Abstract
Epithelial-mesenchymal transition (EMT) and its reversal, mesenchymal-epithelial transition (MET), are essential morphological processes during development and in the regulation of stem cell pluripotency, yet these processes are also activated in pathological contexts, such as in fibrosis and cancer progression. Multi-component signaling pathways cooperate in initiation of EMT and MET programs, via transcriptional, post-transcriptional, translational, and post-translational regulation. EMT is required for tissue regeneration and normal embryonic development as it enables epithelial cells to acquire the mesenchymal phenotype, conferring them migratory and dynamic properties towards forming three-dimensional structures during gastrulation and organ formation. Uncontrolled activation of such phenomenon and the pathways signaling EMT events in adult life, leads to cancer growth and orchestrated by signaling interactions from the microenvironment, epithelial tumor cells with enhanced polarity, become invasive and rapidly metastasize to distant sites. Loss of epithelial markers (E-cadherin) and gain of mesenchymal markers (N-cadherin), at the leading edge of solid tumors is associated with progression to metastasis. This review will explore the contribution of EMT to embryonic development of GU organs and the functional consequences of EMT-MET cycles in prostate tumorigenesis. Recent insights identifying key players driving EMT and its reversal to MET during prostate cancer progression to metastatic castration-resistant disease will be discussed, with specific focus on androgen receptor (AR) and transforming growth factor-β (TGF-β) signaling in the context of their predictive and targeting value in prostate cancer progression.
Keywords: Androgens, cell invasion, adherens junctions, therapeutic targeting, moving front, embryonic growth, predictive markers
Introduction
In 2013 prostate cancer continues to be one of the most commonly diagnosed cancers in men, with an estimated 241,740 men newly diagnosed with prostate cancer in 2012 (1). The majority of deaths associated with prostate cancer are attributed to the failure of current therapies to cure metastatic disease (2). The process of epithelial-mesenchymal transition (EMT) plays a pivotal role in the development of metastatic castration resistant prostate cancer (mCRPC) (3) The androgen receptor (AR) functions not only to control prostate normal development and tumorigenic growth, but also contributes to metastasis by facilitating EMT and promoting signaling interactions (Figure 1).
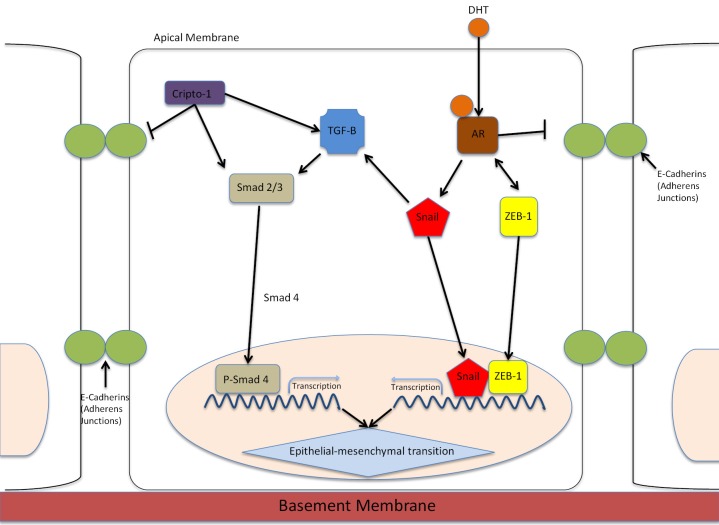
Figure 1.
EMT control by the AR signaling. EMT in prostate epithelial cells is mediated by TGF-β and AR signaling interactions. AR induces EMT through activation of Snail transcription factor or via repression of E-cadherin. Activation of Snail, ZEB-1 or Smad transcription factors initiates allows for mesenchymal gene expression. Snail increases the expression of mesenchymal markers and proteins associated with invasion. E-cadherin is transciptionally suppressed by Snail; this loss of E-cadherin, which mediates intracellular adhesions at adherens junctions, leads to collapse of cell-cell communications and onset of EMT. Cripto-1 is a critical effector of the TGF-β signaling that upregulates mesenchymal expression during embryonic development. ZEB-1 is involved in a feedback loop with AR where downregulation of AR leads to uncontrolled ZEB-1 expression.
