Abstract
Background
Among the various cardiovascular diseases, heart failure (HF) is projected to have the largest increases in incidence over the coming decades; therefore, improving HF prediction is of significant value. We evaluated whether cardiac troponin T (cTnT) measured with a high-sensitivity assay and N-terminal-pro-B-type natriuretic peptide (NT-proBNP), biomarkers strongly associated with incident HF, improve HF risk prediction in the Atherosclerosis Risk In Communities (ARIC) study.
Methods
Using gender-specific models, cTnT and NT-proBNP were added to age and race (“laboratory report” model), and to the ARIC HF model (includes age, race, systolic blood pressure, antihypertensive-medication use, current/former smoking, diabetes, body mass index, prevalent coronary heart disease and heart rate) in 9868 subjects without prevalent HF; area under the receiver operating characteristic curve (AUC), integrated discrimination improvement, net reclassification improvement (NRI) and model fit were described.
Results
Over a mean follow-up of 10.4 years, 970 subjects developed incident HF. Adding cTnT and NT-proBNP to the ARIC HF model significantly improved all statistical parameters (AUCs increased by 0.040 and 0.057; the continuous NRI was 50.7% and 54.7% in women and men, respectively). Interestingly, the simpler laboratory report model was statistically no different than the ARIC HF model.
Conclusion
cTnT and NT-proBNP have significant value in HF risk prediction. A simple gender-specific model that includes age, race, cTnT and NT-proBNP (which can be incorporated in a laboratory report) provides a good model, whereas adding cTnT and NT-proBNP to clinical characteristics results in an excellent HF prediction model.
Keywords: cardiac troponin T, NT-proBNP, heart failure, ARIC, risk prediction
Introduction
Over the next 20 years, the prevalence of heart failure (HF) is projected to increase by 25% (1, 2), the associated direct costs by 200% and indirect costs (loss of productivity) by 80%. Although a number of effective evidence-based therapies have been developed to treat symptomatic HF, long-term prognosis remains poor. Hence, prevention, and prediction, of HF is receiving considerable attention. The American College of Cardiology/American Heart Association (ACC/AHA) (3, 4) proposed a simple new HF staging system (stages A–D) to increase early identification of individuals at risk, in which stages A and B were defined as having the risk factors (or milieu) to develop HF but without clinical symptoms. However, this system classified the majority of individuals aged >45 years as stage A or B (5). Therefore, to improve risk prediction, clinical risk prediction tools such as the Health ABC HF Score (6), the Framingham HF risk score (7) and, more recently, the Atherosclerosis Risk in Communities (ARIC) HF score (8) were developed.
Recently, low levels of circulating cardiac troponin T (cTnT), measured with a novel highly sensitive assay, were shown to be strongly associated with HF outcomes in community-based studies, including the ARIC study (9-11). Similarly, levels of N-terminal pro–B-type natriuretic peptide (NT-proBNP), a biomarker of neurohormonal activation and hemodynamic stress, correlated with incident HF in adults without previously recognized cardiovascular disease (12, 13). In a previous analysis (9), we showed that both biomarkers were associated with coronary heart disease (CHD), mortality and HF and that they seemed to improve HF risk prediction; however, in this previous analysis, our baseline prediction models to which the biomarkers were added were not validated/optimized to predict HF. Therefore, the extent to which cTnT and NT-proBNP improve HF risk prediction beyond clinically validated risk assessment tools remained uncertain. Since our prior analysis (9), a clinical model to predict HF in the ARIC study has been described (8).
Therefore, we performed the current analyses to examine i) whether cTnT and NT-proBNP improve the ARIC HF risk prediction model (8); ii) whether simple models incorporating only age, race, gender, cTnT and NT-proBNP (laboratory report model) perform as well as the ARIC HF model (clinical model); and iii) whether specific cTnT and NT-proBNP cut-points can be identified to help improve prediction of HF risk.
Methods
Study population
As described previously (14) and in Supplemental Data, the ARIC study is a prospective, predominantly biracial study of cardiovascular disease and its predictors in middle-aged individuals(n=15,792) recruited from 4 U.S. communities in 1987–1989.The study was approved by the institutional review boards of the 4 participating centers. For the current analysis, we used the fourth ARIC visit (1996–98) as the baseline (cTnT and NT-proBNP were measured using stored blood samples collected during this visit).
Study population
From the 11,656 individuals attending the fourth ARIC visit, we excluded individuals whose race was neither black nor white (n=31), black participants from the Washington County, MD, or Minneapolis centers (n=38), and participants with prevalent HF at visit 1 (n=410), missing HF status at visit 1 (n=199), HF hospitalization between visits 1 and 4 (n=229), missing covariates for ARIC HF model (n=354) or not having given full consent (n=249). Of these eligible individuals, 268 did not have adequate sample to perform both cTnT and NT-proBNP, and additionally 1 and 8 subjects did not have adequate samples to perform cTnT alone or NT-proBNP alone, which left 9,868 individuals eligible for the current analysis.
