Highlights
-
•
Malignant gliomas are extremely resistant to therapies that induce apoptosis.
-
•
Malignant gliomas are less resistant to therapies that induce autophagy.
-
•
Curcumin induces ROS-dependent induction of autophagy in malignant glioma cells.
-
•
Curcumin induces up-regulation of Par-4 in human malignant glioma cells.
-
•
Curcumin induces tumor suppressive lipid, ceramide in malignant glioma cells.
Abbreviations: AMPK, adenosine monophosphate-activated protein kinase; Cur, curcumin; GSH, glutathione; LKB1, liver kinase B1; mTOR, mammalian target of rapamycin; NAC, N-acetylcysteine; p70S6K, p70S6 kinase; Par-4, prostate apoptosis response 4; PARP, poly (ADP-ribose) polymerase; ROS, reactive oxygen species; z-VAD-fmk, N-benzyloxycarbonyl-Val-Ala-Asp fluoromethylketone
Keywords: Curcumin, Glioma, Autophagy, ROS, Par-4, Ceramide
Abstract
Malignant gliomas are extremely resistant to therapies that induce apoptosis, but are less resistant to therapies that induce autophagy. Therefore, drugs targeting autophagy are promising in the management of malignant gliomas. In this study, we investigated the anti-glioma potential of curcumin in vitro, and further examined the molecular mechanisms of curcumin-induced cell death in human malignant glioma. Here, we provide evidence that curcumin is cytotoxic against human malignant glioma cell lines, and the mechanism of cell death caused by curcumin is associated with features of autophagy. Curcumin suppresses the growth of human malignant glioma cells via ROS-dependent prostate apoptosis response-4 (Par-4) induction and ceramide generation. Extracellular supplementation of antioxidants such as glutathione and N-acetylcysteine to glioma cells abrogated the Par-4 induction, ceramide generation, and in turn, prevented curcumin-induced autophagic cell death. Moreover, tumor cells transfected with Par-4 gene sensitized the curcumin-induced autophagic cell death. Overall, this study describes a novel signaling pathway by which curcumin induces ROS-dependent Par-4 activation and ceramide generation, leading to autophagic cell death in human malignant glioma cells.
1. Introduction
Curcumin (also known as diferuloymethane) is the major yellow pigment extracted from turmeric (Curcuma longa), exerts a wide spectrum of biological activities [1]. Curcumin possesses potent antioxidant, anti-inflammatory, anti-viral and anti-bacterial properties [1]. Indeed, curcumin also possess significant chemopreventive efficacy against various malignancies. A plethora of studies have demonstrated the potent anticancer effect of curcumin both in vitro and in vivo on a wide variety of cancer models [2]. Moreover, curcumin has been shown to induce cell death in apoptosis resistant cancer cells [3,4].
Autophagy is a so-called ‘self-eating’ cellular mechanism that degrades damaged or obsolete proteins and organelles, the product of which are recycled to generate macromolecules and ATP so as to maintain the cellular homeostasis [5] . Recently, interest in autophagy has been renewed amongst cancer biologists, since different types of cancer cells were identified to undergo autophagy in response to anticancer therapies [6]. Malignant glioma cells are more likely to respond to therapy through autophagy than through apoptosis. For instance, Temozolomide, one of the most efficacious chemotherapeutic agents employed in the treatment of glioma, exerts its cytotoxicity by inducing autophagic cell death, and has demonstrated a real therapeutic benefit in apoptosis-resistant glioblastoma patients [7,8]. Thus, identification of novel and efficient pro-autophagic drugs and elucidation of their molecular signaling pathway, undoubtedly, will have a direct impact on future therapies in the fight against malignant glioblastoma.
It is widely accepted that oxidative stress can induce autophagy [9,10]. It has been suggested that ROS have important signaling role in neuronal autophagic cell death in response to nerve growth factor deprivation [11]. Moreover, tumor necrosis factor (TNF)-α has been shown to induce autophagic cell death via a ROS-dependent mechanism [12]. In another study, it has been shown that ROS were both sufficient and essential to induce autophagic cell death in lipopolysaccharide-activated macrophages [13].
The prostate apoptosis response-4 (Par-4), a tumor suppressor protein, was originally discovered in rat prostate cancer cells when they were induced to undergo apoptosis [14,15]. Par-4 can selectively induce apoptosis in a wide variety of cancer cells, leaving the normal cells unaffected. This selective nature of Par-4 makes it an attractive therapeutic option. Recently, it has been reported that low Par-4 expression is associated with increase in breast cancer recurrence [16]. These findings underscore the importance of Par-4 as a tumor suppressor protein.
Ceramide is a sphingolipid which has been shown to exert potent antitumor effect against a variety of cancer cells. A diverse array of stressors, including TNF-α, Fas ligation, UV-irradiation, heat shock, and anticancer drugs were reported to increase intracellular ceramide level leading to the induction of apoptosis [17]. In addition to apoptosis, ceramide has more recently been implicated in the induction of autophagy [18,19]. However, the precise role and mechanism of ceramide in autophagy remains unclear.
To the best of our knowledge, this is the first report to demonstrate that curcumin induces autophagy, which is regulated by the Par-4 up-regulation and ceramide generation via ROS-dependent mechanism. Our finding suggests that curcumin has the potential to be developed into a pro-autophagic drug for the treatment of malignant gliomas.
2. Materials and methods
2.1. Chemicals and antibodies
Curcumin, glutathione (GSH), N-acetyl cysteine (NAC), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT), acridine orange (AO), 3-methyl adenine (3-MA), GW4869, desipramine, phthaldialdehyde (OPA), dimethyl sulfoxide (DMSO), anti-rabbit IgG and anti-mouse IgG were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Oxidation sensitive DCFH-DA (D-399) was from Molecular Probes (Eugene, OR, USA). Dulbecco’s modified essential medium (DMEM), Opti MEM medium, phosphate buffered saline (PBS), trypsin–EDTA and fetal bovine serum (FBS) were from GIBCO BRL (Grand Island, NY, USA). Fumonisin B1, myriocin, and z-VAD-fmk were from Alexis (San Diego, CA, USA). Anti-actin, and anti-MAP LC3β (N-20), anti-p62/SQSTM1, anti-Par-4 and donkey anti-goat IgG antibodies were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Anti-PARP, Anti-phospho AMPK Thr172, Anti-AMPK, Anti-phospho LKB1 Ser428, LKB1, Anti-phospho mTOR Ser2448, anti-mTOR , anti-phospho p70S6K Thr389, anti-p70S6K, anti-TFEB, anti-H3 and anti-LC3B (D11) XP antibodies were from Cell Signaling Technology (Beverly, MA, USA). Hydrogen peroxide was from Merck Millipore. MegaTran 1.0 transfection reagent was from OriGene.
2.2. Glioma cell lines, cell culture conditions and drug treatment
The cell lines U87MG and U118MG (ATCC, Rockville, MD, USA) were grown in DMEM supplemented with 10% heat inactivated FBS. All cell lines were grown without antibiotics in an incubator containing humidified atmosphere of 95% air and 5% CO2 at 37 °C. Curcumin stock solution (20 mM; in DMSO) was kept in a dark colored bottle at −20 °C. Cells were grown to about 70% confluences and then treated with curcumin at different concentrations (0–100 μM) and for different period of time (0–24 h). Cells treated with a medium containing an equivalent amount of DMSO without curcumin was served as control.
2.3. Cell viability and cytotoxicity assay
Cell viability following treatment with curcumin was assessed by trypan blue dye exclusion test. After treatment with curcumin, cells were detached with trypsin EDTA and trypan blue assays were performed as described previously [20].
Cytotoxicity assay were carried out as described previously [20]. After treatment with curcumin, 25 μl of MTT (5 mg/ml in PBS) was added to each well and the assay was performed as described previously [20].