The prostate is an androgen-dependent organ that is regulated by the androgen/AR-signaling axis in both organogenesis and tumorigenesis (4). During the early stages of development, differentiation and development of prostate cells are dependent on androgens. Stimulation of the AR signaling in the urogenital sinus mesenchyme by testicular androgens induces epithelial budding, proliferation and differentiation towards formation of ductal structures. A non-functional AR results in testicular feminization-like syndrome with the absence of the prostate (5). Circulating testosterone is converted into dihydrotestosterone (DHT) via 5-alpha reductase activity, the active metabolite that interacts with the AR to ensure maintenance and function of adult prostate gland. Once bound to DHT, the AR undergoes a conformational change and releases heat shock proteins (HSP). The AR translocates to the nucleus where dimerization, DNA binding, and recruitment of coactivators occurs (6). In men with metastatic prostate cancer, androgen depravation therapy (ADT) improves bone pain, lessens lower UTI symptoms, and increases quality of life (7). Yet, metastatic disease develops in patients failing ADT and emerging with castration-resistant prostate cancer (CRPC), through an aberrant AR signaling mechanism activated despite androgen deprivation. Increased AR expression, elevated intraprostatic androgens and perpetually activated AR signaling in the face of castrate levels of androgens characterize this progression to CRPC (8,9). Prostate tumors employ several mechanisms to bypass or perpetuate AR signaling on the path towards CRPC, including alterations to the AR itself, the synthesis of androgens by prostate cancer cells, or the activation of AR by aberrant signaling pathways (10-12). There are two recognized mechanisms for castration resistance: ligand-dependent and ligand-independent. In the ligand-dependent model, adrenal androgens and intra-tumoral androgens are involved in the development of CRPC. In the ligand-dependent model the increased expression of steroid-5α-reductase isoenzyme-1 allows adrenal androgens to be converted to DHT, bypassing testosterone (13). Adrenal androgens have long been recognized as contributors to androgen production under condition of castration-induced androgen depletion. Still, early attempts to maximize adrenal androgen blockade via adrenalectomy have limited efficacy (14). Recently however, abiraterone acetate, a CYP17A1 inhibitor, blocks the synthesis of adrenal androgens and has been FDA-approved to treat CRPC (15). The presence of residual androgens in the prostate cancer microenvironment points to an autocrine pathway of androgen production that may drive castration resistance (16). Many novel endocrine therapies disruptive to the AR-signaling axis and residual androgen are currently undergoing clinical testing in Phase II clinical trials. The ligand-independent route includes mutations of the AR that may result in the AR responding to AR antagonists, potentially a mechanism underlying the disease remission with the discontinuation of anti-androgen therapy (17,18). Preclinical studies highlight the significance of epigenetic changes such as histone modification and DNA methylation leading to castration resistance (19), supporting optimism towards their therapeutic targeting in clinical trials for the treatment of CRPC (17). Additionally, AR splice variants, unlike wild-type AR, are constitutively active and promote tumor cell growth independent of the ligand (20). The AR signaling inhibitor MDV3100 inhibits the growth of prostate cancer in some cell lines containing the AR splice variants (21). Moreover, the entire AR signaling axis can be bypassed by overexpression of Bcl2, which is an apoptosis blocking protein (22). Innovative therapeutic targeting approaches directly impairing AR activity and localization are currently being interrogated (7).
EMT in control of cell polarity and movement
The onset of mobility requires a relaxation of static actin structures on order to form pliable membrane protrusions. Rigid actin fibers are disassembled upon dorsal circular ruffle formation leaving a fine actin network from which cell membrane protrusions (lamellipodia) are formed (23). Formation of a stable, polarized epithelium requires tight cell-cell and cell-matrix connections. E-cadherin is the major component of epithelial adherens junctions (AJ), which mediate intercellular adhesions. EMT is a cellular process that allows a polarized epithelial cell to assume a mesenchymal phenotype and the first step in this process is the loss of E-cadherin and the collapse of cell-cell communications (24). The downregulation of E-cadherin not only leads to a mechanical disruption of AJ, but it also liberates proteins from the cytoplasmic cell adhesion complex, which exert ambivalent functions depending on the cellular localization. EMT has been identified as an important mechanism in organization of cells within the developing embryo, forming mesenchymal cells following tissue injury, and initiating the invasive and metastatic nature of epithelial cancers (24). EMT and mesenchymal to epithelial transition changes (MET) play critical roles in the development of the prostate gland, the seminal vesicles and kidney. Several studies have established that EMT facilitates malignant transformation of cells and plays an important role in cells’ ability to metastasize (5,25,26). Mesenchymal cells provide support and structure to epithelial cells through the production of an extracellular matrix (ECM) and are highly mobile and invasive, unlike their epithelial counterparts. But, it is believed that insult to cells re-activates these developmental mechanisms out of context in adult cells to trigger oncogenesis. However, EMT is not the only example of epithelial cell plasticity. Another process entails the movement of epithelial cells in a physically and functionally collected group, termed collective migration (27). It is possible that collective migration falls on a spectrum somewhere between EMT and MET. This would make sense in the context of cancer, which lacks the orderly and coordinated induction of EMT (28). In EMT during development, E-cadherin is replaced by N-cadherin and Vimentin and fibronectin replace cytokeratins. These changes also occur in mammary gland tumors undergoing EMT (29).
EMT permits a polarized epithelial cell that normally interacts with a basement membrane via its basal surface to undergo multiple changes that allows it to assume a mesenchymal phenotype. This mesenchymal cell has an elevated resistance to apoptosis, an amplified production of ECM components and has the capacity to migrate and invade (24). One of the classic examples of EMT in organ formation, is the occurrence of EMT in kidney formation, which is driven by genes such as paired box 2 (Pax2), bone morphogenetic protein 7 (Bmp7), and Wilms tumor 1 (Wt1). In fact, several rounds of EMT and MET are necessary to construct the three-dimensional structure of internal organs and to complete the differentiation of specialized cell types (30). Some metastatic cancer cells have shown the ability to re-express E-cadherin after migration and colonization (31) Morphological profiling of EMT consists of several cellular markers. For instance, mesenchymal markers that are increased in EMT include: N-cadherin, Vimentin, Fibronectin, Snail, Slug, Twist, FoxC2, and MMP’s-2, 3, 9. Subsequently, epithelial markers that are decreased in EMT include: E-cadherin, Β-catenin, Cytokeratin, and Desmoplakin (32). Snail and Twist are transcription factors which act as repressors of E-cadherin. TGF-β superfamily members induce Snail1 and Snail2 (33). Moreover microRNAs recently emerged as potent regulators of EMT-MET inter-conversions, with their abilities to target multiple components involved in epithelial integrity or mesenchymal traits, thus impacting tumor progression, metastasis and colonization (34).