Assays
cTnT was measured using a highly sensitive assay (lot number 154102, Elecsys Troponin T; Roche Diagnostics, Indianapolis, IN) on a Cobas e411 automated analyzer. The lower and upper limits of detection of the cTnT assay are 3 and 10,000 ng/L, respectively, and the limit of quantitation (the lowest analyte concentration that can be reproducibly measured with an intermediate-precision coefficient of variation of <10%) is 13 ng/L. NT-proBNP was also measured on the automated Cobas e411 analyzer (Roche Diagnostics) using an electrochemiluminescent immunoassay with a measurement range of 5-35,000 pg/mL and a limit of quantitation of 35 pg/mL. The variability in cTnT and NT-proBNP levels related to freeze–thaw cycles and frozen storage has been previously described (15, 16). The reliability coefficient and inter-assay coefficient of variation for both cTnT and NT-proBNP are presented in the supplemental material.
Incident heart failure
The definitions and methods for identifying incident HF in the ARIC study have previously been described (8). Briefly, hospital discharge records with an ICD-9 code of 428.x in any position or death certificates with an ICD-9 code of 428.x or ICD-10 code of I50 were considered incident HF. Further information about tracking events in ARIC is provided in the Supplemental Data.
Statistical analyses
We evaluated cTnT as 6 categories (undetectable, 3-5 ng/L, 6-8 ng/L, 9-13 ng/L, 14-25 ng/L, ≥26 ng/L; additional details in Supplemental Data). For NT-proBNP, we used the logarithm of NT-proBNP, after Winsorizing 6 large values by setting them to 5000 pg/mL. For individuals with cTnT and NT-proBNP below the lower limits of detection, we assigned a value equal to half of the lower limits of detection. Before finalizing our risk prediction models, we tested for interactions between cTnT, NT-proBNP and the variables used in the risk prediction models and found interactions with gender and other risk factors. cTnT effects were stronger for younger individuals and for women, whereas NT-pro BNP effects were stronger for men. When gender-specific models were used, the interactions with other variables in the risk prediction models were no longer statistically significant. We therefore performed and present gender-specific analyses. We initially described individuals with “stage A/B” HF risk (defined as the presence of any of the following: hypertension, diabetes, obesity, metabolic syndrome and prevalent atherosclerotic cardiovascular disease) and individuals with no risk factors (referred to as stage 0 from here on for simplicity). We then described the cTnT and NT-proBNP distribution by HF stage and incident HF status.
Using Cox proportional hazards models, we described hazards ratios for the associations of cTnT and NT-proBNP with incident HF. Model 1 adjusted for age and race and included either cTnT or NT-proBNP (i.e., when evaluating the hazard ratios of cTnT, NT-proBNP was adjusted for and vice versa); model 2 adjusted for all components of model 1 and additionally included systolic blood pressure, antihypertensive medication use, current/former smoking, diabetes, body mass index, prevalent CHD and heart rate (i.e., other factors used in the ARIC HF risk score).
For HF risk prediction, we also described 4 additional models. The “laboratory report model” added cTnT and NT-proBNP to age and race, and the other models added cTnT and NT-proBNP individually and together to the ARIC HF model.
Comparisons of the ability of these models to improve HF risk prediction were tested by using statistical measures of discrimination and calibration at 10 years of follow-up, including improvements in the area under the receiver operating characteristic curve (AUC), net reclassification improvement (NRI) and integrated discrimination improvement (IDI) (17), all calculated with methods that accounted for censoring (18, 19). We also performed a test of model fit test using the Grønnesby– Borgan test statistic (20), in which higher values of the test statistic and significant p-values are associated with poor model fit. In describing the NRI, because there are no previously described HF risk categories, we used risk categories used in CHD risk prediction, namely, 0–5%, 5–10%, 10–20% and >20% 10-year risk. We also calculated the “continuous NRI” as recently described (21). We performed 1000 bootstraps to adjust for the overoptimism that can occur (22) when model fit is tested in the same data in which models are described and to furnish 95% confidence intervals.
We then described the 10-year risk of HF for the various models by deciles of estimated risk and the estimated percentage of HF events occurring within each decile. Finally, we tried to identify potential cTnT and NT-proBNP cut-points by defining both an unweighted and weighted Youden's index (23). The unweighted Youden's index was defined as sensitivity + specificity – 1, and the weighted Youden's index was described by giving higher importance either to sensitivity [2 * (0.75 * sensitivity + 0.25 * specificity) – 1] or specificity [2 * (0.25 * sensitivity + 0.75 * specificity) – 1] to evaluate potential cut-points to “rule out” and “rule in” incident HF occurrence.
