24 upon in in vitro infection of stimulated CD4+ T cells of HIV-1-exposed infants. In summary, fetal exposure to HIV-1 primes the immune system in the infant leading to an enhanced immune activation and altered T cell homing, with potential ramifications regarding T cell responses and the acquisition of HIV-1 as an infant.
Annually, more than 1.5 million HIV-1-infected women give birth to 330,000 HIV-1-infected children1. Long-term combination antiretroviral therapy (cART) during pregnancy, reduces mother-to-child transmission of HIV-1 from 25% to <1%2. Since 2010, long-term cART during pregnancy is therefore recommended for all HIV-1-infected women, also in resource-poor settings, reducing the number of children infected with HIV-1 at birth3,4. However, this also implies that the number of uninfected children born to HIV-1-infected women is rapidly increasing2,3.
Although these children are uninfected and considered to be healthy, they are exposed during in utero development to maternal HIV-1 and cART. There is accumulating data suggesting that exposure to maternal HIV-1-infection and cART in utero has an effect on their immune system and susceptibility to infections5,6. Children born to HIV-1-infected mothers with a CD4+ T cell count less than 200 cells/μL, have reduced lymphocyte counts compared to infants born to mothers with a CD4+ T cell count above 200 cells/μL7,8,9. Furthermore, exposure to cART in utero is associated with reduced numbers of lymphocytes and neutrophils up to at least 8 years of age7,10,11. These observations suggest that key components of the immune system of HIV-1-exposed children are substantially altered with clinical consequences; indeed, an increased susceptibility to common infections has been reported in uninfected HIV-1-exposed children, in particular in countries with a high burden of infectious diseases5,12. Alterations in the infant's immune system could also affect postnatal transmission of HIV-1, as many of these children are breastfed with continuous exposure to HIV-1 after birth up to at least 6 months of age and potentially as an adult.
Toxicity of cART on blood stem cells could affect numbers of circulating CD4+ T cells; however, this does not explain the correlation between infant and maternal CD4+ T cell counts8,9. Due to in utero exposure to HIV-1, altered migratory patterns of CD4+ T cells from blood into tissues could also influence CD4+ T cell counts in the circulation of HIV-1-exposed infants. Homing to specific anatomical sites depends on the expression of specific chemokine receptors on CD4+ T cells13. Expression of specific chemokine receptors allows functional characterization of CD4+ T cells14,15,16. For example, CCR7 is specifically expressed by naive CD4+ T cells to direct these cells to lymph nodes, where their cognate antigen may be presented14,15,16. Other chemokine receptors are associated with specific immune responses upon infection, such as CXCR6, in the case of Th1 responses, and direct CD4+ T cells towards the infected tissues14,15,16. Furthermore, increased expression of certain chemokine receptors, especially those upregulated during inflammatory responses, also play a role in the pathogenesis of inflammatory diseases17,18. The evaluation of chemokine receptor expression on CD4+ T cells can, therefore, provide insight into the homing pattern as well as the immune response of CD4+ T cell in an individual and help elucidate the observed differences in CD4+ T cell count in HIV-1-exposed infants.
In the study reported here, we investigated the expression of chemokine receptors on CD4+ T cells from cord blood from infants born to HIV-1-infected women and healthy women. Although small, we detected a higher expression of chemokine receptors, normally upregulated under inflammatory conditions already at birth. However, after in vitro stimulation with a broad variety of cytokines and stimuli, including IL-1β, chemokine receptors were greatly upregulated and the differences between HIV-1-exposed infants and controls became strikingly evident. Furthermore, the enhanced state of activation of CD4+ T cells of infants born to HIV-1-infected women was associated with an increased susceptibility to HIV-1 infection compared to healthy controls. Combined, these data not only show an upregulation of chemokine receptors on CD4+ T cells upon in utero HIV-1 exposure, which allows egress from blood in tissues, but also an overall activated state, which affects postnatal susceptibility to HIV-1 and potentially other infections.
Results
CD4+ T cells have a largely naïve phenotype in HIV-1-exposed infants and healthy controls at birth
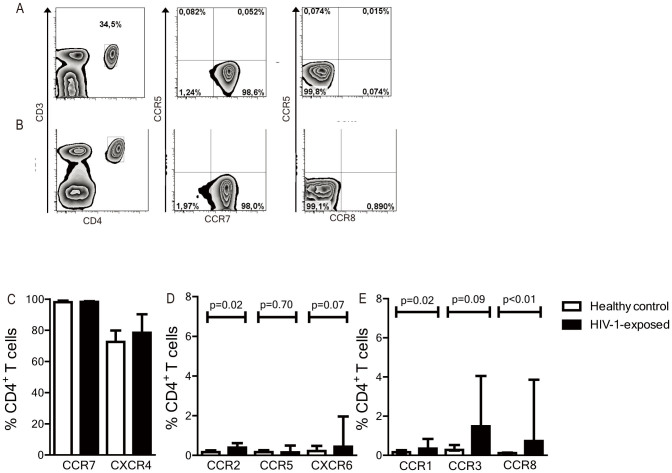
CD4+ T cells derived from cord blood were analyzed by flow cytometry to determine baseline chemokine receptor expression profiles at birth. CD4+ T cells from both healthy controls and uninfected HIV-1-exposed infants had a largely naive, undifferentiated phenotype (CD45RO−CD27+) (data not shown) with high expression of CCR7 and CXCR4 (Figures 1A–C). In accordance, only few CD4+ T cells expressed chemokine receptors associated with inflammatory responses, such as CCR2, CCR5 and CXCR6 that are predominantly expressed by Th1 cells (Figure 1D), and CCR1, CCR3 and CCR8, which are expressed by Th2 cells as shown in (Figure 1E)14,15,16,19. Although percentages were very small, significantly more CD4+ T cells from HIV-1-exposed infants expressed chemokine receptors associated with inflammatory responses than from the healthy controls (Figures 1D, E). These data suggest that CD4+ T cells from both healthy and HIV-1-exposed infants have a largely naïve phenotype, however small percentages of CD4+ T cells of HIV-1-exposed infants maybe activated in utero.