2.4. Protein lysate preparation and Western blot analysis
Whole cell lysate with or without curcumin treatments were prepared and performed Western blot analysis as described previously [20]. For preparing nuclear and cytosolic fractions cells were washed twice in PBS and resuspended in 500 μl RSB buffer (10 mM NaCl, 3 mM MgCl2 and 10 mM Tris–HCl, pH 7.4 and EDTA free Rosh protease inhibitor tablets per 20 ml buffer) for 10 min on ice. Cells were disrupted with a Dounce homogenizer until over 90% of the cells were disrupted. The homogenate was centrifuged at 1000×g for 5 min at 4 °C. The supernatant (cytosolic fraction) was transferred and nuclear pellet was washed with RSB buffer and proteins were extracted with RIPA lysis buffer.
2.5. Measurement of intracellular ROS Levels
After the curcumin treatment, generation of ROS in the cells was measured by oxidation-sensitive fluorescent probe DCFH-DA as described previously [20].
2.6. DNA fragmentation analysis
After the curcumin treatment, cells were washed with PBS and incubated with 200 μl of lysis buffer (50 mM Tris–HCl (pH 7.5), 3% non-ionic detergent IGEPAL CA-630 and 20 mM EDTA) for 10 min. DNA fragments were isolated from the cells and analyzed as described previously [21].
2.7. Intracellular ceramide measurement
Intracellular ceramide was measured as described previously [22]. After the curcumin treatment, cells were washed in PBS and lysed using ceramide assay lysis buffer (50 mM Tris (pH-7.4) containing 0.4% IGEPAL CA 630) by freeze and thaw method. The final concentration of IGEPAL CA 630 in the assay was 0.2%. The lysate was then heated at 70 °C for 5 min in a water bath and centrifuged at 12000 rpm for 10 min at 4 °C. The reaction was started by mixing 10 μl of supernatant with 10 ng recombinant human neutral ceramidase enzyme (10 μl), and incubated for 1 h at 37 °C. After stopping the reaction by adding 55 μl of stopping buffer (1:9, 0.07 M potassium hydrogen phosphate buffer: methanol), the released SPH was derivatized with OPA as described previously [22]. After incubation for 30 min at room temperature in the dark, an aliquot of 25 μl was used for the ceramide analysis. HPLC analyses were conducted using Waters 1525 binary pump system. Waters XTerra RP18 (5 μm, 3 × 250 mm) column was equilibrated with a mobile phase (20% methanol, 80% 1:9 stopping buffer) at a flow rate of 0.5 ml/min. The fluorescence detector (Waters 2475) was set at an excitation wavelength of 340 nm and an emission wavelength of 455 nm.
2.8. Plasmids and transient transfection
pCMV6-XL6-Par-4 (SC110969) and pCMV6-Myc-DDK-tagged PAR-4 (RC202733) were purchased from OriGene Technologies, Inc. The green fluorescent protein (GFP)-tagged microtubule-associated protein light chain 3 (LC3) expression vector, pEGFP-LC3 were obtained from Addgene 21073 (Dr. Tamotsu Yoshimori’s laboratory, Osaka University, Osaka, Japan). DNA transfection to cells was performed by using MegaTran 1.0 transfection reagent as described in the manufacture’s protocol.
2.9. GFP-LC3 dot assay
U87MG and U118MG cells (stably transfected with pEGFP-LC3 using MegaTran 1.0 transfection reagent according to the manufacture’s instruction) were treated with curcumin and formation of LC3 puncta was examined under Olympus DP71 fluorescent microscope. To quantify LC3 puncta, 100 GFP-LC3 cells that had more than 10 bright GFP-LC3 punctate spots were counted.
2.10. Detection and quantification of acidic vesicular organelles (AVOs) with AO staining
Cells were incubated with AO (1 μg/ml) for 20 min and examined under Olympus DP71 fluorescent microscope. To quantify the development of AVOs, after incubation with AO, cells were removed from the plate using trypsin–EDTA, resuspended in phenol red free DMEM and analyzed by BD FACSCanto II using BD FACSDiva software. A minimum of 10,000 cells within the gated region were analyzed.
2.11. Statistical analysis
Each experiment was performed at least three times and data are shown as the mean ± standard deviation. Statistical analysis was performed using Graph pad Prism 5.0 by one-way anova and Bonferroni post hoc test. A difference was considered significant when p < 0.05.
3. Results
3.1. Curcumin activates autophagy in human malignant glioma cells
Autophagy has been postulated as a potential mechanism whereby eukaryotic cells commit suicide by degrading their own cytoplasmic organelles. In initial study, malignant glioma cells over expressing GFP-LC3 were treated with curcumin in order to know whether they undergo autophagy in response to the treatment. LC3 is the mammalian homologue of yeast Atg8. During autophagy, GFP-LC3 converts from diffuse pattern to the accumulation of puncta in the cytoplasm which represents an effective way of detecting autophagosome formation [23]. Treatment of GFP-LC3 stable U87MG and U118MG cells with curcumin resulted in significant dose-dependent increase in the number of cells with punctate fluorescence (Fig. 1A).
Fig. 1.
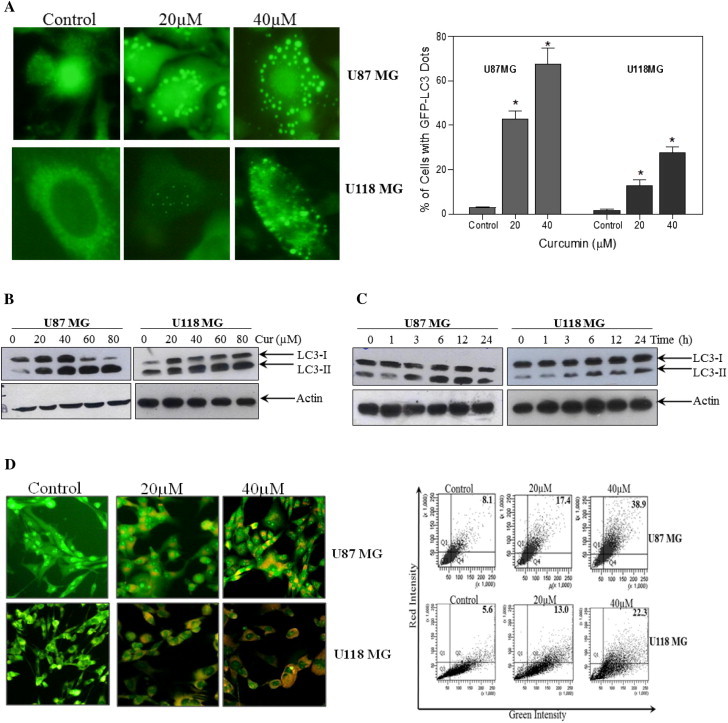
Induction of autophagy in glioma cells treated with curcumin. (A) Glioma cells stably transfected with GFP-LC3 were treated with indicated concentration of curcumin for 24 h. After the treatment, curcumin-induced autophagy manifesting as GFP-LC3 puncta was assessed by fluorescence microscopy (left). Quantitatively, puncta were counted as described in the Section 2 (right). Data shown are means ± SD (n = 3) (∗p < 0.01, verses control). (B) Glioma cells were treated with indicated concentration of curcumin for 24 h and (C) with 60 μM curcumin for indicated time period. Western blot analysis of LC3-I and LC3-II were detected. Actin was used as loading control. (D) Cells were treated with indicated concentration of curcumin for 24 h followed by staining with 1 μg/ml AO for 20 min at 37 °C. A representative slide under fluorescence microscopy shows curcumin-induced AVOs displaying red bright fluorescence staining (left). Quantitative analysis of cells with red bright fluorescence using fluorescence activated cell sorter (right). (E) Cells were treated with 40 μM curcumin in the presence or absence of Baf A1 (250 nM) followed by Western blot analysis of LC3-II. Actin was used as loading control. (F) Glioma cells were treated with indicated concentration of curcumin for 24 h and (G) with 60 μM curcumin for indicated time period. After the treatment, level of p62/SQSTM1 was detected. Actin was used as loading control. (H) Cells were subjected to nuclear and/or cytosolic fractionation and blotted with antibody against TFEB. H3 and actin were used as the loading control for nuclear and cytosolic fractions, respectively. (I) Cells were treated with 60 μM concentration of curcumin for 24 h and Western blot analysis of p-LKB1, LKB1, p-AMPK, AMPK, p-mTOR, mTOR, p-p70S6K and p70SK were performed. Actin was used as loading control.