EMT-MET cycles in urogenital growth and organ development
The cycling of EMT-MET processes, is instrumental in inprinting urogenital growth during embryomic development. The signaling activities of mesenchymal cells facilitate migration and survival of epithelial cells in an anchorage-independent environment. Expression of the transcription factors Snail1 and Snail2, is necessary for gastrulation to proceed during embryogenesis, by signaling TGF-β mediated EMT. Elegant studies have established that Snail-deficient embryos fail to gastrulate and mesodermal-like cells that are unable to downregulate E-cadherin accumulate at the gonadal streak. These mesodermal cells eventually undergo MET to become the notochord, the somites and the precursors of the urogenital system (30). In male reproductive tracts the Mullerian-inhibiting substance induces EMT in the Mullerian duct, causing its regression. Testicular cords form following the mesonephric endothelial cells that have undergone EMT (30). Snail is thus considered a “master regulator” that upregulates expression of mesenchymal proteins associated with invasion such as: vimentin, fibronectin, metalloproteinase-2, -9, ZEB1 And LEF-1 (35).
EGF-CFC proteins have been implicated as essential signaling cofactors for Nodal, a transforming growth factor β family member whose expression has been defined as embryo specific. Cripto-1 (CR-1), an embryonic gene that encodes for an epidermal growth factor-CFC (EGF-CFC) family member, performs key functions during embryonic development, while it dramatically disappears in normal adult tissues, with the possible exception the stem cells (36). Cripto-1 is highly expressed in a subpopulation of human embryonal carcinoma cells with prostate cancer stem-like characteristics (37). Cripto-1 re-expression in human tumors promotes cell proliferation, migration, invasion, EMT and angiogenesis. This diversity of biological effects is functionally dictated by the interaction of Cripto-1 with an extensive array of signaling molecules. Specifically, Cripto-1 modulates signaling of TGF-β family members, including Nodal, GDF-1/-3, Activin, and TGF-β1, activates c-src/MAPK/Protein Kinase B (AKT) pathway in a Glypican-1 and GRP78-dependent manner (36). It also are cross-talks with erbB4, Wnt/β-catenin, Notch, Caveolin-1, and ALK4 on the cell membrane of epithelial conferring a mesenchymal phenotype. Nodal is coexpressed with Cripto-1 in the mammary gland, and Cripto-1 can phosphorylate the Smad-2 TGF-β signaling effector in epithelial cells (in presence of ALK4) promoting induction of EMT. Cripto-1 contributes to an upregulation of mesenchymal markers including vimentin, Snail and N-cadherin, while it reduces expression of epithelial markers such as E-cadherin during mammary cancer development (38). Cripto-1 expression initially detected in the blastocyst during early embryonic mouse development, is indeed high in stem-like cells in embryonal, melanoma, prostate, and pancreatic cancer cells. Its essential role and contribution to embryogenesis is revealed by genetic studies identifying the embryonic lethality of Cripto-1 knockout mice (38).
The prostate gland is formed during embryonic development from the urogenital sinus, a midline structure with an endodermally derived epithelium surrounded by a mesodermally derived mesenchyme (25). SRY-related high-mobility-group box (Sox) transcription factors are transcription factors that regulate development during male differentiation. One of those transcription factors, SOX9, is found in basal epithelial cells in a normal prostate and is essential for prostate development. SOX9 is highly expressed in fetal prostate cells as the epithelium is expanding into the mesenchyme (39). Moreover, SOX9 stimulates expression of anti-Mullerian hormone in the developing gonad (40), and is also elevated in recurrent prostate cancer (41). SOX9 is a critical signaling partner of both the Wnt/Β-catenin and fibroblast growth factor signaling pathways and can induce AR expression, ultimately impacting growth and progression of metastatic tumors (41). During prostatic development, androgenic action within smooth muscle cells is suppressed by TGF-β via translocation of nuclear AR into the cytoplasm (26). TGF-β is an essential cytokine necessary for embryogenesis, as TGF-β null mutation mice embryos die within several weeks due to an excessive inflammatory response (42). TGF-β signaling is required for testis development also by virtue of its effect in navigating EMT (43). During kidney development, the gene family encoding Snail are downregulated and Snail expression correlates with Cadherin-16 expression in a renal tissue specific pattern towards EMT outcomes (44). Testis cords form via an endothelial to mesenchyme transition following endothelial cell migration. Endothelial cells express cell surface factors that influence surrounding cells as well as remodeling of the surrounding ECM during development (45).