Results
The mean age of the study population at ARIC visit 4 was 62.7 years; 44% were males and 80% were white (Table 1). In all, 46% were hypertensive, 16% had diabetes and 7% (n=701) had prevalent CHD. cTnT and NT-proBNP were detectable in 6677 and 9563 subjects, respectively, with 93 and 98 subjects, respectively having values greater than the 99th percentile (as defined in the ARIC population). The 99th percentile for cTnT published by the manufacturer [14 ng/L] corresponds to approximately the 92nd percentile in our analysis. Over a mean follow-up of 10.4 years, there were 970 hospitalizations or deaths with HF (195 in individuals with CHD at baseline). Overall, 74% of the subjects (n=7,278) had at least 1 risk factor which qualified as stage A HF (diabetes, hypertension, obesity, metabolic syndrome or prevalent cardiovascular disease) and 26% (n=2,590) had none of these risk factors (i.e., stage 0). Individuals with stage 0 and stage A HF who developed incident HF had higher cTnT and NT-proBNP levels (Supplemental Data Tables 1A and 1B) than those who did not develop HF. After adjusting for age and race, we estimated that 3.0% and 8.9% of women and 3.5% and 12.7% of men in stages 0 and A, respectively, will develop HF within 10 years.
Table 1.
Baseline characteristics*: ARIC study visit 4 (n=9868)
| Demographics | |
| Age, years | 62.7 (5.65) |
| White race, % | |
| Male gender, % | 44.3 |
| Body mass index, kg/m2 | 28.6 (5.44) |
| Medical History | |
| Hypertension, % | 45.7 |
| Diabetes mellitus, % | 15.6 |
| Systolic blood pressure, mm Hg | 127.3 (18.91) |
| Diastolic blood pressure, mm Hg | 71.0 (10.24) |
| Current smoking, % | 14.7 |
| Former smoking, % | 43.4 |
| Laboratory Data | |
| Total cholesterol, mg/dL£ | 201.4 (36.91) |
| HDL-C, mg/dL£ | 50.2 (16.54) |
| Triglycerides, mg/dL£ | 142.9 (86.97) |
| eGFR, mL/min/1.73 m2 | 82.3 (18.96) |
| hs-CRP, mean [median] (SD), mg/L | 4.3 [2.4] (6.44) |
| NT-proBNP, mean [median] (SD),† pg/m L† | 122.1 [66.7] (259.36) |
| cTnT, mean [median] (SD), ng/L† | 6.5 [5.0] (17.0) |
| Medications | |
| Aspirin, %‡ | 56.1 |
| Antihypertensives, % | 34.3 |
| Statins, %‡ | 10.9 |
| Nonstatin lipid-lowering drugs, % | 3.0 |
| Others parameters | |
| Left ventricular hypertrophy by ECG, %§ | 3.0 |
Abbreviations: cTnT, cardiac troponin T measured by a highly sensitive assay; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; NT-proBNP, N-terminal pro–B-type natriuretic peptide
Data reported as unadjusted mean (standard deviation) unless otherwise specified.
Persons with levels below detectable limits were assigned half the lower limits of detection.
Information available in 9848 subjects.
Information available in 9864 subjects.
To convert cholesterol values to mmol/L divide by 38.6 and to convert triglyceride values to mmol/L divide the triglyceride value by 88.5.
In evaluating the hazards for incident HF, any detectable level of cTnT in men and cTnT levels >5 ng/L in women were associated with incident HF in a minimally adjusted model and in a model adjusted for variables used in the ARIC HF score and NT-proBNP (Supplemental Data Table 2). Overall, the hazards for incident HF increased with increasing cTnT levels, with hazard ratios (in models adjusted for the ARIC HF score + NT-proBNP) of 4.3 (95% confidence interval 2.6, 7.1) in men and 5.3 (95% confidence interval 3.3, 8.4) in women for cTnT values >25 ng/L (Supplemental Data Table 2). Similarly, NT-proBNP levels were associated positively with incident HF in both men and women for both the minimally adjusted model and the fully adjusted ARIC HF + cTnT models (Supplemental Data Table 3).