LC3 protein is known to exist in two forms, LC3-I residing in the cytosol and its phosphatidylethanolamine conjugated form LC3-II, which is the major component of autophagosome. The amount of LC3-II formation is considered as a good early marker for the autophagy induction [23]. Treatment of glioma cells with curcumin resulted in a significant increase in LC3-II levels in both dose- and time-dependent manner (Fig. 1B and C). To further clarify the ability of curcumin to induce autophagy, we examined the development of AVOs with AO staining, followed by fluorescent microscopy and flow cytometry as reported previously [24]. Treatment of glioma cells with curcumin led to accumulation of AO-stained orange–red acidic vesicles resembling autolysosomes (Fig. 1D). The quantitative analysis of AO-stained cells demonstrated a dramatic increase in red fluorescence in U87MG cells from 8.1% to 38.9%, and in U118MG cells from 5.6% to 22.3% (Fig. 1D).
Next, we examined the autophagic flux by LC3 turnover assay, which measures the autolysosomal degradation of LC3-II by comparing the amount of LC3-II in the presence and absence of the autolysosome formation inhibitor bafilomycin A1 (BafA1). Treatment with BafA1 resulted in a significant increase in endogenous LC3-II accumulation. The BafA1-induced LC3-II accumulation was apparently augmented in cells exposed to curcumin (Fig. 1E), suggesting an increase in the autophagic flux. To further confirm the autophagic flux, the effects of curcumin on the expression of p62/SQSTM-1, an LC3-intracting protein degraded in the autolysosomes [23] was examined. Treatment of U87MG and U118MG cells with curcumin resulted in a dose- and time-dependent decrease in detectable p62/SQSTM1 protein level (Fig. 1F and G). Altogether, these data illustrate the ability of curcumin to increase autophagy in human malignant glioma cells.
It has been reported that transcription factor EB (TFEB), a master gene for lysosomal biogenesis, has an important role in the induction of autophagy by positively regulating the expression of several autophagy genes [25]. To achieve this TFEB, which normally resides in the cytosol, must translocate to the nucleus [25]. In order to investigate whether TFEB translocates to the nucleus during the curcumin-induced autophagy, both glioma cells were treated with 0–40 μM curcumin for 24 h. After the treatment, nuclear fractions were isolated and Western blot analysis was performed. As shown in the Fig. 1H, treatment of glioma cells with curcumin, indeed, resulted in an efficient TFEB nuclear translocation.
Previously, it has been reported that LKB1/AMPK activation leads to the induction of autophagy through negative regulation of mTOR [26,27]. Therefore, we examined whether phosphorylation of LKB1 and AMPK are involved in the curcumin-induced autophagy in human malignant glioma cells. As shown in the Fig. 1I, treatment of both U87MG and U118MG cells with curcumin resulted in the phosphorylation of LKB1 and its downstream target AMPK in a time dependent manner. In line with this, curcumin treatment also resulted in the dephosphorylation of mTOR and its downstream target p70S6K in human malignant glioma cells (Fig. 1I). Taken together, these findings indicate that LKB1/AMPK/mTOR/p70S6K pathway is involved in the curcumin-induced autophagy in human malignant glioma cells.
3.2. Curcumin induces caspase-independent cell death associated with features of autophagy
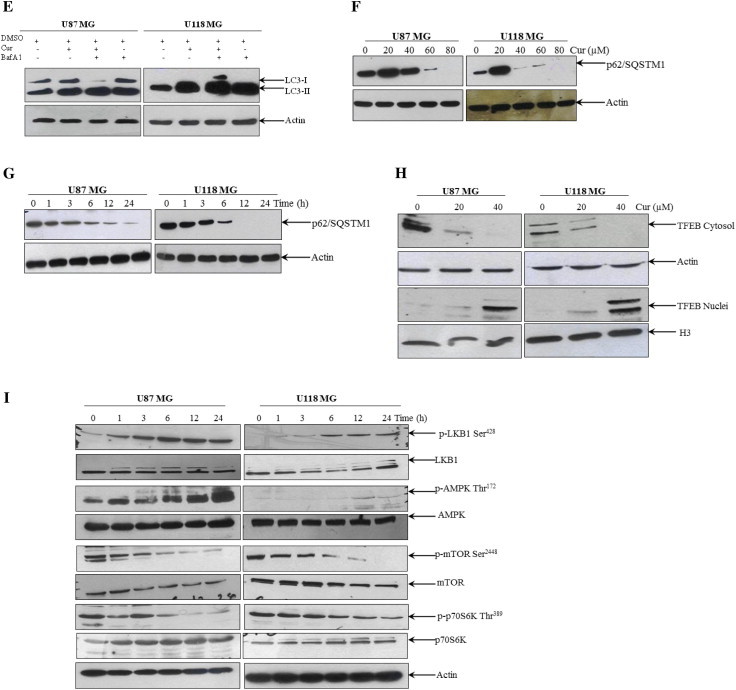
To ascertain the antitumor action of curcumin, the effect of curcumin on the viability of both U87MG and U118MG cells were examined by using MTT assay. As shown in Fig. 2A and B, exposure of glioma cells to curcumin resulted in both a dose- and a time-dependent reduction in viability. Since changes in cellular metabolism may affect the MTT assay, cell viability were also measured using trypan blue dye exclusion test. As shown in Fig. 2C, trypan blue data correlates to the MTT data. Morphological analysis shows that curcumin treated cells were rounded up, swelled and detached from the dish, without the presence of apoptotic bodies (Fig. 2D). In malignant cells, curcumin has also been reported to induce apoptosis which is characterized by caspase-3 activation and PARP cleavage, prime hallmark of apoptotic cell death [28]. In order to know whether the cytotoxic effect of curcumin on these glioma cells is associated with induction of apoptosis, U87MG and U118MG cells were treated with increasing concentrations of curcumin for 24 h, and PARP cleavage was examined. As shown in the Fig. 2E, curcumin treatment did not induce PARP cleavage in these two glioma cells tested. Whereas, a solid PARP cleavage was evident in Jurkat cells which is used as a positive control [28]. In line with this, curcumin treatment also failed to induce “DNA ladder” fragmentation, a typical marker for cells undergoing apoptosis (Fig. 2F). Whereas, a pattern of apoptotic DNA fragmentation was evident in Jurkat cells treated with curcumin. Additionally, we performed MTT viability assay in cells stimulated with curcumin in the presence and absence of pan caspase inhibitor z-VAD-fmk. Cell death induced by curcumin was not attenuated by the z-VAD-fmk in malignant glioma cells (Fig. 2G). However, pretreatment with z-VAD-fmk significantly inhibited the curcumin-induced cell death in Jurkat cells (Fig. 2G). Taken together, these data strongly suggest that curcumin induces caspase-independent death in human malignant glioma cells.
Fig. 2.