Figure 1. Chemokine receptor expression on cord blood CD4+ T cells from HIV-1-infants and healthy controls assessed with flow cytometry.
CD4+ T cells of a healthy control (A) and an HIV-1-exposed infant (B) at birth were analysed with flow cytometry. Expression of CCR7 was high in both groups, whereas inflammatory response-related chemokine receptors CCR5 and CCR8 were nearly absent. (C) High expression of CXCR4 and CCR7 was detected in both groups and did not differ significantly. (D) Th1-inflammatory response-related chemokine receptors CCR2, CCR5 and CXCR6 were nearly absent on CD4+ T cells in both HIV-1-exposed infants and healthy controls. (E) Th2- inflammatory response-related chemokine receptors CCR1 and CCR8 were marginally expressed on CD4+ T cells in both HIV-1-exposed infants and healthy controls, but were significantly higher in HIV-1-exposed infants. CCR6, CCR9 and CCR10 were not depicted as these were expressed at extremely low percentages of CD4+ T cells in both groups (<0.4%). N = 12 (healthy control) and 10 (HIV-1-exposed infants). Median is depicted with interquartile range (IQR). Statistical analyses with Man-Whitney U-test.
Chemokine receptors upregulated upon infection or inflammation are upregulated upon stimulation of cord blood CD4+ T cells in vitro
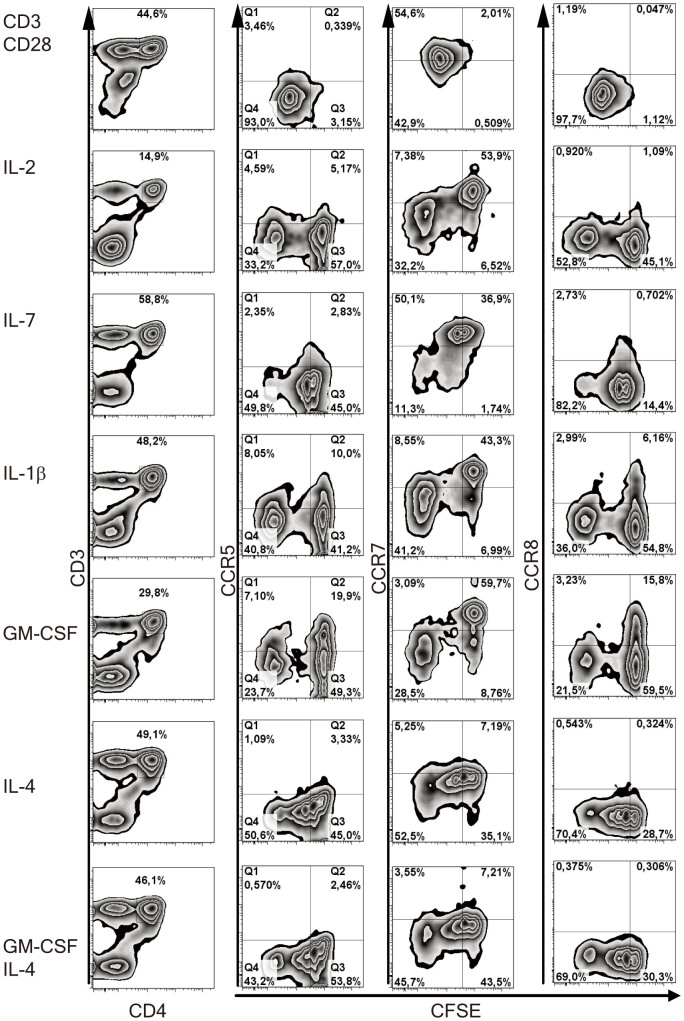
Adult naïve CD4+ T cells cultured with cytokines and dendritic cells increase expression of inflammatory response-related chemokine receptors20. We explored whether upon stimulation with cytokines chemokine receptors could be upregulated on naïve cord blood CD4+ T cells from healthy infants and HIV-1-exposed infants. The cord blood mononuclear cell fractions of healthy controls and HIV-1-exposed infants were labelled with CFSE to assess proliferation and cultured for 6 days with one of the following stimuli: IL-2, IL-4, IL-7, IL-15, IL1-β, GM-CSF, or a combination of monoclonal antibodies against CD3 and CD28 to simulate TCR stimulated activatin. Chemokine receptor expression on and CFSE dilution in CD4+ T cells were measured by flow cytometry. In vitro proliferation of cord blood CD4+ T cells varied among the different cultures; in culture with IL-15 10% (IQR 9–35%) of the CD4+ T cells had proliferated, whereas 73% (IQR 59–82%) when cultured with IL-4 and almost all CD4+ T cells (>99%) had undergone multiple cycles of proliferation, when cultured in the presence of monoclonal antibodies against CD3 and CD28 (Figure 2).