Benign prostatic hyperplasia (BPH) is an abnormal prostate growth condition that resembles embryonic awakening of the gland. Until recently EMT has only casually been linked to the onset of BPH, potentially dictated by macrophages associated with the benign growth of the prostate (24). Morphological-based evidence suggested that accumulation of mesenchymal cells derived from the prostatic epithelium contributes to BPH development, implicating TGF-β mediated EMT in the etiology the disease (46). EMT markers such as N-cadherin, TGF-β2, and Snail are overexpressed in prostate specimens derived from BPH patients and functional communication between macrophages and prostate epithelial cells may lead to induction of TGF-β2 (47). Mechanistically, modulation of AR activity in BPH induces characteristic EMT changes suggesting that AR in prostate epithelial cells may promote macrophage-mediated EMT in BPH (47).
EMT in prostate tumorigenesis
The cytoskeletal rearrangements that tumor cells endure during EMT and blood vessel invasion determine the cell-in-motion plasticity and sensitivity to ECM-adhesion-detachment. Snail and Slug are zinc-finger transcription factors that are instrumental in embryonic development via their regulatory roles in EMT. The functional requirement of the significant players is reflected by the knowledge that Snail knockout mice are embryonically lethal (48). Snail, expressed in response to FGF, binds to the promoter region of the E-cadherin gene to silence E-cadherin expression and induce EMT during gastrulation (49). The Wnt signaling pathway is involved in embryonic development and tumorigenic growth and progression. Components of the Wnt signaling pathway, including Β-catenin, glycogen synthase kinase, lymphoid-enhancer binding factor 1 and cyclin D1 by functionally engaging the AR signaling axis, are capable of modulating the AR-driven transcriptional activity (50). β-catenin is a leading cell-cell adhesion molecule that navigates the dynamics of the actin cytoskeleton remodeling to cell behavior via its functional interaction with E-cadherin. β-catenin acts to link the cytoplasmic domain of the cadherin family of transmembrane proteins to α-catenin and ultimately connecting the adhesion complex to the actin cytoskeleton. Non-invasive cells tend to exhibit β-catenin on the membrane, as opposed to invasive cells that have undergone EMT changes where β-catenin is confined more to the cytosol and the nucleus (51). Expression of β-catenin is distributed in two different cellular localizations: in the cellular membrane associated with E-cadherin, and in the cytoplasm or nucleus in functionally association with an activated Wnt signaling pathway. It is regulated by several signaling pathways through binding to other protein partners including Tcf/LEF family members, axin, APC, and cadherins. In an unorthodox twist of interaction, β-catenin binds to the AR but not to other nuclear steroid receptors, such as the progesterone receptor, the estrogen receptor or the glucocorticoid receptor. When bound to AR, β-catenin is able to translocate into the nucleus, where it increases the ligand-dependent transcriptional activity of AR, increasing expression of AR-dependent promoters such as MMTV and the PSA gene, and consequently contributing to a highly malignant and invasive phenotype. The effect of β-catenin on AR is further enhanced in cells not harboring E-cadherin expression (52). Since elevated β-catenin in association with increased AR has been detected in CRPC, the evidence implicates a value of β-catenin protein expression as potential predictive marker of prostate cancer progression. Indeed, overexpression of β-catenin may explain a mechanism for the emergence of the androgen independent-castration resistant state of prostate cancer (53). Attractive as this concept might be, one must also consider the differential cellular “zip code” of β-catenin that impacts the high expression of nuclear protein in BPH and low-grade prostate cancers, while a decreased level of nuclear β-catenin is associated with increasing Gleason scores and tumor progression to metastasis (53), potentially via deregulation of the EMT-MET phenotypic switching navigated by AR signaling. TGF-β overexpression is detected in the serum of patients with advanced prostate cancer (54). The fluidity of the microenvironment dynamic is enhanced in prostate tumors, as the cancer epithelial cells counterbalance the signals from the cancer-associated fibroblasts and neighboring endothelial cells. In a pre-clinical model of prostate cancer metastasis, the tumor suppressor DAB2IP, can reverse EMT and prevent circulating tumor cells from spreading (55). This is evidence not only identifies a tumor suppressor that controls scaffolding, but it also provides a new platform for targeting tumor-circulating cells undergoing EMT at initiation of metastasis.
TGF-β and AR navigate EMT during tumor progression to metastasis
TGF-β is a multifunctional cytokine that controls critical cellular processes including apoptosis, immune responses, and EMT (56). The TGF-β serine threonine membrane kinases that activate intracellular Smad2/3 proteins, forming a complexthat upon nuclear translocation is responsible for transcription of TGF-β responsive genes. TGF-β promotes EMT and consequently leads to generation of cells with stem cell like properties (57). Moreover, this EMT protagonist (TGF-β) induces proliferation in mesenchymal cells, while it inhibits growth in epithelial cells (58). Cripto-1 is a cell-signaling marker that may play a role in the reversal of TGF-β from a tumor-suppressing gene to a tumor promoter (38). Twist is a helix-loop-helix transcription factor that imparts migratory and invasive characteristics to cells and controls multiple aspects of EMT, primarily by repressing E-cadherin expression (59), while concomitantly induces mesenchymal gene expression (60). Twist is indicative of high-grade tumors, malignant disease progression and resistance to anti-cancer therapies (61), via its ability to suppress apoptosis and promote angiogenesis. Moreover its significant regulatory role in EMT, has rendered Twist an attractive molecular target for CRPC treatment (32).