Heart failure risk prediction
Several models for HF risk prediction were compared (Tables 2 and 3). The model that added cTnT and NT-proBNP to the ARIC HF model was the best model (in terms of the statistical metrics) for predicting HF risk. Adding cTnT and NT-proBNP to the ARIC HF model increased the AUC from 0.779 to 0.836 in men and from 0.776 to 0.817 in women (Table 3). In all, 38% of men and 32% of women were reclassified through the addition of cTnT and NT-proBNP to the ARIC HF model with a resultant NRI of 19.6% in men and 19.9% in women (Table 2). Given that risk categories for HF prediction do not exist and that we created these risk categories based on CHD risk categories, we also described the continuous NRI, which was 54.7% for men and 50.7% for women. Addition of cTnT to a model that included the ARIC HF model + NT-proBNP improved risk prediction, as did adding NT-proBNP to a model that included ARIC HF model + cTnT (Table 2).
Table 2.
Model comparisons with differences in AUC, net reclassification improvement (NRI) and integrated discrimination improvement (IDI)
| Men: | |||||
|---|---|---|---|---|---|
| Model comparisons | AUC difference, 95% CI | IDI | NRI, % | Continuous NRI, % | % reclassified |
| ARIC HF model vs. ARIC HF + biomarker model | 0.057 (0.044, 0.073) | 0.101 (0.079, 0.132) | 19.6 (12.4, 28.3) | 54.7 (42.8, 67.6) | 37.9 |
| ARIC HF model vs. lab model | 0.010 (–0.015, 0.032) | 0.029 (–0.007, 0.063) | –3.7 (–14.6, 8.0) | 2.1 (–18.1, 18.9) | 56.4 |
| Lab model vs. ARIC HF + biomarker model | 0.047 (0.036, 0.063) | 0.073 (0.057, 0.098) | 24.5 (15.9, 32.6) | 53.9 (47.4, 70.8) | 40.4 |
| ARIC HF model + cTnT vs. ARIC HF model + cTnT + NT-proBNP | 0.025 (0.016, 0.035) | 0.049 (0.032, 0.071) | 7.5 (2.1, 15.0) | 41.5 (29.9, 55.7) | 27.3 |
| ARIC HF model + NT-proBNP vs. ARIC HF model + cTnT + NT-proBNP | 0.014 (0.008, 0.023) | 0.031 (0.018, 0.048) | 8.29 (0.1, 11.9) | 23.1 (4.2, 41.9) | 20.0 |
| Women | |||||
|---|---|---|---|---|---|
| Model comparisons | AUC difference, 95% CI | IDI | NRI (%) | Continuous NRI (%) | % reclassified |
| ARIC HF model vs. ARIC HF + biomarker model | 0.040 (0.030, 0.055) | 0.078 (0.060, 0.104) | 19.9 (12.0, 28.3) | 50.7 (38.8, 62.3) | 31.5 |
| ARIC HF model vs. Lab model | –0.009 (–0.034, 0.012) | 0.023 (–0.009, 0.052) | –4.9 (–16.4, 6.3) | –8.1 (–27.6, 6.3) | 48.9 |
| Lab model vs. ARIC HF + biomarker model | 0.050 (0.038, 0.068) | 0.055 (0.042, 0.080) | 27.5 (19.2, 36.2) | 66.1 (55.3, 78.0) | 36.7 |
| ARIC HF model + cTnT vs. ARIC HF model + cTnT +NT-proBNP | 0.012 (0.006, 0.022) | 0.027 (0.015, 0.042) | 7.3 (1.5, 14.0) | 24.5 (15.8, 39.4) | 20.9 |
| ARIC HF model + NT-proBNP vs. ARIC HF model + cTnT + NT-proBNP | 0.012 (0.005, 0.022) | 0.030 (0.016, 0.047) | 7.3 (0.1, 13.6) | 39.7 (16.3, 60.0) | 21.6 |
Table 3.
Area under the receiver operating characteristic curve (AUC) and the goodness of fit test statistic
| AUC | Goodness of model fit: Grønnesby–Borgan test statistic | |||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Model 1 | 0.653 (0.628, 0.676) | 0.658 (0.634, 0.682) | 9.33 (p=0.41) | 18.32 (p=0.03) |
| Model 2 (ARIC HF model) | 0.779 (0.763, 0.800) | 0.776 (0.760, 0.797) | 18.12 (p=0.03) | 21.91 (p=0.01) |
| Model 3 (lab model) | 0.789 (0.767, 0.812) | 0.767 (0.745, 0.789) | 14.35 (p=0.11) | 5.80 (p=0.76) |
| Model 4 (ARIC HF + biomarkers model) | 0.836 (0.821, 0.857) | 0.817 (0.803, 0.837) | 14.60 (p=0.10) | 18.31 (p=0.03) |
| Model 2 + cTnT | 0.811 (0.797, 0.833) | 0.804 (0.790, 0.825) | 15.95 (p=0.07) | 20.39 (p=0.02) |
| Model 2 + NT-proBNP | 0.822 (0.805, 0.843) | 0.804 (0.789, 0.826) | 7.96 (p=0.54) | 19.64 (p=0.02) |
Model 1: Age + race
Model 2: ARIC HF model
Model 3: Model 1+ cTnT + NT-proBNP (lab model)
Model 4: Model 2 + cTnT + NT-proBNP (ARIC HF + biomarkers model)
Given past difficulties in the implementation of risk scores in clinical practice, we evaluated how a simplified approach (more likely to be used in clinical practice) to HF risk prediction would compare. Overall, the laboratory report model was comparable to the ARIC HF model (Tables 2 and 3) with no statistically significant differences in AUC, NRI or IDI. The beta coefficients, “baseline” values of the exposure variables and “baseline” survival probabilities to apply the proportional hazards assumption to calculate t-year risks are provided in Supplemental Data Table 4.