Figure 2. Chemokine receptor expression on CD4+ T cells after in vitro culture analysed with flow cytometry.
Mononuclear cell fractions derived from cord blood of either healthy controls or HIV-1-exposed infants were labelled with CFSE and cultured for 6 days with various stimuli: IL-2, IL-4, IL-7, IL-15, IL-1β GM-CSF and T cell receptor (TCR)-mediated activation with monoclonal antibodies against CD3 and CD28. Expression of chemokine receptors and CFSE was measured by flow cytometry. CD4+ T cells are shown of a HIV-1-exposed infant after stimulation. Stimulation with monoclonal antibodies against CD3 and CD28 induced proliferation in almost all CD4+ T cells (>99%), measured by dilution of CFSE. Culture with IL-1β or GM-CSF induced the highest upregulation of CCR5 and CCR8. Proliferating cells in all cultures except in cultures with antibodies against CD3 and CD28 downregulated CCR7 expression.
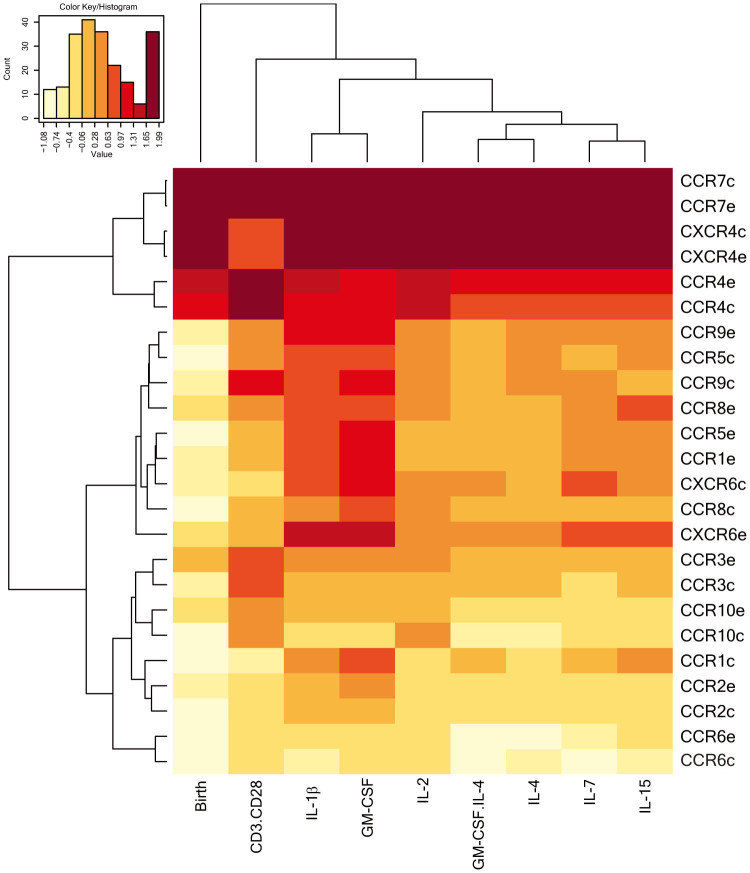
Chemokine receptors related to inflammatory responses such as CCR3, CCR5, CCR8 and CXCR6 were upregulated on CD4+ T cells in cultures with in particular IL1-β and GM-CSF (Figure 2). CCR7, expressed by almost all CD4+ T cells before culture (>98% (median)), was downregulated on proliferating cells upon culture. To distinguish patterns in the regulation of chemokine receptors by the stimuli, we performed a hierarchical clustering between the expression of the chemokine receptors on the CD4+ T cells and the different stimuli (Figure 3). Two groups were identified: 1) chemokine receptors mainly expressed by naïve cells such as CCR7 and CXCR4, which were downregulated in cultures with the stimuli; and 2) chemokine receptors associated with inflammatory responses, i.e. CCR5, CXCR6 and CCR8 which were upregulated upon culture.
Figure 3. Hierarchical clustering of the expression of the chemokine receptors on CD4+ T cells at baseline and after stimulation.
The heatmap shows the hierarchical clustering of the expression levels of the chemokine receptors on CD4+ T cells at baseline and after culture with various stimuli, measured by flow cytometry. The -e- after chemoreceptor indicates HIV-1-exposed infants and the -c- healthy controls. CCR7, CXCR4 grouped together, as well as chemokine receptor-related inflammatory responses, i.e. CCR5, CCR8 and CXCR6. The expression of inflammatory response-related chemokine receptors such as CCR5, CCR8 and CXCR6 increased in in vitro cultures in particular with IL-1β and GM-CSF, clustering these two cytokines together. N = 7 in both groups.
Specific stimuli clustered together based on the pattern of expression of specific chemokine receptors on CD4+ T cells in these cultures. For example, IL-2 and the other γc chain receptor (CD132)-restricted cytokines, IL-4, IL-7 and IL-15 induced similar expression profiles of chemokine receptors. Cultures with IL1-β and GM-CSF also clustered together, inducing the highest expression of inflammatory response-related chemokine receptors on CD4+ T cells.
Thus, stimulation of cord blood CD4+ T cells with specific stimuli is associated with specific chemokine expression patterns. Moreover, inflammatory response-related chemokine receptors can be upregulated on cord blood CD4+ T cells.