EMT-based molecular signatures as biomarkers for prostate cancer
The loss of E-cadherin is a hallmark of EMT induction, in association with changes in several interconnected signaling pathways that frame the cellular landscape in advanced tumors (Figure 1). E-cadherin loss correlates with prostate tumor progression and Gleason grade, establishing this EMT player as a prognostic factor for clinical disease progression (62). Elevated N-Cadherin has been shown to be a significant predictor of clinical recurrence in prostate cancer patients following radical prostatectomy (63), as well as an effective therapeutic target in CRPC (64). Activated AR decreases E-cadherin in metastatic prostate and breast cancer cells, leading to a mesenchymal phenotype (65).
TGF-β serves as suppressor of tumorigenesis via apoptosis induction and inhibition of proliferation, but during tumor progression, cells become insensitive to the growth suppressor actions of TGF-β, that it functionally switches to a metastasis promoter leading to tumor invasion and metastasis (66,67). In the prostate TGF-β takes a lead role in tumor-stroma interactions and input of the tumor microenvironment to the metastatic progression of primary cancer epithelial cells to metastasis (68). The stroma induces expression of TGF-β via multilayered signaling events that impact apoptosis, angiogenesis and adhesion, ultimately promoting prostate cancer progression (69,70). In a dynamic cross-talk, androgens enhance the apoptotic effects elicited by TGF-β in prostate cancer cells. Changes within the androgen signaling axis in prostate tumors occur due to altered stromal-epithelial cell interactions and aberrant recruitment of AR co-regulators towards enhanced vascularity and invasion (71). In a dynamic frunctional cross-talk AR enables prostate cancer cells to overcome the apoptotic and EMT action of TGF-β (70,72). Elevated TGF-β ligand correlates with increasing tumor grade in prostate cancer (73) and a dysfunctional TGF-β receptor signaling accelerates prostate cancer progression in a mouse model via EMT and cytoskeleton changes in the tumor microenvironment (68). ZEB-1 is a zinc-finger transcription factor that plays a role in loss of adherens junctions (AJ) during EMT (Figure 1). ZEB1 represses E-cadherin expression, facilitates transendothelial migration, and mediates progression to metastasis (74). Consequential to loss of ZEB1 in prostate cancer cells already undergone EMT, there is a moderate re-expression of E-cadherin, enabling the acquisition of epithelial characteristics (74). Of translational significance is the correlation between elevated ZEB1 expression, induced by androgens, and high Gleason scores in prostate cancer, evidence implicating its biomarker value in predicting the onset of metastatic spread (75), potentially via the involvement of ZEB1 with AR in a feedback loop. Downregulation of AR during ADT leads to unchecked ZEB1 expression, ultimately promoting EMT and metastasis (76). Mechanistic evidence supports an AR function, similar to Snail, in repressing E-cadherin expression and independently triggering EMT (65). In androgen sensitive and TGF-β responsive human prostate cancer cells, Snail is upregulated at the transcriptional and translational level by androgens alone or in combination with TGF-β, while Snail2 expression can be directly enhanced by AR (68,70,77). Work from this laboratory first demonstrated that human prostate cancer cells undergo changes consistent with EMT and cytoskeleton reorganization in response to androgens (70). Interestingly these androgen-mediated EMT changes occur independently of TGF-β and promote aggressive tumor cell behavior (70). The inverse relationship between AR expression and EMT induction suggests that a threshold level of AR observed after the onset of ADT in patients (18), may enhance prostate tumor cell metastatic spread (70). Activation of the β-catenin pathway dictated by AR signaling provides another mechanistic platform via which androgens indirectly impact EMT in prostate tumors (70). The central role played by the AR in EMT enables new insights into therapeutic targeting of androgen axis and AR function in CRPC (78).
Circulating tumor cells (CTCs) and bone turnover markers have been identified as possible biomarkers in the blood that could be used as potential surrogates for clinical benefit in men with CRPC. Detectable levels of CTCs are however found in only 50% of patients with widespread metastases. The problem may be linked to CTC’s undergoing EMT, which could cause underdetection (78). Improved methods of capturing CTCs could enhance the promise of its biomarker value. Bone turnover markers such as bone type 1 collagen breakdown product N-telopeptide have been linked to survival, however the metastasis predictive value is limited by its normal values in patients with bone metastasis (79).
Most of the predictive markers for high-risk disease maybe indirectly associated with EMT-MET interconversions during progression to CRPC (80,81). Prostate Cancer Antigen 3 (PCA3), a noncoding RNA with expression confined to the prostate, is overexpressed in 95% of prostate cancers compared with normal or BPH (82). Progensa PCA3 is a commercially available diagnostic test that detects PCA3 RNA expression in urine and prostatic fluid, with high sensitivity and specificity for prostate cancer (83). The notoriety of the transmembrane protease serine 2 v-ets erythroblastosis virus E26 oncogene homolog (TMPRSS2-ERG) translocation cannot be bypassed, as these gene fusions are detected in more than 50% of human prostate tumors, pointing to a specificity for prostate cancer detection (80). The gene coding AKR1C3, the enzyme responsible for the conversion of adrenal androgens to DHT, is elevated at the mRNA and protein level in CRPC compared to BPH or primary prostate cancer (84). The “universal” presence of AR gene changes in prostate cancer, potentially navigating EMT in disease progression to mCRPC, enables new avenues for therapeutic targeting and predictive screening (85). Decreasing costs of whole-genome sequencing and technology-driven discovery of molecular profiles, lead new avenues of identification of gene signature-based classification markers of prostate cancer progression, as well as therapeutic guidance towards treatment optimization of metastatic disease (18).