Curcumin induces caspase-independent cell death in human malignant glioma cells. (A) Glioma cells were treated with the indicated concentration of curcumin for 24 h and (B) with 60 μM curcumin for the indicated time period. Cell viability was assessed by MTT assay. Data shown are means ± SD (n = 3). (C) Cells were treated with indicated concentrations of curcumin for 24 h and cell viability was assessed by trypan blue dye exclusion test. (D) Morphological changes by curcumin treatment in U87MG and U118MG cells. (E) PARP cleavage was determined by Western blot analysis. (F) DNA fragmentation was analyzed as described in the Section 2. Jurkat cells, treated or untreated with curcumin (25 μM), were used as a positive control for apoptotic PARP cleavage and DNA fragmentation. (G) Cells were pretreated with z-VAD-fmk (50 μM) for 1 h before curcumin (60 μM) treatment for further 24 h and cell viability was measured using MTT assay. Similar experiment in Jurkat cells using 25 μM curcumin were used as a positive control. Data shown are means ± SD (n = 3) (∗p < 0.01).
3.3. 3-Methyladenine decreases curcumin-induced autophagy and cytotoxicity
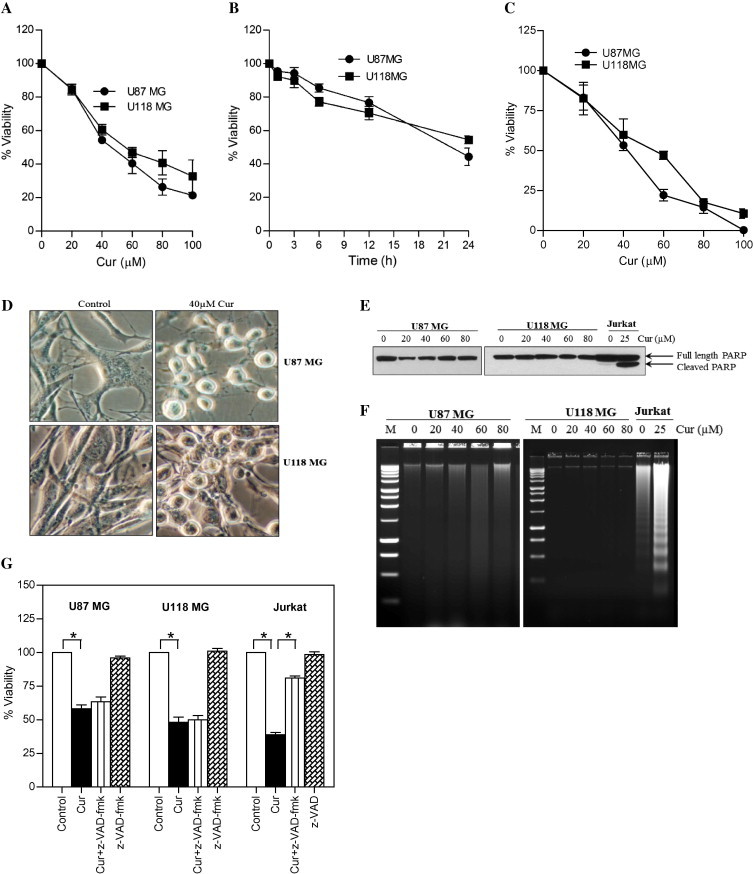
Pretreatment with 3-MA (a most commonly used autophagy inhibitor) significantly reduced the curcumin-induced GFP-LC3 puncta formation (Fig. 3A), AVOs formation (Fig. 3B), and also partly protected the curcumin-induced cytotoxicity (Fig. 3C). These findings indicate that, the mechanism of anticancer activity of curcumin in glioma cells is partially attributable to the induction of autophagy.
Fig. 3.
Effect of 3-MA on curcumin-induced autophagic cell death in human malignant glioma cells. (A) Glioma cells stably transfected with GFP-LC3 were pretreated with 3-MA (2.5 mM) for 1 h followed by curcumin (40 μM) treatment for 24 h. After the treatment, curcumin-induced GFP-LC3 dots were assessed by fluorescence microscopy (left). Quantitatively, puncta were counted as described in Section 2 (right). Data shown are means ± SD (n = 3). (B) U87MG and U118MG cells were pretreated with 3-MA (2.5 mM) for 1 h followed by curcumin (40 μM) treatment for 24 h. After the treatment, cells were stained with 1 μg/ml AO for 20 min at 37 °C. A representative slide under fluorescence microscopy shows curcumin-induced AVOs displaying red bright fluorescence (left). Quantitative analysis of cells with red bright fluorescence using fluorescence activated cell sorter (right). (C) Cell viability was assessed by MTT assay. Data shown are means ± SD (n = 3) (∗p < 0.01 and #p < 0.05).
3.4. Curcumin triggers intracellular ROS generation in human malignant glioma cells
Recently, several reports provided strong evidence for the involvement of ROS in the induction of autophagy in response to chemotherapy-induced stress [9,10]. Furthermore, curcumin has shown to induce rapid accumulation of ROS which leads to caspase-dependent and -independent apoptosis [20]. In order to understand the involvement of ROS in curcumin-induced autophagy, both U87MG and U118MG cells were stained with DCFH-DA followed by curcumin treatment. Curcumin treatment resulted in an increase in DCFH-DA derived fluorescence by 7-fold in U87MG cells within 30 min, and by 2.5-fold in U118MG cells within 5 min (Fig. 4A and B). To know if ROS has any role in curcumin-induced autophagy, both U87MG and U118MG cells were pretreated with thiol-based ROS scavengers, namely GSH (10 mM) or NAC (10 mM), followed by the treatment with curcumin. Attenuation of ROS levels by GSH and NAC almost completely abolished curcumin-induced GFP-LC3 puncta formation (Fig. 4C), LC3-II conversion and p62/SQSTM1 degradation (Fig. 4D), AVOs formation (Fig. 4E) and cell death (Fig. 4F).
Fig. 4.
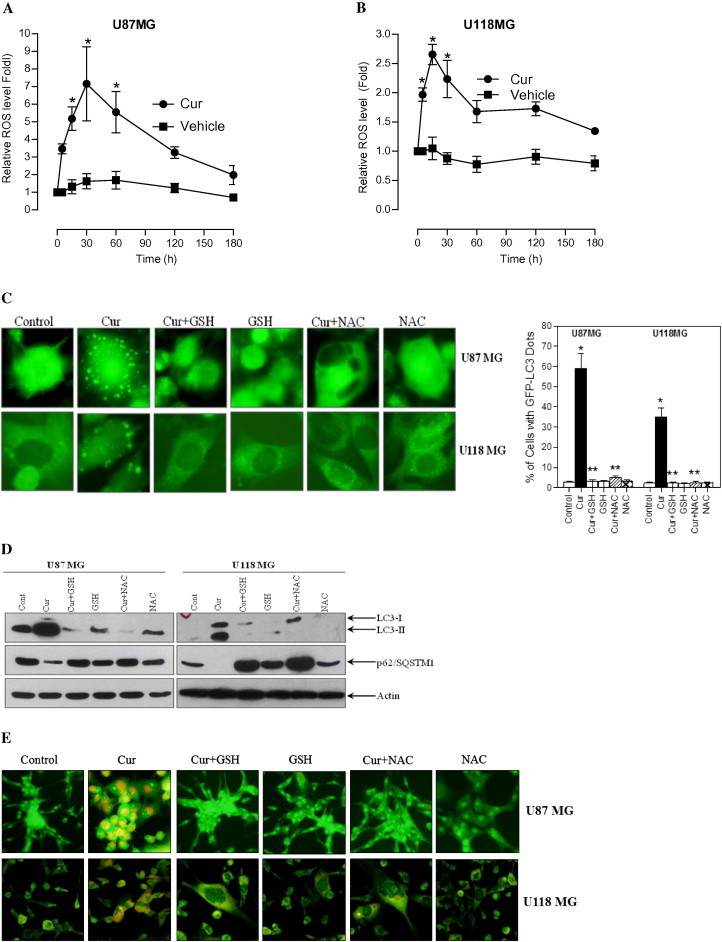
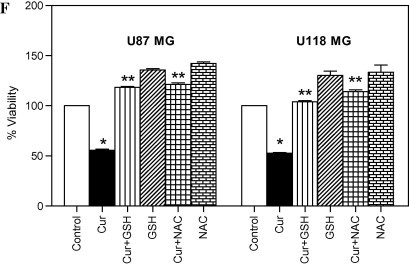
Curcumin induces intracellular ROS-mediated autophagy and cell death in human malignant glioma cells. (A) U87MG and (B) U118MG cells were treated with curcumin (60 μM) for indicated time period. Following the treatment, cells were stained with DCFH-DA and analyzed by fluorometry. Data shown are means ± SD (n = 3) (∗p < 0.05, verses control). (C) Glioma cells stably transfected with GFP-LC3 were pretreated with GSH (10 mM) and NAC (10 mM) for 1 h followed by curcumin (40 μM) treatment for 24 h. After the treatment, curcumin-induced GFP-LC3 dots were assessed by fluorescence microscopy (left). Quantitatively, puncta were counted as described in Section 2 (right). Data shown are means ± SD (n = 3). (∗p < 0.01, verses control and ∗∗p < 0.01, verses curcumin). (D) Cells were pretreated with GSH (10 mM) and NAC (10 mM) for 1 h followed by curcumin (40 μM) treatment for 24 h. After the treatment, cell extracts were prepared for the detection of LC3-II and p62/SQSTM1 by Western blot analysis. Actin was used as loading control. (E) Cells were stained with 1 μg/ml AO for 20 min at 37 °C. A representative slide under fluorescence microscopy shows curcumin-induced AVOs displaying red bright fluorescence. (F) Cell viability was assessed by MTT assay. Data shown are means ± SD (n = 3) (∗p < 0.01, verses control and ∗∗p < 0.01, verses curcumin).