Increased frequencies of CD4+ T cells expressing inflammatory response-related chemokine receptors in HIV-1-exposed children compared to healthy controls upon stimulation
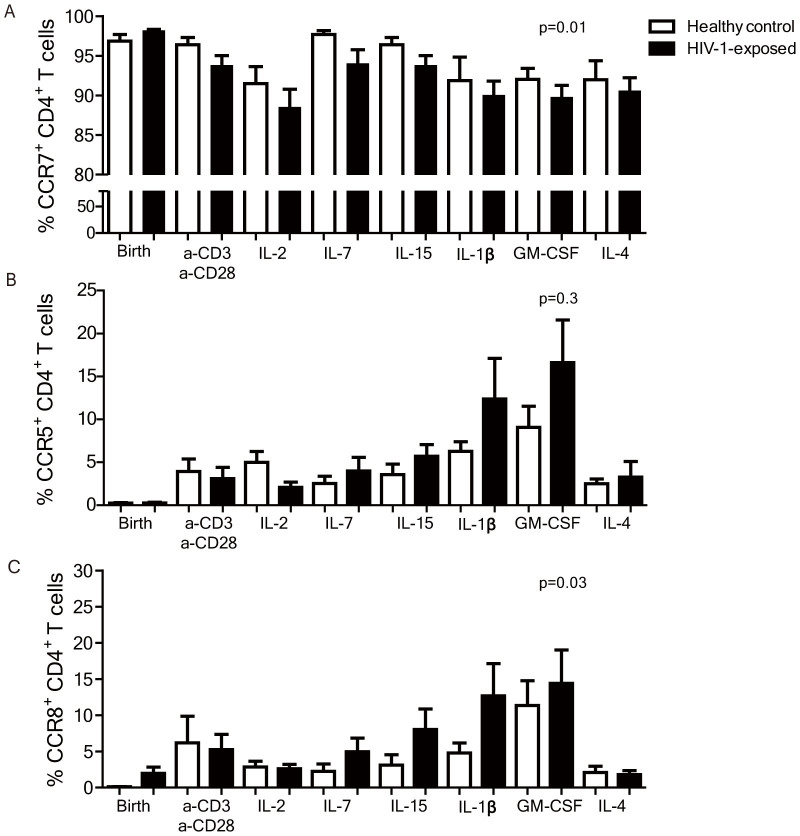
In the above analyses we showed that chemokine receptors can be upregulated on cord blood derived CD4+ T cells and specific patterns are induced by specific stimuli. Next, we compared chemokine receptor regulation on CD4+ T cells from uninfected HIV-1-exposed infants to those from healthy controls. An ANOVA analysis per chemokine receptor, including all the cultures, showed that upon stimulation in vitro a significantly larger percentage of CD4+ T cells of HIV-1-exposed infants expressed inflammatory response-related chemokine receptors than CD4+ T cells of control infants; CCR1 (p = 0.04), CCR3 (p < 0.01), CCR4 (p < 0.01), CCR8 (p = 0.03) and CXCR6 (p < 0.01) (Fig. 4A, C). At the same time CCR7 expression was significantly lower on CD4+ T cells from HIV-1-exposed infants compared to healthy controls after culture (p = 0.01) (Figures 4A–B). The expression of CCR5 did not significantly differ between the groups (p = 0.3). The proliferation capacity as measured by CFSE dilution in CD4+ T cells from HIV-1-exposed infants was similar to the controls. These data strongly indicate that upon activation with cytokines CD4+ T cells of HIV-1-exposed infants behave differently compared to controls; the CD4+ T cells have an enhanced state of activation as reflected by the increased upregulation of inflammatory response-related chemokine receptors and decreased expression of chemokine receptors expressed by naive CD4+ T cells14,15,16,19.
Figure 4. ANOVA analyses of chemokine receptor expression of CD4+ T cells from HIV-1-exposed infants and healthy controls.
(A) CCR7 was significantly lower on cultured CD4+ T cells from HIV-1-exposed infants compared to healthy controls in an ANOVA analysis (p = 0.01). (B) CCR5 expression on CD4+ T cells of HIV-1-exposed infants and healthy controls after a 6 day culture with various stimuli did not differ significantly across all cultures. (C) CCR8 expression on CD4+ T cells of HIV-1-exposed infants and healthy controls after a 6 day culture with various stimuli was significantly higher across all cultures (p = 0.03). N = 7 in both groups. Medians are depicted with interquartile range.