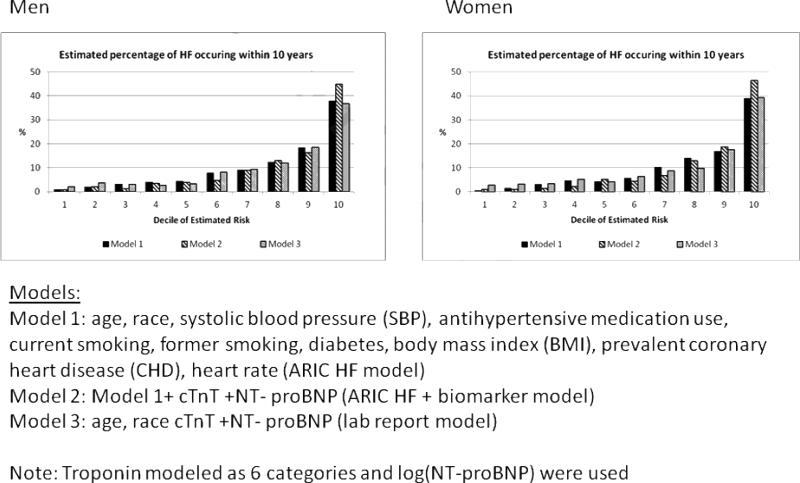
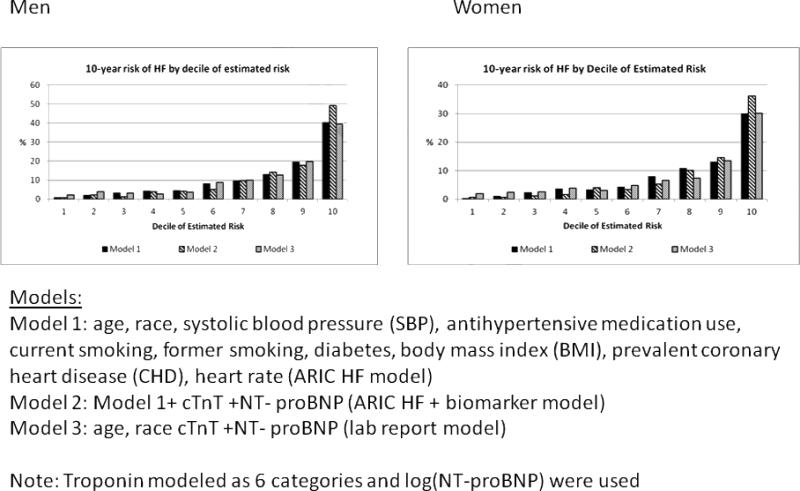
For all the models (ARIC HF, laboratory report and ARIC HF + cTnT +NT-proBNP), in men and women (Figures 1 and 2), the majority of incident HF events occurred in the highest 2 deciles of estimated risk. Figure 1 describes how many of 100 HF events occur by each decile of predicted risk over a 10-year period. For example, in men, approximately 40 events (out of 100) occur in the highest decile of risk. Figure 2 on the other hand describes the number of individuals in each decile of risk who will have incident HF in 10 years. For example, in women, out of every 100 persons whose predicted risk is in the highest decile, 30–35% (depending on the model) will have an HF event within 10 years. Supplemental Data Table 5 provides the cut-points for the various deciles of risk, which can allow the identification and definition of risk categories (low, intermediate and high) if needed.
Figure 1. Distribution (%) of HF events within 10 years over deciles of estimated risk.
In this figure, we describe, in men and women, how many of 100 HF events occur by each decile of predicted risk over a 10-year period.
Figure 2. 10-year risk of HF by decile of estimated risk.