Summary
EMT is a morphological phenomenon involving disruption of cell polarity, acquisition of mesenhymal phenotype, and cytoskeleton organization remodeling. The process of EMT is necessary for normal embryonic development and GU organ differentiation, but it is hijacked by mechanisms that promote tumor initiation and progression. EMT in tumor epithelial cells results from transcriptional reprogramming of abnormal survival signals via growth factor receptor signaling regulating apoptosis, survival and cytoskeletal organization. Metastatic CRPC is driven by EMT, a process facilitating epithelial-derived tumors to invade and rapidly metastasize. EMT-MET interconversions via aberrant TGF-β and androgen signaling pathways confer distinct survival and invasive abilities to prostate tumor epithelial cells. How can we, in view of such uninhibited and functionally promiscuous behavior at the cellular level, build a case for EMT as an effective therapeutic target and attractive diagnostic test in patients with advanced prostate cancer? A morphologic reflection of transcriptional events governed by the prostate microenvironment and dictate tumor cell behavior in a controlled pattern of EMT-MET cycling that produces metastasis, may provide valuable insights. Therapeutic targeting of EMT in mRCPC by a proteasome inhibitor suppressing Snail and reducing RKIP is promising (35). Interrogation of disruptive mechanisms via which AR induces EMT under conditions of androgen depletion in CRPC, may define an EMT-MET signature interconversion that would predict therapeutic resistance and facilitate treatment. The tumor suppressor DAB2IP provides scaffolding to modulate prostate tumor EMT towards MET, blocking the metastatic spread at initiation point (55). Thus in a personalized medicine approach, directed by EMT profiling of individual tumors impacting tumor survival pathways and cytoskeleton remodeling, as validated in pre-clinical models of tumor progression (68,80), EMT mechanistic exploitation in prostate cancer metastasis is unlikely to be clinically insignificant.
Acknowledgements
Funding: This work was supported by a grant from the National Institutes of Health, R01 DK083761.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220-41. [DOI] [PubMed] [Google Scholar]
- 2.Taichman RS, Loberg RD, Mehra R, et al. The evolving biology and treatment of prostate cancer. J Clin Invest 2007;117:2351-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matuszak EA, Kyprianou N. Androgen regulation of epithelial-mesenchymal transition in prostate tumorigenesis. Expert Rev Endocrinol Metab 2011;6:469-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaeffer EM, Marchionni L, Huang Z, et al. Androgen-induced programs for prostate epithelial growth and invasion arise in embryogenesis and are reactivated in cancer. Oncogene 2008;27:7180-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayward SW, Cunha GR. The prostate: development and physiology. Radiol Clin North Am 2000;38:1-14. [DOI] [PubMed] [Google Scholar]
- 6.Quigley CA, De Bellis A, Marschke KB, et al. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev 1995;16:271-321. [DOI] [PubMed] [Google Scholar]
- 7.Friedlander TW, Ryan CJ. Targeting the androgen receptor. Urol Clin North Am 2012;39:453-64. [DOI] [PubMed] [Google Scholar]
- 8.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med 2004;10:33-9. [DOI] [PubMed] [Google Scholar]
- 9.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer 2001;1:34-45. [DOI] [PubMed] [Google Scholar]
- 10.Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res 2008;68:6407-15. [DOI] [PubMed] [Google Scholar]
- 11.Culig Z, Hobisch A, Cronauer MV, et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res 1994;54:5474-8. [PubMed] [Google Scholar]
- 12.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res 2006;66:2815-25. [DOI] [PubMed] [Google Scholar]
- 13.Chang KH, Li R, Papari-Zareei M, et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci U S A 2011;108:13728-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhanalaph T, Varkarakis MJ, Murphy GP. Current status of bilateral adrenalectomy or advanced prostatic carcinoma. Ann Surg 1974;179:17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res 2004;10:7121-6. [DOI] [PubMed] [Google Scholar]
- 17.Tsao CK, Galsky MD, Small AC, et al. Targeting the androgen receptor signalling axis in castration-resistant prostate cancer (CRPC). BJU Int 2012;110:1580-8. [DOI] [PubMed] [Google Scholar]
- 18.Logothetis CJ, Gallick GE, Maity SN, et al. Molecular classification of prostate cancer progression: foundation for marker-driven treatment of prostate cancer. Cancer Discov 2013;3:849-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry AS, Watson RW, Lawler M, et al. The epigenome as a therapeutic target in prostate cancer. Nat Rev Urol 2010;7:668-80. [DOI] [PubMed] [Google Scholar]
- 20.Dehm SM, Schmidt LJ, Heemers HV, et al. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res 2008;68:5469-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson PA, Chen YF, Balbas MD, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A 2010;107:16759-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colombel M, Symmans F, Gil S, et al. Detection of the apoptosis-suppressing oncoprotein bc1-2 in hormone-refractory human prostate cancers. Am J Pathol 1993;143:390-400. [PMC free article] [PubMed] [Google Scholar]