Levels of IL-1β and IL-8 are increased in plasma from HIV-1-exposed infants
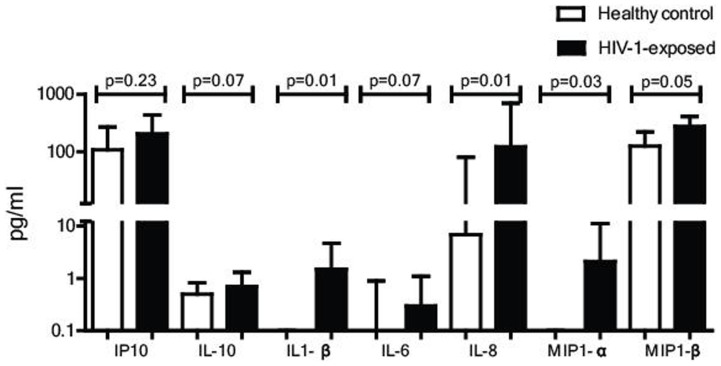
T cell responses are shaped by signals from innate cells, including cytokines released by macrophages or dendritic cells. We investigated whether levels of soluble factors, such as cytokines in plasma of HIV-1-exposed infants were also indicative of an enhanced immune activation, using a Luminex assay assessing a panel of soluble factors in plasma at birth. Out of the 17 cytokines and chemokines we assessed, most were undetectable in plasma, including IFN-α and IFN-γ. However, the cytokines associated with inflammation, IL-1β and IL-8, were detectable and significantly higher in HIV-1-exposed infants than in healthy controls (Figure 5). IP10 (CXCL10) and IL-10 were also detected, however these did not differ between the two groups. These data demonstrate that inflammatory cytokines IL-1β and IL-8 are increased in plasma of HIV-1-exposed children at birth, supporting the model of an enhanced immune activation in these infants.
Figure 5. Levels of soluble factors in plasma of HIV-1-exposed infants and healthy controls.
Cytokines, chemokines and growth factors (IL1-β IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IL-17A, IL-18, IFN-α, IFN-γ, IP-10, TNF-α, MIP-1α, MIP-1β, RANTES and GM-CSF) were measured in plasma at birth from 20 healthy controls and 20 HIV-1-exposed infants by LUMINEX analyses. Cytokines associated with acute phase inflammatory responses, IL-1β and IL-8 were significantly higher in HIV-1-exposed infants. Cytokines related to antiviral or regulatory responses were low and not significantly different in the plasma of both healthy controls and HIV-1-exposed children. Medians are depicted with interquartile range. Statistical analyses performed with Man-Whitney U-test.
Increased susceptibility to HIV-1-infection of CD4+ T cells from HIV-1-exposed infants compared to controls
Immune activation is critical for HIV-1 infection as highly activated CD4+ T cells are more susceptible to HIV-1 and supportive of HIV-1 replication, than resting CD4+ T cells. We therefore explored whether CD4+ T cells in HIV-1-exposed infants, due to their enhanced activation, would be more permissive to HIV-1 infection compared to CD4+ T cells from controls. Cord blood mononuclear cells were inoculated directly after isolation with YU2 (R5-strain) for 12 hours or after stimulation for 4 days with IL-2 100 IU/ml or GM-CSF. As described earlier non-stimulated CD4+ T cells from HIV-1-as well as healthy controls could not be infected with HIV-1 (data not shown)21. However, after immune activation by IL-2 or GM-CSF, infection was detected (Figure 6). Strikingly, levels of p24 were significantly higher in cell cultures from HIV-1-exposed infants compared to healthy controls. Together, these data demonstrate that the enhanced immune activation in HIV-1-exposed infants leads to increased HIV-1 susceptibility of CD4+ T cells.
Figure 6. In vitro HIV-1 infection of CD4+ T cells from HIV-1-exposed infants and healthy controls.
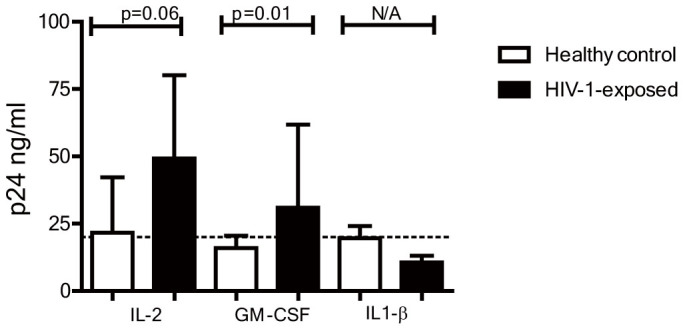
Isolated cord blood mononuclear cells from HIV-1-exposed infants and healthy children were directly infected with YU2 or after a 4 day culture with, IL-2 or GM-CSF. HIV-1 infection was determined using an ELISA for p24 at day 6 after infection. After pre-culture with IL-2 or GM-CSF significantly higher p24 levels were detected in cultures from HIV-1-exposed infants than in the healthy controls. Median levels of p24 in cultures with IL1-β were under the detection limit of the ELISA (25 ng/ml) and as such can not be statistically compared. Median is depicted with inter quartile range. Statistical analyses with Man-Whitney U-test.
Discussion
Although uninfected HIV-1-exposed children are considered to be healthy, there is accumulating data suggesting that exposure to maternal HIV-1 infection and cART in utero has an effect on their immune system7,10,11. In this study, we demonstrated that chemokine receptors associated with inflammatory responses were expressed on a significantly larger percentage of CD4+ T cells from HIV-1-exposed infants compared to controls at birth, in particular after culture with stimuli released upon inflammation in vivo. At the same time chemokine receptors indicating mainly naïve cells such as CCR7 were expressed by fewer CD4+ T cells in HIV-1-exposed infants.
These data strongly indicate that, already at baseline but particularly upon stimulation, a larger percentage of CD4+ T cells of HIV-1-exposed infants express chemokine receptors associated with an inflammatory response. In vivo a similar situation is expected during common viral or bacterial infections. Consequently, a larger number of CD4+ T cells egress from blood into immune active sites, such as the intestinal tract, resulting in lower lymphocyte counts in blood and providing a mechanistic explanation for the epidemiological observations in HIV-1-exposed children.