In this figure, we describe, in men and women, the number of individuals in each decile of risk who will have incident HF in 10 years
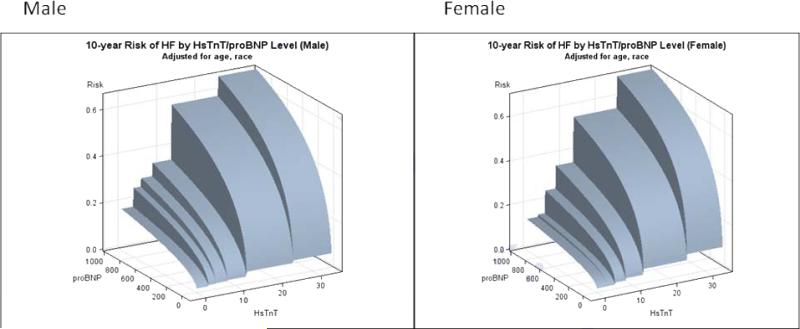
We next investigated cut-points and described the Youden's index (both unweighted and weighted). Because of the continuous, rather monotonic association of cTnT and NT-proBNP with HF events (Figure 3), no clear cut-points emerged (Supplemental Data Table 6). The negative predictive values were, however, uniformly high.
Figure 3. 10-year risk of HF by cTnT/NT-proBNP levels in men and women.
In this figure, we present the 10-year risk of HF (adjusted for age and race) by both cTnT and NT-proBNP level.
Discussion
Among cardiovascular diseases, HF is projected to have the largest increases in incidence over the coming decades (1). HF prevention has therefore gained importance. Applying the ACC and AHA HF classification (3, 4) in a random population of individuals ≥45 years of age identified 56% to be stages A and B (i.e. with risk factors or asymptomatic left ventricular dysfunction but without manifest symptoms of HF (5). Similarly, in our current analysis of middle-aged to older adults, 74% had at least 1 risk factor used to identify HF stage A. Approximately 10% of our entire cohort, initially free of HF, developed incident HF over a mean follow-up of 10.4 years. If the great majority of asymptomatic individuals are classified as “at risk” and only a minority develop incident HF, clearly additional risk stratification is needed to identify individuals at higher risk and direct preventive therapies to these individuals. Several HF risk prediction scores have been developed in the last decade, including ones from Health ABC (6), the Framingham Heart Study (7) and more recently the ARIC study (8). Our current work expanded on the ARIC model and found that adding cTnT measured with a highly sensitive assay and NT-proBNP significantly improved HF risk prediction.
Although our prior work (9) suggested that cTnT and NT-proBNP likely improve HF prediction, our current work studied the value of cTnT and NT-proBNP in detail and reported that these biomarkers, individually and together, added significantly to the ARIC HF prediction model (8). We also evaluated a simpler, perhaps more clinically usable laboratory report model, which included age, race, cTnT and NT-proBNP. Interestingly, the laboratory report model was largely comparable to the ARIC HF model. However, adding both cTnT and NT-proBNP to the ARIC HF model resulted in the best statistical HF risk prediction model.
Although our recommendation and desire is the use of the best available risk prediction score (in our analysis the ARIC HF score + cTnT + NT-proBNP), we recognize that adoption of clinical risk scores in practice has been poor. For example, only 50% of physicians who provided primary care incorporated National Cholesterol Education Program Adult Treatment Panel III guidelines, Joint National Committee on the Prevention, Detection, and Treatment of High Blood Pressure 7 guidelines or American Heart Association Evidence-Based Guidelines for Women in their practices (24). European studies have reported even less use of risk scores (25, 26). An important barrier reported in clinical implementation of guidelines was lack of time (24). While the advent of electronic medical records may help reduce this barrier (for example, risk estimation could be programmed and automatically calculated), simplified approaches, such as our laboratory report model, may also warrant consideration. Providing actuarial risk estimates for HF based on our laboratory report model would be simple and could be implemented automatically on a laboratory report as is currently done in most institutions for estimation of glomerular filtration rate (eGFR). When eGFR (along with various cut-points) reporting was required with each measurement of serum creatinine, several reports suggested a beneficial/positive impact in clinical practice (27, 28). In primary care, prescriptions of NSAIDs and metformin in patients with chronic renal disease were reduced and eGFR increased over time (27). Although the same level of improvement could not be maintained in a follow-up study (29), these studies suggest the potential value of laboratory reporting in calling risk to the attention of clinicians and patients.
We were unable to identify distinct cut-points using Youden's index because of the rather monotonic association between the biomarkers and incident HF. However, a laboratory-based report of risk, factoring in basic information available to the laboratory (i.e., age, race and gender) and the biomarker values (i.e., the “laboratory” report model), could be a good starting point for clinicians to evaluate a patient's HF risk. Furthermore, availability of a risk score in a laboratory report (to which the patient can have easy access) may empower the patient to discuss this further with their physician.