- 23.Condeelis J, Singer RH, Segall JE. The great escape: when cancer cells hijack the genes for chemotaxis and motility. Annu Rev Cell Dev Biol 2005;21:695-718. [DOI] [PubMed] [Google Scholar]
- 24.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009;119:1420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marker PC, Donjacour AA, Dahiya R, et al. Hormonal, cellular, and molecular control of prostatic development. Dev Biol 2003;253:165-74. [DOI] [PubMed] [Google Scholar]
- 26.Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation 2008;76:641-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rørth P. Collective cell migration. Annu Rev Cell Dev Biol 2009;25:407-29. [DOI] [PubMed] [Google Scholar]
- 28.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia 2010;15:117-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kokkinos MI, Wafai R, Wong MK, et al. Vimentin and epithelial-mesenchymal transition in human breast cancer-- observations in vitro and in vivo. Cells Tissues Organs 2007;185:191-203. [DOI] [PubMed] [Google Scholar]
- 30.Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871-90. [DOI] [PubMed] [Google Scholar]
- 31.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev 2009;28:151-66. [DOI] [PubMed] [Google Scholar]
- 32.Wallerand H, Robert G, Pasticier G, et al. The epithelial-mesenchymal transition-inducing factor TWIST is an attractive target in advanced and/or metastatic bladder and prostate cancers. Urol Oncol 2010;28:473-9. [DOI] [PubMed] [Google Scholar]
- 33.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 2005;132:3151-61. [DOI] [PubMed] [Google Scholar]
- 34.Korpal M, Ell BJ, Buffa FM, et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med 2011;17:1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baritaki S, Chapman A, Yeung K, et al. Inhibition of epithelial to mesenchymal transition in metastatic prostate cancer cells by the novel proteasome inhibitor, NPI-0052: pivotal roles of Snail repression and RKIP induction. Oncogene 2009;28:3573-85. [DOI] [PubMed] [Google Scholar]
- 36.Nagaoka T, Karasawa H, Castro NP, et al. An evolving web of signaling networks regulated by Cripto-1. Growth Factors 2012;30:13-21. [DOI] [PubMed] [Google Scholar]
- 37.Bianco C, Rangel MC, Castro NP, et al. Role of Cripto-1 in stem cell maintenance and malignant progression. Am J Pathol 2010;177:532-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rangel MC, Karasawa H, Castro NP, et al. Role of Cripto-1 during epithelial-to-mesenchymal transition in development and cancer. Am J Pathol 2012;180:2188-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Leav I, Ibaragi S, et al. SOX9 is expressed in human fetal prostate epithelium and enhances prostate cancer invasion. Cancer Res 2008;68:1625-30. [DOI] [PubMed] [Google Scholar]
- 40.De Santa Barbara P, Bonneaud N, Boizet B, et al. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol Cell Biol 1998;18:6653-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, McKnight NC, Zhang T, et al. SOX9 is expressed in normal prostate basal cells and regulates androgen receptor expression in prostate cancer cells. Cancer Res 2007;67:528-36. [DOI] [PubMed] [Google Scholar]
- 42.Kulkarni AB, Huh CG, Becker D, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A 1993;90:770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarraj MA, Escalona RM, Umbers A, et al. Fetal testis dysgenesis and compromised Leydig cell function in Tgfbr3 (beta glycan) knockout mice. Biol Reprod 2010;82:153-62. [DOI] [PubMed] [Google Scholar]
- 44.Boutet A, De Frutos CA, Maxwell PH, et al. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J 2006;25:5603-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Combes AN, Wilhelm D, Davidson T, et al. Endothelial cell migration directs testis cord formation. Dev Biol 2009;326:112-20. [DOI] [PubMed] [Google Scholar]
- 46.Alonso-Magdalena P, Brössner C, Reiner A, et al. A role for epithelial-mesenchymal transition in the etiology of benign prostatic hyperplasia. Proc Natl Acad Sci U S A 2009;106:2859-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu T, Lin WJ, Izumi K, et al. Targeting androgen receptor to suppress macrophage-induced EMT and benign prostatic hyperplasia (BPH) development. Mol Endocrinol 2012;26:1707-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 2009;119:1438-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell 2001;1:37-49. [DOI] [PubMed] [Google Scholar]
- 50.Robinson DR, Zylstra CR, Williams BO. Wnt signaling and prostate cancer. Curr Drug Targets 2008;9:571-80. [DOI] [PubMed] [Google Scholar]
- 51.Polette M, Mestdagt M, Bindels S, et al. Beta-catenin and ZO-1: shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs 2007;185:61-5. [DOI] [PubMed] [Google Scholar]
- 52.Yang F, Li X, Sharma M, et al. Linking beta-catenin to androgen-signaling pathway. J Biol Chem 2002;277:11336-44. [DOI] [PubMed] [Google Scholar]
- 53.Whitaker HC, Girling J, Warren AY, et al. Alterations in beta-catenin expression and localization in prostate cancer. Prostate 2008;68:1196-205. [DOI] [PubMed] [Google Scholar]
- 54.Ivanović V, Todorović-Raković N, Demajo M, et al. Elevated plasma levels of transforming growth factor-beta 1 (TGF-beta 1) in patients with advanced breast cancer: association with disease progression. Eur J Cancer 2003;39:454-61. [DOI] [PubMed] [Google Scholar]