The increased expression of inflammatory response-related chemokine receptors in HIV-1-infants and inflammatory cytokine levels suggest that the immune activation is enhanced in these infants. Previous data also indicated towards this, with more effector memory CD4+CD27− T cells and CD4+ CD38bright HLA-DRbright cells at birth in HIV-1-exposed infants compared to healthy controls, even though the percentages were small in both groups (<1%)9,22,23. The mechanism behind the enhanced immune activation in HIV-1-exposed children is unknown. In HIV-1-infected patients, enhanced immune activation is an important characteristic of infection24,25. In utero recognition of viral components, which have crossed the placenta or maternal immune inflammatory stimuli could induce infant innate cells, e.g. dendritic cells or macrophages, to produce inflammatory cytokines including IL-1β, which we detected in a higher concentration in plasma of HIV-1-exposed infants compared to healthy infants26. These acute phase cytokines could shape the innate and adaptive immune responses towards an inflammatory profile in HIV-1-infants and priming T cells. Furthermore, these data also explain the correlation between CD4+ T cell counts in the mother and the child, as mothers with decreased counts will have a higher immune activation and viral load, which activates the infant's immune system and affects chemokine receptor expression. As these observations are preserved past the neonatal period, viral exposure in utero may lead to epigenetic changes regulating inflammatory responses in infants over longer times and sustaining altered inflammatory responses beyond the duration of viral and immune activation exposure in utero27,28,29.
In particular, Th2-related chemokine receptors, CCR1, CCR3 and CCR8 were increased on CD4+ T cells in HIV-1-exposed infants, and not Th1-related chemokine receptors, CCR5 and CXCR6. This pattern closely resembles the altered Th1/Th2 ratio in HIV-1-infected patients, which is also observed in HIV-1-infected pregnant women30,31,32. A preferential Th2 response concurs with the higher IgG antibody titers observed in uninfected HIV-1-infants compared to controls33,34. However, this may have implications when children are infected with intracellular pathogens, such as viruses when Th1 responses are required.
Furthermore, the enhanced immune activation may have direct clinical implications for these children in the context of mother-to-child transmission of HIV-1 as we also detected an increased susceptibility to HIV-1 infection in uninfected HIV-1-exposed infants. The majority of the children born to HIV-1-infected mothers are breastfed for at least 6 months and consequently are continuously exposed and at risk for viral transmission. Recently, we have shown that already at birth activated memory CD4+ CCR5+ T cells, exemplary target cells for HIV-1, are almost exclusively present in the gut. Due to the enhanced immune activation, children exposed to HIV-1 in utero are expected to have greater percentages of these target cells in the gut facilitating HIV-1 transmission during delivery or breastfeeding35. Interestingly, although culture with IL1-β leads to an increase of CCR5 this did not lead to increase of p24 production, in contrast to IL-2. It is known that IL-2 at several stages of viral replication positively impacts virus production, 1. by increasing nucleotide pools for reverse transcription and 2. increasing NF-κB induced transcription36,37,38,39. Taken together CCR5 expression is important for infection, but additional factors are needed for successful viral replication.
Our studies were performed on a relatively small number of infants; the mothers had only very mild HIV-1 disease and low viral load, which would limit inflammatory responses in the infant. Nonetheless, our results already show an evident difference in the response of the CD4+ T cell compartment of these HIV-1-exposed infants upon stimulation. Although we have not investigated this, we conjecture that in regions with a higher microbial load and more advanced HIV-1 disease these differences would probably be more explicit and have clinical implications. Studies investigating uninfected children born to HIV-1 infected women detected more severe sequelae of infections with intracellular pathogens such as CMV in infants6,12,40. This suggests that similar to common viral infections, fetal HIV-1 exposure may have clinical consequences41.
In conclusion, fetal exposure to maternal HIV-1 infection results in an enhanced immune activation with increased expression of inflammatory response-related chemokine receptors in HIV-1-exposed infants. This affects the homing of CD4+ T cells in tissues and provides a mechanistic explanation for the low blood lymphocyte counts at birth and thereafter in these children. The consequences of this finding reach beyond our research question and are expected to have clinical implications, regarding the immune responses generated in HIV-1-exposed infants upon common infections and susceptibility to HIV-1 later in life as well other pathogens.
Methods
Subjects
This study was carried out in the Emma Children's Hospital at the Academic Medical Centre (AMC), Amsterdam, the Netherlands. Prospectively, cord blood samples from uninfected children born to HIV-1-infected women (N = 10) and from children born to healthy mothers (N = 12) were obtained, for the chemokine receptor expression at birth. The study was approved by our local ethics committee and parental consent provided.
cART was initiated in the HIV-1-infected mothers, from 20 weeks gestation and consisted of zidovudine, lamivudine and nelfinavir or lopinavir boosted with ritonavir. All mothers acquired an undetectable viral load before delivery and had a CD4+ T cell count > 200 cells/μL. The newborn infants were treated for 4 weeks with zidovudine and lamivudine orally, according to national guidelines. HIV-1 infection was diagnosed with a positive HIV-1 RNA PCR in the first 18 months after birth and confirmed at 18 months with a negative HIV-1 p24 antibody test. The uninfected infants born to HIV-1-infected women were included in the study.
All infants were born after 37 weeks of gestational age and had a birth weight above 2500 grams. None of the children suffered from an infection at birth. The children in the comparison group consisted of healthy children born to healthy mothers and were matched for ethnicity.