Improved risk prediction does not necessarily translate into improved disease prevention. In fact, a relative paucity of studies that have reported on the use of risk prediction algorithms in clinical practice demonstrated improvement in cardiovascular disease outcomes, although preventive strategies such as statins have had a major impact in reducing the incidence of cardiovascular disease. Furthermore, primordial prevention (i.e., preventing the development of risk factors) is clearly associated with marked decreases in the incidence of various cardiovascular diseases, including HF (30), and should be the overall focus. However, it is also important to note that currently very few individuals in the US population (0.1%) (31) have “ideal” cardiovascular health as identified by the American Heart Association (32), and therefore the general population is likely to have an increasing risk for HF in the years to come. Therapies to prevent the onset of HF must therefore be identified and developed. Good risk prediction tools will help us to identify the highest-risk individuals, who would be expected to have the largest benefit from preventive therapies; additionally, accurate quantitative estimation of HF risk may also help with selection of clinical trial cohorts. For example, based on our models of risk prediction, 10% of the population (i.e., top decile of risk) had an annual HF incidence of 3–4% (Figure 1), which may allow for the effective and efficient design of clinical trials targeting HF prevention. Finally, although cost–benefit analysis is an important aspect of any additional risk prediction test, it is beyond the scope of our analysis. However, identifying individuals at higher risk based on a laboratory test or risk score may alleviate the challenges faced by practicing physicians in selecting individuals with risk factors for HF (such as diabetes or hypertension) who may benefit from further testing with cardiovascular imaging tests such as echocardiograms. Individuals in stage A/B HF form a majority of the middle-aged and older population (~74% in our study), and imaging all of them is not practical. However, a selective approach of identifying the highest-risk individuals using a clinical/laboratory report or combination approach such as ours may identify those at the highest risk who may possibly benefit from additional imaging. Clearly, such strategies will need to be tested before being recommended for clinical use.
Our study had several and strengths and limitations that merit consideration. Our sample size was large as were the number of incident HF events. Further, the ARIC study is well characterized, biracial and has good representation from both genders. The addition of both cTnT and NT-proBNP to clinical predictors in the prediction of HF is novel and finally the exploration of several models is a strength. Both cTnT and NT-proBNP were measured in 2009–2010 from samples obtained in 1996–1998 (ARIC visit 4) and were therefore subject to possible degradation as with any stored sample. Further, intra-individual variability (biological variability) has been noted to be high for NT-proBNP and we had only one measure; however, this mirrors what happens in a clinical setting. Therapies and risk factors may have changed during the follow-up period of 10.4 years, and changes were not accounted for. However, this is the case with any risk prediction tool. Imaging studies such as an echocardiogram may have added value but were not available in the ARIC study. Nonhospitalized, nonfatal HF was missed, but this should be a relatively small proportion of total HF. We did not have information related to all the risk factors that would identify an individual as having stage A HF (e.g., use of chemotherapy agents); however, if anything this would have increased the number of individuals in stage A, further strengthening our argument that better risk prediction tools are required. Additionally, we were unable to classify individuals as stage B HF since we did not have adequate methods to assess for structural heart disease. Therefore some of the individuals we labeled as stage A may have in fact been stage B HF. Also, we were unable to distinguish between HF with and without preserved ejection fraction. Finally, the cost-effectiveness of such a strategy could not be evaluated at this time and will need to be considered in future analyses.
In conclusion, cTnT, measured with a highly sensitive assay, and NT-proBNP are biomarkers strongly associated with incident HF and improved HF risk prediction. A simplified laboratory report model performs similar to the validated ARIC HF model, although the best performance was seen when cTnT and NT-proBNP were added to the ARIC HF model. Further research into the clinical implementation of HF risk prediction models and evaluation of therapies based on predicted risk will be needed.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. The authors would like to thank Kerrie C. Jara for her editorial assistance.
Funding information: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Drs. Astor and Coresh are supported by the National Institute of Diabetes and Digestive and Kidney Diseases (1 R01 DK076770-01). Dr. Nambi is supported by a National Heart, Lung, and Blood Institute grant (5K23HL096893-02). Dr. Virani is supported by a Department of Veterans Affairs Health Services Research and Development Services (HSR&D) Career Development Award (CDA 09-028).
