Abstract
Purpose of Review:
This article provides an overview for understanding the diagnosis, pathogenesis, and management of diabetic neuropathy.
Recent Findings:
New information about the pathogenesis of diabetic neuropathy continues to emerge, which will lead to identifying new drug targets. It is clear that the natural history of diabetic neuropathy is changing and the rate of progression is slowing. This is likely because of a combination of earlier diagnosis, improved glycemic management, and improved control of related complications such as hyperlipidemia and hypertension. Early diagnosis is critical, and small fiber neuropathy or subclinical diabetic neuropathy may be reversed or significantly improved with appropriate intervention. The American Academy of Neurology recently published guidelines for the treatment of painful diabetic neuropathy.
Summary:
Diabetic neuropathy is common and can present with varied clinical presentations discussed in this article. Although treatment currently focuses on pain management, attention should be paid to potential risk factors for neuropathy. For example, glycemic control, hyperlipidemia, and hypertension should be managed with diet, exercise, and medications. Class I or II clinical studies indicate that pregabalin, duloxetine, amitriptyline, gabapentin, and opioids are effective in the management of diabetic neuropathic pain.
INTRODUCTION
The most common form of diabetes mellitus, type 2 diabetes mellitus, is projected to affect an estimated 366 million people worldwide by 2030.1 The lifetime incidence of neuropathy is approximately 45% for patients with type 2 diabetes mellitus and 54% to 59% for patients with type 1 diabetes mellitus.2 Studies of nerve conduction tests performed at the time of diabetes mellitus diagnosis demonstrate that neuropathy is already present in patients when the neuropathy is still subclinical, and these tests show improvement with intensive control of glycemia.3 Significant neuropathic pain occurs in 7.5% to 24% of all patients with diabetes mellitus.2 Neuropathic pain is also one of the most common presentations in impaired glucose tolerance and impaired fasting glucose.4 Interestingly, although pain-specific medications are required to treat the discomfort, therapies that ameliorate the underlying neuropathy also reduce the severity of the neuropathic pain.
CLASSIFICATION OF DIABETES MELLITUS AND PREDIABETES
Type 2 diabetes mellitus accounts for the majority (90% to 95%) of individuals with diabetes mellitus. A strong genetic predisposition for this disease exists, although the genetics are not fully understood. The risk of developing type 2 diabetes mellitus increases with age, obesity, and lack of physical activity. Hyperglycemia often develops gradually, and early symptoms are often not recognized or reported.
Type 1 diabetes mellitus accounts for 5% to 10% of people with diabetes mellitus; its hallmark is a deficiency of insulin production caused by cellular-mediated autoimmune destruction of pancreatic β-cells. Its onset is typically seen in childhood or adolescence, but it can occur at any age. Multiple genetic predispositions exist in addition to poorly defined environmental factors, but the cause can also be idiopathic. Autoantibodies can be found in 85% to 90% of patients, with strong human leukocyte antigen (HLA) associations. Patients with type 1 diabetes mellitus are prone to developing other autoimmune disorders.
Diabetes mellitus is defined by a 2-hour plasma glucose of greater than or equal to 200 mg/dL during an oral glucose tolerance test, fasting glucose greater than or equal to 126 mg/dL, or glycosylated hemoglobin (HbA1c) greater than or equal to 6.5%. Patients with classic hyperglycemic symptoms and a random plasma glucose greater than or equal to 200 mg/dL also meet diagnostic criteria for diabetes mellitus. Recently, there has been a greater emphasis placed on identifying patients who are at an elevated risk for developing diabetes mellitus. These individuals demonstrate elevated glucose levels but not to the degree that is required for the diagnosis of diabetes mellitus. They are defined as having either impaired fasting glucose (fasting plasma glucose between 100 mg/dL and 125 mg/dL) or impaired glucose tolerance (2-hour glucose value in an oral glucose tolerance test of 140 mg/dL to 199 mg/dL). Although less sensitive, a HbA1c value from 5.7% to 6.4% can also be used to identify patients who are at risk for developing diabetes mellitus. Both glucose measurements and HbA1c values have a curvilinear relationship with the risk of developing diabetes mellitus. As their values rise, the risk of diabetes mellitus rises disproportionately. In practice, a combination of the oral glucose tolerance test and the HbA1c is used.