Cell isolation and cultures
Cord blood mononuclear cells were isolated using Ficoll gradients and were directly stained with monoclonal antibodies for flow cytometry analyses. From 7 HIV-1-exposed infants and 7 controls the remaining cells were used in the cytokine stimulation assay and HIV-1 infection assay. The cells were labeled with CFSE and cultured for 6 days in IMDM, supplemented with 10% heat-inactivated fetal bovine plasma, penicillin (50 U/mL), streptomycin (50 mg/mL) and various stimuli; either IL-2 (100 IU/ml, Chiron Benelux), IL-1β (10 ng/ml), IL-4 (10 ng/ml), IL-7 (20 ng/ml), and IL-15 (20 ng/nl), GM-CSF (10 ng/ml) (all from R&D systems) or anti-CD3 and anti-CD28 (both from Sanquin).
Flow cytometry
The expression of surface markers on single cells was determined with a FACS CANTO (BD Biosciences) using fluorochrome-conjugated monoclonal antibodies. The antibodies included CD3 (Sanquin), CD4 (Pharmingen), CCR1, CCR2, CCR3, CCR4, CCR5, CCR6, CCR7, CCR8, CCR9, CCR10, CXCR4 and CXCR6 (all from R&D Systems).
HIV-1 infection assay
After isolation from cord blood, mononuclear cells from 7 HIV-1-exposed infants and 7 healthy controls were inoculated directly with YU2 (R5-strain) at a multiplicity of infection of 1 for 12 hours at 37°C or after a stimulation for 4 days with IL-2 (100 IU/ml, Chiron Benelux), GM-CSF and IL-1β (10 ng/ml, RnD systems). After removal of virus, the cells were cultured for 6 days in IMDM, supplemented with 10% heat-inactivated fetal bovine plasma, penicillin (50 U/mL), streptomycin (50 mg/mL) and with either IL-2 (100 IU/ml) or GM-CSF (10 ng/ml). Supernatant was analyzed for p24 with a standardized ELISA to determine HIV-1-infection in the mononuclear cell culture.
Luminex
A multiparameter Luminex bead assay was used to assess levels of soluble factors indicative a various immune responses; IL1-β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IL-17A, IL-18, IFN-α, IFN-γ, IP-10, TNF-α, MIP-1α, MIP-1β, RANTES and GM-CSF, as indicated by the manufacturer (Invitrogen).
Statistical analyses
Flow cytometry data were analyzed with FlowJo (Tree Star Inc) and statistically investigated using Graphpad Prism Software (GraphPad Software Inc.). Medians are depicted with interquartile ranges (IQR) To compare continuous data, a Mann-Whitney U-test was performed between the two groups. In a two-way ANOVA analysis, we compared the expression of specific chemokine receptors between controls and HIV-1-exposed children across all cultures. Tree analyses were performed using Los Alomos tools (http://www.hiv.lanl.gov/content/sequence/HEATMAP/heatmap_mainpage.html).
Author Contributions
M.J.B. and T.W.K. are the principal investigators who conceived and designed the study. M.J.B. and K.B. acquired the samples. N.A.K. helped designing the HIV-1 infection experiment. M.J.B., J.L.H. and M.J. performed the experiments. M.J.B. devised and performed the analyses and wrote the first draft of the manuscript with input from all authors supervised by T.W.K. All authors approved the final manuscript revisions.
Acknowledgments
The project is funded by the NWO AGIKO-Stipendium (92003417) and the Dutch AIDS Foundation (2006015).
References
- UNAIDS. 2011 report on the global AIDS epidemic. (2012).
- European Collaborative Study. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 40, 458–465 (2005). [DOI] [PubMed] [Google Scholar]
- WHO, UNAIDS, & UNICEF. Global HIV/AIDS response: progress report. (2011).