List of abbreviations
- HF
Heart failure
- ACC
American College of Cardiology
- AHA
American Heart Association
- ARIC
Atherosclerosis Risk in Communities
- cTnT
Cardiac troponin T
- NT-proBNP
N-terminal pro–B-type natriuretic peptide
- CHD
Coronary heart disease
- AUC
Area under the receiver operating characteristic curve
- NRI
Net reclassification improvement
- IDI
Integrated discrimination improvement
Footnotes
Previous presentation: Results based on this analysis were presented at the Scientific Sessions of the American Heart Association in Los Angeles, November 2012
Disclosures: Roche Diagnostics provided reagents and loan of an instrument through a grant to Baylor College of Medicine to conduct the highly sensitive cTnT and NT-proBNP assays.
Dr James DeLemos reports receiving grants from Roche and Abbott.
Vijay Nambi, Xiaoxi Liu, Lloyd Chambless, Ron C Hoogeveen and Christie Ballantyne along with Roche and Baylor College of Medicine have filed a provisional patent (patent #61721475) entitled ” Biomarkers to Improve Prediction of Heart Failure Risk.”
References
- 1.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–44. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, et al. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation. 2001;104:2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 5.Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC, Jr., Rodeheffer RJ. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115:1563–70. doi: 10.1161/CIRCULATIONAHA.106.666818. [DOI] [PubMed] [Google Scholar]
- 6.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, et al. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008;1:125–33. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, et al. Prediction of Incident Heart Failure in General Practice: The Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5:422–9. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–63. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 13.Hill SA, Balion CM, Santaguida P, McQueen MJ, Ismaila AS, Reichert SM, et al. Evidence for the use of B-type natriuretic peptides for screening asymptomatic populations and for diagnosis in primary care. Clin Biochem. 2008;41:240–9. doi: 10.1016/j.clinbiochem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 14.The ARIC investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Agarwal SK, Avery CL, Ballantyne CM, Catellier D, Nambi V, Saunders J, et al. Sources of variability in measurements of cardiac troponin T in a community-based sample: the Atherosclerosis Risk in Communities study. Clin Chem. 2011;57:891–7. doi: 10.1373/clinchem.2010.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowatzke WL, Cole TG. Stability of N-terminal pro-brain natriuretic peptide after storage frozen for one year and after multiple freeze-thaw cycles. Clin Chem. 2003;49:1560–2. doi: 10.1373/49.9.1560. [DOI] [PubMed] [Google Scholar]
- 17.Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 18.Chambless LE, Cummiskey CP, Cui G. Several methods to assess improvement in risk prediction models: extension to survival analysis. Stat Med. 2011;30:22–38. doi: 10.1002/sim.4026. [DOI] [PubMed] [Google Scholar]
- 19.Chambless LE, Diao G. Estimation of time-dependent area under the ROC curve for long-term risk prediction. Stat Med. 2006;25:3474–86. doi: 10.1002/sim.2299. [DOI] [PubMed] [Google Scholar]
- 20.Grønnesby JK, Borgan O. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal. 1996;2:315–28. doi: 10.1007/BF00127305. [DOI] [PubMed] [Google Scholar]
- 21.Pencina MJ, D'Agostino RB, Sr., Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Mosca L, Linfante AH, Benjamin EJ, Berra K, Hayes SN, Walsh BW, et al. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation. 2005;111:499–510. doi: 10.1161/01.CIR.0000154568.43333.82. [DOI] [PubMed] [Google Scholar]
- 25.Bonnevie L, Thomsen T, Jorgensen T. The use of computerized decision support systems in preventive cardiology--principal results from the national PRECARD survey in Denmark. Eur J Cardiovasc Prev Rehabil. 2005;12:52–5. [PubMed] [Google Scholar]
- 26.Hobbs FD, Erhardt L. Acceptance of guideline recommendations and perceived implementation of coronary heart disease prevention among primary care physicians in five European countries: the Reassessing European Attitudes about Cardiovascular Treatment (REACT) survey. Fam Pract. 2002;19:596–604. doi: 10.1093/fampra/19.6.596. [DOI] [PubMed] [Google Scholar]
- 27.Wyatt C, Konduri V, Eng J, Rohatgi R. Reporting of estimated GFR in the primary care clinic. Am J Kidney Dis. 2007;49:634–41. doi: 10.1053/j.ajkd.2007.02.258. [DOI] [PubMed] [Google Scholar]
- 28.Levin A, Stevens PE. Early detection of CKD: the benefits, limitations and effects on prognosis. Nat Rev Nephrol. 2011;7:446–57. doi: 10.1038/nrneph.2011.86. [DOI] [PubMed] [Google Scholar]
- 29.Wentworth AL, Fox CH, Kahn LS, Glaser K, Cadzow R. Two years after a quality improvement intervention for chronic kidney disease care in a primary care office. Am J Medical Qual. 2011;26:200–5. doi: 10.1177/1062860610381916. [DOI] [PubMed] [Google Scholar]
- 30.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–7. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–6. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.