Extracellular addition of H2O2 can be used to mimic the effects of intracellularly produced ROS [10,29]. In order to further confirm the involvement of ROS in curcumin-induced autophagy, U87MG cells were treated with 2 mM H2O2 for 24 h. As expected, H2O2 treatment resulted in an increase in GFP-LC3 puncta formation (Supplementary Fig. 1A), AVOs formation (Supplementary Fig. 1B) and cell death (Supplementary Fig. 1C). All together, these findings suggest the prime role of ROS in curcumin-induced autophagic cell death in malignant glioma.
3.5. Curcumin-induced autophagic cell death is associated with the ROS-dependent induction of Par-4 in human malignant glioma cells
Prostate apoptosis response-4 is a pro-apoptotic tumor-suppressor protein that selectively induces apoptosis in cancer cells [14]. Previously, we have reported that curcumin induces caspase-3-dependent cleavage of Par-4 that leads to the induction of apoptosis [21]. In order to know whether Par-4 is involved in curcumin-induced autophagic cell death, the effect of curcumin on Par-4 expression was tested. Curcumin treatment resulted in significant up-regulation of Par-4 in a dose- and time-dependent manner in both glioma cells tested (Fig. 5A and B).
Fig. 5.
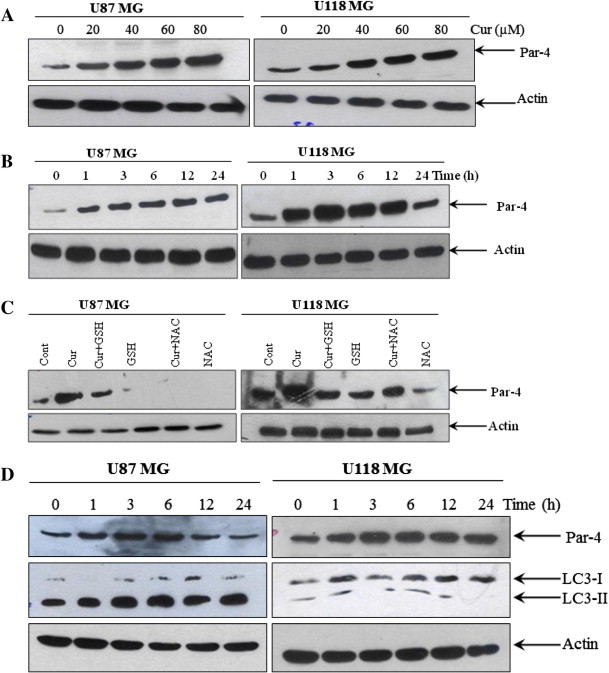
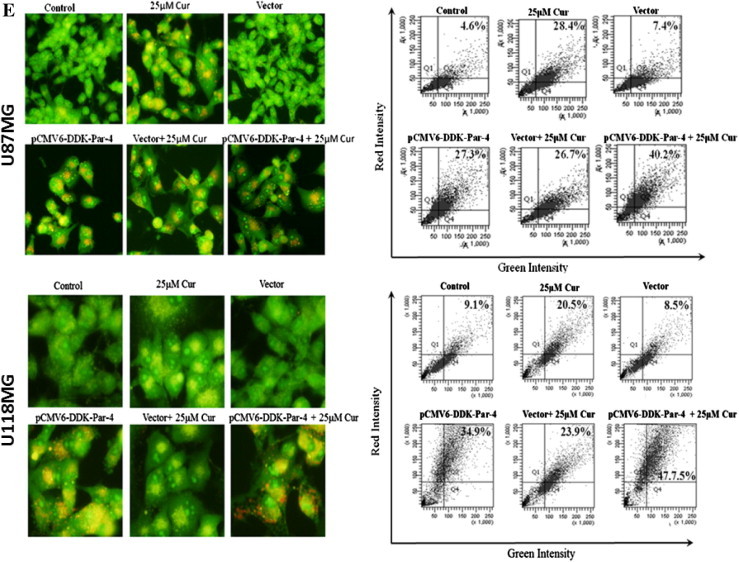
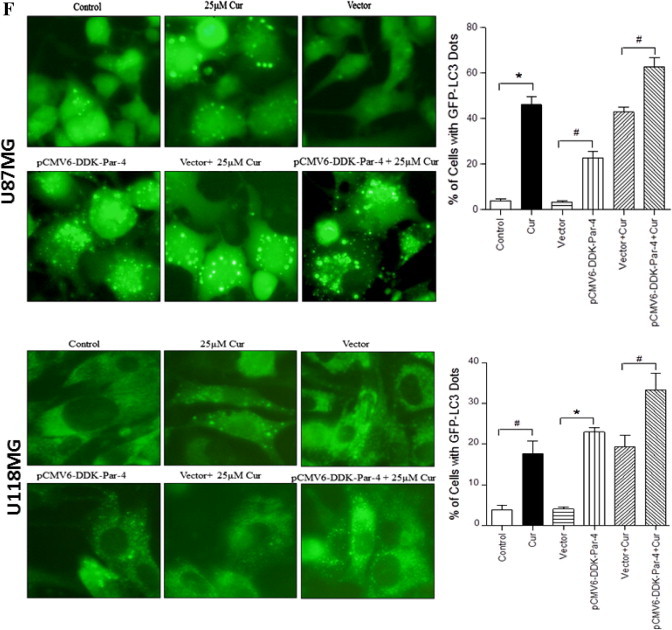
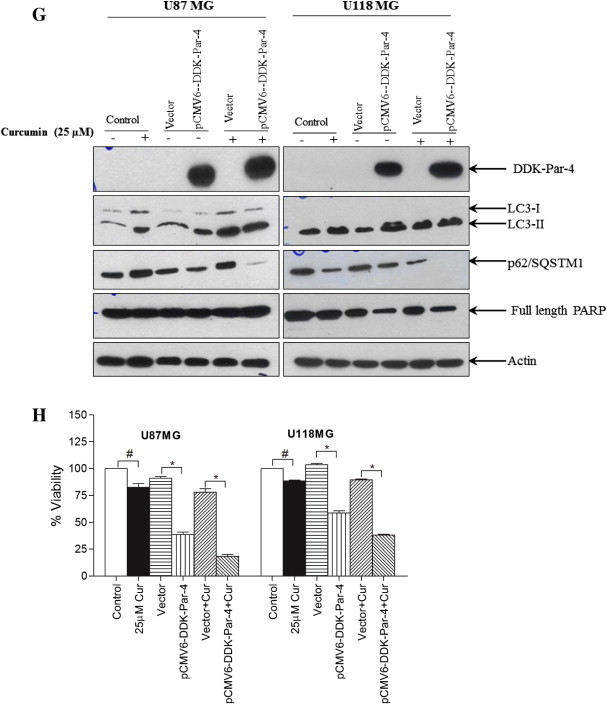
Involvement of Par-4 in curcumin-induced autophagy in human malignant glioma cells. (A) U87MG and U118MG cells were treated with indicated concentration of curcumin for 24 h and (B) with 60 μM curcumin for indicated time period. Western blot analysis of Par-4 was detected. Actin was used as loading control. (C) Cells were pretreated with GSH (10 mM) and NAC (10 mM) for 1 h followed by curcumin (60 μM) treatment for 24 h. Western blot analysis of Par-4 was detected. Actin was used as loading control. (D) U87MG and U118MG cells were treated with 2 mM and 0.5 mM H2O2, respectively followed by Western blot analysis of Par-4 and LC3-II. Actin was used as loading control. (E) pCMV6-DDK-Par-4 and empty vector transiently transfected U87MG and U118MG cells were treated with either vehicle or curcumin (25 μM) for 24 h. After the treatment, cells were stained with 1 μg/ml AO for 20 min at 37 °C. A representative slide under fluorescence microscopy shows H2O2-induced AVOs (left). Quantitative analysis of cells with red bright fluorescence using fluorescence activated cell sorter (right). (F) GFP-LC3 dots were assessed by fluorescence microscopy (left). Quantitatively, puncta were counted as described in Section 2 (right). Data shown are means ± SD (n = 3) (∗p < 0.01 and #p < 0.05). (G) The levels of DDK-Par-4, LC3-II, p62/SQSTM1 and PARP were detected using the corresponding antibodies. Actin was used as loading control. (H) Cell viability was assessed by MTT assay. Data shown are means ± SD (n = 3) (∗p < 0.01 and #p < 0.05).
Since curcumin-induced ROS generation was an early event, it is possible that ROS generation could lead to Par-4 induction. In order to test this, both glioma cells were pretreated with GSH and NAC for 1 h, followed by treatment with curcumin for 24 h, and the expression of Par-4 was measured. As shown in the Fig. 5C, both GSH and NAC completely abrogated the curcumin-induced Par-4 induction. To confirm this further, U87MG and U118MG cells were treated with 2 mM and 0.5 mM H2O2 respectively, and the expression of Par-4 and LC3-II were analyzed. As shown in the Fig. 5D, H2O2 treatment resulted in significant induction of Par-4 expression and LC3-II formation in both glioma cells. All together, these data indicate that curcumin-induced Par-4 induction was mediated via ROS generation.