- WHO. Antiretroviral therapy for HIV infection in infants and children: Towards universal access. 2010. [PubMed]
- Epalza C. et al. High incidence of invasive group B streptococcal infections in HIV-exposed uninfected infants. Pediatrics 126, e631–e638 (2010). [DOI] [PubMed] [Google Scholar]
- Mussi-Pinhata M. M. et al. Infectious disease morbidity among young HIV-1-exposed but uninfected infants in Latin American and Caribbean countries: the National Institute of Child Health and Human Development International Site Development Initiative Perinatal Study. Pediatrics 119, e694–e704 (2007). [DOI] [PubMed] [Google Scholar]
- Bunders M. J. et al. Haematological parameters of HIV-1-uninfected infants born to HIV-1-infected mothers. Acta Paediatr. 94, 1571–1577 (2005). [DOI] [PubMed] [Google Scholar]
- Bunders M., Thorne C. & Newell M. L. Maternal and infant factors and lymphocyte, CD4 and CD8 cell counts in uninfected children of HIV-1-infected mothers. AIDS 19, 1071–1079 (2005). [DOI] [PubMed] [Google Scholar]
- Clerici M. et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood 96, 3866–3871 (2000). [PubMed] [Google Scholar]
- Le Chenadec C. J., Mayaux M. J., Guihenneuc-Jouyaux C. & Blanche S. Perinatal antiretroviral treatment and hematopoiesis in HIV-uninfected infants. AIDS 17, 2053–2061 (2003). [DOI] [PubMed] [Google Scholar]
- Bunders M., Cortina-Borja M., Thorne C., Kuijpers T. W. & Newell M. L. Levels and patterns of neutrophil cell counts over the first 8 years of life in children of HIV-1-infected mothers. AIDS 18, 2009–2017 (2004). [DOI] [PubMed] [Google Scholar]
- Slogrove A. L., Cotton M. F. & Esser M. M. Severe infections in HIV-exposed uninfected infants: clinical evidence of immunodeficiency. J. Trop. Pediatr. 56, 75–81 (2010). [DOI] [PubMed] [Google Scholar]
- Kim C. H. Chemokine-chemokine receptor network in immune cell trafficking. Curr. Drug Targets. Immune. Endocr. Metabol. Disord. 4, 343–361 (2004). [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Forster R., Lipp M. & Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999). [DOI] [PubMed] [Google Scholar]
- Rivino L. et al. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J. Exp. Med. 200, 725–735 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V., van Lier R. A., Sallusto F. & Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry 73, 975–983 (2008). [DOI] [PubMed] [Google Scholar]
- Tarrant T. K. & Patel D. D. Chemokines and leukocyte trafficking in rheumatoid arthritis. Pathophysiology 13, 1–14 (2006). [DOI] [PubMed] [Google Scholar]
- Gerard C. & Rollins B. J. Chemokines and disease. Nat. Immunol. 2, 108–115 (2001). [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Mackay C. R. & Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 187, 875–883 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geginat J., Sallusto F. & Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J. Exp. Med. 194, 1711–1719 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunders M. J. et al. Memory CD4(+)CCR5(+) T cells are abundantly present in the gut of newborn infants to facilitate mother-to-child transmission of HIV-1. Blood 120, 4383–4390 (2012). [DOI] [PubMed] [Google Scholar]
- Miles D. J. et al. Human immunodeficiency virus (HIV) infection during pregnancy induces CD4 T-cell differentiation and modulates responses to Bacille Calmette-Guerin (BCG) vaccine in HIV-uninfected infants. Immunology 129, 446–454 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich K. C., Siegel J. N., Jennings C., Rydman R. J. & Landay A. L. Function and phenotype of immature CD4+ lymphocytes in healthy infants and early lymphocyte activation in uninfected infants of human immunodeficiency virus-infected mothers. Clin. Diagn. Lab Immunol. 4, 358–361 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin N. L. & Walker B. D. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat. Med. 9, 861–866 (2003). [DOI] [PubMed] [Google Scholar]
- Stevenson M. HIV-1 pathogenesis. Nat. Med. 9, 853–860 (2003). [DOI] [PubMed] [Google Scholar]
- Kanneganti T. D. Central roles of NLRs and inflammasomes in viral infection. Nat. Rev. Immunol. 10, 688–698 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. K. Environmental epigenomics and disease susceptibility. EMBO Rep. 12, 620–622 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. K. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics 6, 838–842 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan R. S. et al. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature 487, 249–253 (2012). [DOI] [PubMed] [Google Scholar]
- Clerici M. et al. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J. Clin. Invest. 91, 759–765 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M. & Shearer G. M. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol. Today 14, 107–111 (1993). [DOI] [PubMed] [Google Scholar]
- Fiore S. et al. Antiretroviral therapy-associated modulation of Th1 and Th2 immune responses in HIV-infected pregnant women. J. Reprod. Immunol. 70, 143–150 (2006). [DOI] [PubMed] [Google Scholar]
- Bunders M., Pembrey L., Kuijpers T. W. & Newell M. L. Evidence of impact of maternal HIV infection on immunoglobulin levels in HIV-exposed uninfected children. AIDS Res. Hum. Retroviruses 26, 967–975 (2010). [DOI] [PubMed] [Google Scholar]
- Jones C. E. et al. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA 305, 576–584 (2011). [DOI] [PubMed] [Google Scholar]
- Cowan F. M. et al. Maternal Herpes simplex virus type 2 infection, syphilis and risk of intra-partum transmission of HIV-1: results of a case control study. AIDS 22, 193–201 (2008). [DOI] [PubMed] [Google Scholar]
- Chun T. W., Engel D., Mizell S. B., Ehler L. A. & Fauci A. S. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J. Exp. Med. 188, 83–91 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun T. W. et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat. Med. 5, 651–655 (1999). [DOI] [PubMed] [Google Scholar]
- Bayard-McNeeley M. et al. Differential effects of interleukin-12, interleukin-15, and interleukin-2 on human immunodeficiency virus type 1 replication in vitro. Clin. Diagn. Lab Immunol. 3, 547–553 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd B., Pope J. H. & Georghiou P. Interleukin-2 enhances production in 24 hours of infectious human immunodeficiency virus type 1 in vitro by naturally infected mononuclear cells from seropositive donors. Arch. Virol. 121, 227–232 (1991). [DOI] [PubMed] [Google Scholar]
- Gompels U. A. et al. Human cytomegalovirus infant infection adversely affects growth and development in maternally HIV-exposed and unexposed infants in Zambia. Clin. Infect. Dis. 54, 434–442 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchide N., Ohyama K., Bessho T., Takeichi M. & Toyoda H. Possible roles of proinflammatory and chemoattractive cytokines produced by human fetal membrane cells in the pathology of adverse pregnancy outcomes associated with influenza virus infection. Mediators. Inflamm. 2012, 270670 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]