- 55.Xie D, Gore C, Liu J, et al. Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis. Proc Natl Acad Sci U S A 2010;107:2485-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massagué J. TGFbeta in Cancer. Cell 2008;134:215-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wendt MK, Allington TM, Schiemann WP. Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future Oncol 2009;5:1145-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moses HL, Yang EY, Pietenpol JA. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell 1990;63:245-7. [DOI] [PubMed] [Google Scholar]
- 59.Cakouros D, Raices RM, Gronthos S, et al. Twist-ing cell fate: mechanistic insights into the role of twist in lineage specification/differentiation and tumorigenesis. J Cell Biochem 2010;110:1288-98. [DOI] [PubMed] [Google Scholar]
- 60.Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004;117:927-39. [DOI] [PubMed] [Google Scholar]
- 61.Yuen HF, Chua CW, Chan YP, et al. Significance of TWIST and E-cadherin expression in the metastatic progression of prostatic cancer. Histopathology 2007;50:648-58. [DOI] [PubMed] [Google Scholar]
- 62.Makrilia N, Kollias A, Manolopoulos L, et al. Cell adhesion molecules: role and clinical significance in cancer. Cancer Invest 2009;27:1023-37. [DOI] [PubMed] [Google Scholar]
- 63.Gravdal K, Halvorsen OJ, Haukaas SA, et al. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res 2007;13:7003-11. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka H, Kono E, Tran CP, et al. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth,metastasis and castration resistance. Nat Med 2010;16:1414-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu YN, Liu Y, Lee HJ, et al. Activated androgen receptor downregulates E-cadherin gene expression and promotes tumor metastasis. Mol Cell Biol 2008;28:7096-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akhurst RJ, Derynck R. TGF-beta signaling in cancer--a double-edged sword. Trends Cell Biol 2001;11:S44-51. [DOI] [PubMed] [Google Scholar]
- 67.Niu YN, Xia SJ. Stroma-epithelium crosstalk in prostate cancer. Asian J Androl 2009;11:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pu H, Collazo J, Jones E, et al. Dysfunctional transforming growth factor-beta receptor II accelerates prostate tumorigenesis in the TRAMP mouse model. Cancer Res 2009;69:7366-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang F, Tuxhorn JA, Ressler SJ, et al. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res 2005;65:8887-95. [DOI] [PubMed] [Google Scholar]
- 70.Zhu ML, Kyprianou N. Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells. FASEB J 2010;24:769-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cano P, Godoy A, Escamilla R, et al. Stromal-epithelial cell interactions and androgen receptor-coregulator recruitment is altered in the tissue microenvironment of prostate cancer. Cancer Res 2007;67:511-9. [DOI] [PubMed] [Google Scholar]
- 72.van der Poel HG. Androgen receptor and TGFbeta1/Smad signaling are mutually inhibitory in prostate cancer. Eur Urol 2005;48:1051-8. [DOI] [PubMed] [Google Scholar]
- 73.Bierie B, Moses HL. TGF-beta and cancer. Cytokine Growth Factor Rev 2006;17:29-40. [DOI] [PubMed] [Google Scholar]
- 74.Drake JM, Strohbehn G, Bair TB, et al. ZEB1 enhances transendothelial migration and represses the epithelial phenotype of prostate cancer cells. Mol Biol Cell 2009;20:2207-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anose BM, LaGoo L, Schwendinger J. Characterization of androgen regulation of ZEB-1 and PSA in 22RV1 prostate cancer cells. Adv Exp Med Biol 2008;617:541-6. [DOI] [PubMed] [Google Scholar]
- 76.Sun Y, Wang BE, Leong KG, et al. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res 2012;72:527-36. [DOI] [PubMed] [Google Scholar]
- 77.Bolton EC, So AY, Chaivorapol C, et al. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev 2007;21:2005-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raimondi C, Gradilone A, Naso G, et al. Epithelial-mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res Treat 2011;130:449-55. [DOI] [PubMed] [Google Scholar]
- 79.Armstrong AJ, Eisenberger MA, Halabi S, et al. Biomarkers in the management and treatment of men with metastatic castration-resistant prostate cancer. Eur Urol 2012;61:549-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choudhury AD, Eeles R, Freedland SJ, et al. The role of genetic markers in the management of prostate cancer. Eur Urol 2012;62:577-87. [DOI] [PubMed] [Google Scholar]
- 81.Bernard D, Pourtier-Manzanedo A, Gil J, et al. Myc confers androgen-independent prostate cancer cell growth. J Clin Invest 2003;112:1724-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salagierski M, Schalken JA. Molecular diagnosis of prostate cancer: PCA3 and TMPRSS2:ERG gene fusion. J Urol 2012;187:795-801. [DOI] [PubMed] [Google Scholar]
- 83.van Gils MP, Hessels D, van Hooij O, et al. The time-resolved fluorescence-based PCA3 test on urinary sediments after digital rectal examination; a Dutch multicenter validation of the diagnostic performance. Clin Cancer Res 2007;13:939-43. [DOI] [PubMed] [Google Scholar]
- 84.Hamid AR, Pfeiffer MJ, Verhaegh GW, et al. Aldo-keto reductase family 1 member C3 (AKR1C3) is a biomarker and therapeutic target for castration-resistant prostate cancer. Mol Med 2013;18:1449-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010;18:11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]