Overexpression of Par-4 sensitizes cancer cells to apoptosis in response to various apoptosis inducing agents [30]. In order to examine whether Par-4 overexpression leads to induction of autophagy, glioma cells transiently expressing Par-4 or cells expressing the empty vector control, were exposed to curcumin treatment. The cells were then stained with AO, and analyzed by fluorescent microscopy and FACS analysis. As shown in Fig. 5E, curcumin treatment resulted in a marked increase in the orange–red acidic vesicles in Par-4 overexpressed cells. More importantly, Par-4 overexpression alone was sufficient to induce autophagic features in both glioma cell types. To confirm our observation by additional methods, cells were subjected to LC3 puncta analysis and immuno blot analysis of LC3 conversion and p62/SQSTM1 degradation. As expected, expression of Par-4 significantly increased the number of autophagic LC3 puncta (Fig. 5F), LC3-II formation (Fig. 5G) and p62/SQSTM1 degradation (Fig. 5G). Moreover, the curcumin-induced cell death was potentiated in the presence of Par-4 (Fig. 5H). Since the role of Par-4 is best-known in promoting apoptosis, we further investigated to know whether sensitization of curcumin-induced cell death by Par-4 is as a result of apoptosis. As shown in Fig. 5G, Par-4 overexpression together with curcumin treatment did not cause the PARP cleavage, a prime marker for apoptosis. Taken together, ROS-dependent up regulation of Par-4 regulates curcumin-induced autophagy in glioma cells.
3.6. Curcumin induces autophagy concomitant with ceramide generation in human malignant glioma cells
In addition to its role in apoptosis, ceramide has more recently been implicated in the induction of autophagy [18,19]. To investigate the involvement of ceramide in curcumin-induced autophagic cell death, both U87MG and U118MG cells were treated with various concentrations of curcumin (0–80 μM for 24 h), and for different time periods (60 μM for 0–24 h), and ceramide generation was examined. Curcumin treatment resulted in a dose- and time-dependent accumulation of ceramide in both glioma cell lines (Fig. 6A and B).
Fig. 6.
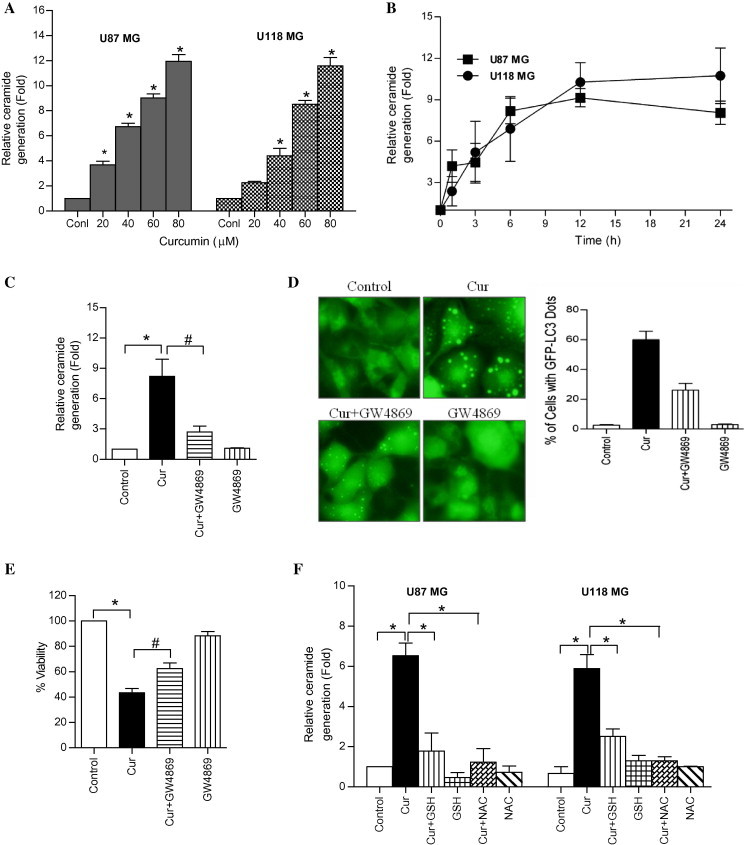
Curcumin induces ceramide generation in human malignant glioma cells. (A) U87MG and U118MG cells were treated with indicated concentration of curcumin for 24 h and (B) with 60 μM curcumin for indicated time period. The ceramide levels were measured as described in the Section 2. Data represent the mean ± SD (n = 3) (∗p < 0.01, verses control) (C) U87MG cells were pretreated with GW4869 (25 μM) for 1 h followed by curcumin (60 μM) treatment for 24 h. After the treatment, ceramide levels were measured as described in the Section 2. Data represent the mean ± SD (n = 3) (∗p < 0.01 and #p < 0.05). (D) The dots of GFP-LC3 were assessed by fluorescence microscopy (left). Quantitatively, puncta were counted as described in Section 2 (right). Data shown are means ± SD (n = 3). (E) Cell viability was assessed by MTT assay. Data represent the mean ± SD (n = 3) (∗p < 0.01 and #p < 0.05). (F) U87MG and U118MG cells were pretreated with GSH (10 mM) and NAC (10 mM) for 1 h followed by curcumin (60 μM) treatment for 24 h. After the treatment, ceramide levels were measured as described in the Section 2. Data represent the mean ± SD (n = 3) (∗p < 0.01).
Ceramide generation may proceed from either the de novo synthesis, or sphingomyelin hydrolysis [17]. To know which of these pathways is involved in curcumin-induced ceramide generation, U87MG cells were pretreated with inhibitors targeting these pathways followed by treatment with curcumin. The de novo synthesis inhibitors such as fumonisin B1 and myriocin, and acid sphingomyelinase inhibitor, desipramine exerted no effects on curcumin-induced ceramide generation, autophagy and cell death (Supplementary Fig. 2A, B, and C). In contrast, neutral sphingomyelinase inhibitor GW4869 [31], partially but significantly blocked curcumin-induced ceramide generation (Fig. 6C), autophagy (Fig. 6D) and cell death (Fig. 6E). This suggests that neutral sphingomyelinase-mediated ceramide generation, at least in part, plays a role in curcumin-induced cell death in U87MG malignant glioma cells.
The oxidative stress and ceramide pathways have been linked in number of previous studies [32]. Since curcumin-induced ROS generation was rapid in comparison with ceramide accumulation, it is plausible that ROS generation precedes the ceramide accumulation. In order to examine this possibility, both glioma cells were pretreated with GSH and NAC for 1 h followed by treatment with curcumin for 24 h, and ceramide levels were measured. As shown in Fig. 6F, GSH and NAC pretreatment, completely abrogated the ceramide accumulation, indicating that curcumin induces ROS-dependent ceramide generation and autophagic cell death in human malignant glioma cells.
4. Discussion
Autophagy plays critical role in the generation of antineoplastic responses in malignant glioma cells [6–8]. The mechanism by which autophagy mediates cell death is entirely different from that of apoptosis.
In the present study, we investigated the ability of curcumin to induce autophagic cell death in human malignant glioma cells and the functional consequences of such an induction. Our results show that curcumin exhibits a potent pro-autophagic effect on human malignant glioma cells, as demonstrated by AVOs formation, GFP-LC3 punctate formation, increased LC3-II conversion, p62/SQSTM1 degradation, and TFEB nuclear translocation. Curcumin-induced autophagy and cell death was marginally rescued by a well-known early phase autophagy inhibitor 3-MA. We also provide evidence that pro-autophagic effect of curcumin occurs via rapid generation of ROS, leading to the up-regulation of Par-4 and ceramide. This is extremely important, since Par-4 and ceramide were considered as important tumor suppressor protein and lipid, respectively [14,15,17].
An emerging body of evidence indicate that activation of LKB1/AMPK pathway is associated with autophagy in cancer cells. Activated AMPK induces autophagy by inhibiting mTOR, a key negative regulator of autophagy [26,27]. Our results demonstrate that treatment of glioma cells with curcumin resulted in activation of LKB/AMPK, which in turn leads to inactivation of mTOR and its downstream target p70S6K.
Accumulating evidence suggest that ROS generation is an important regulator for autophagy [9,10]. Some of the anticancer agents have been shown to induce ROS generation on its en route to induce autophagic cell death [33–35]. Blocking the ROS generation by using antioxidants inhibited the autophagic cell death, indicating the prime role that ROS in mediating the autophagic response [33–35]. In this regard, it was of interest to determine the functional link between ROS and autophagy in curcumin-induced cell death. In the present study, we demonstrate a rapid accumulation of ROS following curcumin treatment. Inhibition of this ROS accumulation with GSH and NAC almost completely abrogated the curcumin-induced autophagy and cell death. Results from this study are consistent with previous reports in which anticancer drugs were demonstrated to induce ROS production, and a resultant accelerated autophagy and cell death [34,35].
The tumor suppressor protein Par-4 is considered as an important target for cancer therapeutic intervention, since it selectively induces apoptosis in cancer cells; but not in normal cells [15]. Investigations are still undergoing to elucidate the mechanism by which Par-4 exhibits its function in the cell. Earlier studies indicate that Par-4 is strongly up-regulated in cells undergoing apoptosis [14]. In contrast, our data shows that Par-4 is significantly up-regulated in curcumin-induced autophagic cell death in human malignant glioma cells. Very recently, Wang et al. reported the simultaneous induction of apoptosis and autophagy by Par-4 in hypopharyngeal carcinoma cells [36]. Given that curcumin induces Par-4 expression in human malignant glioma cells, we investigated whether Par-4 has any role in autophagy. Our experiment shows that overexpression of Par-4 sensitizes curcumin-induced autophagy and cell death. Moreover, Par-4 overexpression alone was sufficient to induce cell death which is associated with features of autophagy as evidenced by increased AVOs formation, GFP-LC3 punctate formation, LC3-II conversion and p62/SQSTM1 degradation. Antioxidants such as GSH and NAC completely inhibited the curcumin-mediated Par-4 induction. Extracellular treatment of H2O2 also induced Par-4 and demonstrated features of autophagic cell death in human malignant glioma cells. Taken together these data clearly suggest that Par-4 has a vital role in curcumin-induced autophagic cell death in malignant glioma cells.
Ceramide has emerged as an important lipid mediator in the induction of apoptotic and autophagic cell death in variety of human tumor cells [18,19]. Recently, we have reported that curcumin induces caspase-dependent ceramide generation and apoptosis in human leukemic cells [28]. In this study, we demonstrate that curcumin treatment resulted in a dose- and time-dependent accumulation of ceramide content in both cell lines. The fact that the kinetics of autophagic cell death and ceramide generation parallels each other, suggests an involvement of ceramide in curcumin-induced autophagy. In this context, our results show that curcumin-induced rapid ROS generation, increases intracellular ceramide level, and specifically induces autophagy. Additionally, GW4869, an inhibitor of neutral sphingomyelinase, significantly blocked curcumin-induced ceramide generation, autophagy and subsequently decreased cell death in U87MG cells. We also demonstrate that extracellular supplementation of antioxidants to human malignant glioma cells completely inhibited the ceramide production. All together, these studies support a significant role for ceramide in triggering curcumin-induced autophagy in human malignant glioma cells.
The present study provides the first mechanistic evidence that curcumin is highly effective in suppressing the growth of human malignant glioma cells via autophagic cell death, which is associated with ROS-dependent up-regulation of Par-4, and ceramide generation. These findings not only add a novel concept to our understanding of curcumin-induced cell death pathway but also may serve as a powerful stimulus to design a curcumin based pro-autophagic drug for the treatment of brain tumor.
Disclosure of conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was financially supported by grants from UAEU-NRF (31M097), The Sheikh Hamdan Award for Medical Sciences (MGR-60/2011-2012), Terry Fox Foundation for Cancer Research (SGC-pmr-7091-2004) and in part from College of Medicine and Health Sciences, UAE University grant. S. G. is supported by Al Jalila Foundation for Medical Education and Research.
Contributor Information
Faisal Thayyullathil, Email: t.faisal@uaeu.ac.ae.
Anees Rahman, Email: anees@uaeu.ac.ae.
Siraj Pallichankandy, Email: sirajpk@uaeu.ac.ae.
Mahendra Patel, Email: cslpatel@gmail.com.
Sehamuddin Galadari, Email: sehamuddin@uaeu.ac.ae.
Appendix A. Supplementary data
This document contains supplementary Figs. 1 and 2.
References
- 1.Aggarwal B.B., Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol. Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Ravindran J., Prasad S., Aggarwal B.B. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009;11:495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magalska A., Sliwinska M., Szczepanowska J., Salvioli S., Franceschi C., Sikora E. Resistance to apoptosis of HCW-2 cells can be overcome by curcumin- or vincristine-induced mitotic catastrophe. Int. J. Cancer. 2006;119:1811–1818. doi: 10.1002/ijc.22055. [DOI] [PubMed] [Google Scholar]
- 4.Aoki H., Takada Y., Kondo S., Sawaya R., Aggarwal B.B., Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol. Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 5.Levine B., Klionsky D.J. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 6.Kaza N., Kohli L., Roth K.A. Autophagy in brain tumors: a new target for therapeutic intervention. Brain Pathol. 2012;22:89–98. doi: 10.1111/j.1750-3639.2011.00544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanzawa T., Germano I.M., Komata T., Ito H., Kondo Y., Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–457. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 8.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 9.Scherz-Shouval R., Shvets E., Elazar Z. Oxidation as a post-translational modification that regulates autophagy. Autophagy. 2007;3:371–373. doi: 10.4161/auto.4214. [DOI] [PubMed] [Google Scholar]
- 10.Scherz-Shouval R., Shvets E., Fass E., Shorer H., Gil L., Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkland R.A., Adibhatla R.M., Hatcher J.F., Franklin J.L. Loss of cardiolipin and mitochondria during programmed neuronal death: evidence of a role for lipid peroxidation and autophagy. Neuroscience. 2002;115:587–602. doi: 10.1016/s0306-4522(02)00512-2. [DOI] [PubMed] [Google Scholar]
- 12.Djavaheri-Mergny M., Amelotti M., Mathieu J., Besançon F., Bauvy C., Souquère S. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J. Biol. Chem. 2006;281:30373–30382. doi: 10.1074/jbc.M602097200. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y., Kim S.O., Li Y., Han J. Autophagy contributes to caspase-independent macrophage cell death. J. Biol. Chem. 2006;281:19179–19187. doi: 10.1074/jbc.M513377200. [DOI] [PubMed] [Google Scholar]
- 14.Sells S.F., Wood D.P., Jr., Joshi-Barve S.S., Muthukumar S., Jacob R.J., Crist S.A., Humphreys S., Rangnekar V.M. Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ. 1994;5:457–466. [PubMed] [Google Scholar]
- 15.El-Guendy N., Zhao Y., Gurumurthy S., Burikhanov R., Rangnekar V.M. Identification of a unique core domain of par-4 sufficient for selective apoptosis induction in cancer cells. Mol. Cell. Biol. 2003;23:5516–5525. doi: 10.1128/MCB.23.16.5516-5525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez J.V., Pan T.C., Ruth J., Feng Y., Zhou A., Pant D. Par-4 downregulation promotes breast cancer recurrence by preventing multinucleation following targeted therapy. Cancer Cell. 2013;24:30–44. doi: 10.1016/j.ccr.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannun Y.A., Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 18.Sentelle R.D., Senkal C.E., Jiang W., Ponnusamy S., Gencer S., Selvam S.P. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat. Chem. Biol. 2012;8:831–838. doi: 10.1038/nchembio.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taniguchi M., Kitatani K., Kondo T., Hashimoto-Nishimura M., Asano S., Hayashi A. Regulation of autophagy and its associated cell death by “sphingolipid rheostat”: reciprocal role of ceramide and sphingosine 1-phosphate in the mammalian target of rapamycin pathway. J. Biol. Chem. 2012;287:39898–39910. doi: 10.1074/jbc.M112.416552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thayyullathil F., Chathoth S., Hago A., Patel M., Galadari S. Rapid reactive oxygen species (ROS) generation induced by curcumin leads to caspase-dependent and -independent apoptosis in L929 cells. Free Radic. Biol. Med. 2008;45:1403–1412. doi: 10.1016/j.freeradbiomed.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Thayyullathil F., Pallichankandy S., Rahman A., Kizhakkayil J., Chathoth S., Patel M. Caspase-3 mediated release of SAC domain containing fragment from Par-4 is necessary for the sphingosine-induced apoptosis in Jurkat cells. J. Mol. Signal. 2013;8:2. doi: 10.1186/1750-2187-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thayyullathil F., Chathoth S., Kizhakkayil J., Galadari A., Hago A., Patel M. Glutathione selectively inhibits Doxorubicin induced phosphorylation of p53Ser, caspase dependent ceramide production and apoptosis in human leukemic cells. Biochem. Biophys. Res. Commun. 2011;411:1–6. doi: 10.1016/j.bbrc.2011.05.156. [DOI] [PubMed] [Google Scholar]
- 23.Klionsky D.J., Abdalla F.C., Abeliovich H., Abraham R.T., Acevedo-Arozena A., Adeli K. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paglin S., Hollister T., Delohery T., Hackett N., McMahill M., Sphicas E. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- 25.Settembre C., Di Malta C., Polito V.A., Garcia Arencibia M., Vetrini F., Erdin S. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z., Klionsky D.J. An overview of the molecular mechanism of autophagy. Curr. Top. Microbiol. Immunol. 2009;335:1–32. doi: 10.1007/978-3-642-00302-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He C., Klionsky D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kizhakkayil J., Thayyullathil F., Chathoth S., Hago A., Patel M., Galadari S. Glutathione regulates caspase-dependent ceramide production and curcumin-induced apoptosis in human leukemic cells. Free Radic. Biol. Med. 2012;52:1854–1864. doi: 10.1016/j.freeradbiomed.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Kim E.H., Sohn S., Kwon H.J., Kim S.U., Kim M.J., Lee S.J. Sodium selenite induces superoxide-mediated mitochondrial damage and subsequent autophagic cell death in malignant glioma cells. Cancer Res. 2007;67:6314–6324. doi: 10.1158/0008-5472.CAN-06-4217. [DOI] [PubMed] [Google Scholar]
- 30.Boehrer S., Nowak D., Puccetti E., Ruthardt M., Sattler N., Trepohl B. Prostate-apoptosis-response-gene-4 increases sensitivity to TRAIL-induced apoptosis. Leuk. Res. 2006;30:597–605. doi: 10.1016/j.leukres.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Luberto C., Hassler D.F., Signorelli P., Okamoto Y., Sawai H., Boros E. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J. Biol. Chem. 2002;277:41128–41139. doi: 10.1074/jbc.M206747200. [DOI] [PubMed] [Google Scholar]
- 32.Dumitru C.A., Zhang Y., Li X., Gulbins E. Ceramide: a novel player in reactive oxygen species-induced signaling? Antioxid. Redox Signal. 2007;9:1535–1540. doi: 10.1089/ars.2007.1692. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y., Azad M.B., Gibson S.B. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16:1040–1052. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- 34.Gao M., Yeh P.Y., Lu Y.S., Hsu C.H., Chen K.F., Lee W.C. OSU-03012, a novel celecoxib derivative, induces reactive oxygen species-related autophagy in hepatocellular carcinoma. Cancer Res. 2008;68:9348–9357. doi: 10.1158/0008-5472.CAN-08-1642. [DOI] [PubMed] [Google Scholar]
- 35.Fu J., Shao C.J., Chen F.R., Ng H.K., Chen Z.P. Autophagy induced by valproic acid is associated with oxidative stress in glioma cell lines. Neuro Oncol. 2010;12:328–340. doi: 10.1093/neuonc/nop005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L.J., Chen P.R., Hsu L.P., Hsu W.L., Liu D.W., Chang C.H. Concomitant induction of apoptosis and autophagy by prostate apoptosis response-4 in hypopharyngeal carcinoma cells. Am. J. Pathol. 2014;184:418–430. doi: 10.1016/j.ajpath.2013.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This document contains supplementary Figs. 1 and 2.