Natural history studies of impaired glucose tolerance have shown that it is a fluctuating and reversible state. The Diabetes Prevention Program study randomized 3244 patients with impaired glucose tolerance to treatment with placebo, metformin, or intensive diet and exercise counseling. Nearly 30% of 1082 subjects receiving placebo progressed from impaired glucose tolerance to type 2 diabetes in 3 years, but during this same period, 25% reverted to postprandial normoglycemia.5 Similar results have been demonstrated in other studies. Based on the natural history studies, most patients will slowly progress toward greater glycemic dysregulation. Unmonitored patients probably experience many years of occult insulin resistance and postprandial hyperglycemia before developing typical symptoms of diabetes mellitus.
Thus, although blood glucose values are used to define impaired fasting glucose, impaired glucose tolerance, or type 2 diabetes mellitus, these definitions are artificial because they fail to recognize that impaired glucose regulation is a dynamic surrogate marker for an underlying metabolic disturbance and the glucose level fluctuates depending on changes in insulin resistance.
CLASSIFICATION OF DIABETIC NEUROPATHIES
Diabetes mellitus can cause several different types of neuropathy (Table 3-1). Most recently, the Toronto Expert Panel on Diabetic Neuropathy6,7 has provided criteria for the diagnosis of diabetic neuropathy (Table 3-2).
Table 3-1.
Neuropathies Associated With Diabetes Mellitus
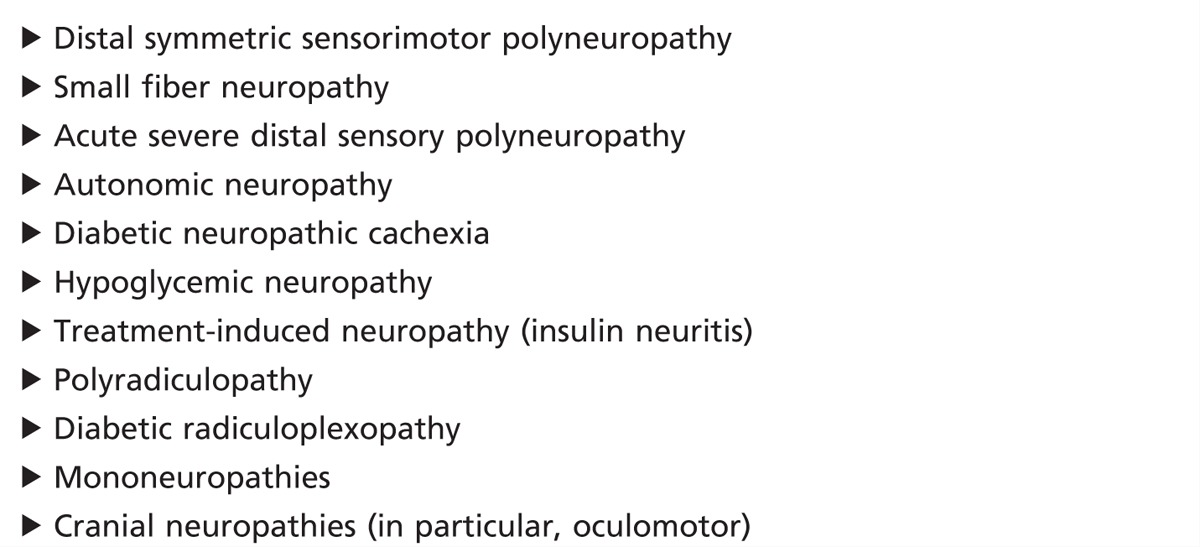
Table 3-2.
Diagnostic Criteria for Diabetic Neuropathy
DISTAL SYMMETRIC POLYNEUROPATHY
Approximately half of all patients with diabetes mellitus have a distal symmetric polyneuropathy. This is the most common presentation of diabetic neuropathy. It is typically a slowly progressive sensory predominant neuropathy. Patients initially experience sensory loss in the toes and feet that results from length-dependent dysfunction of nerve fibers (Case 3-1). This distal “dying back” type of neuropathy is consistent with a metabolic disturbance in the peripheral nervous system. Symptoms may include “negative” symptoms, such as decreased sensation and numbness, or “positive” symptoms such as prickling, burning, or aching sensations. Small myelinated and unmyelinated fibers convey sensations of light touch, pain, and temperature, while large fibers are responsible for vibratory sensation and joint position sense. Significant weakness is not a common finding in early diabetic neuropathy. There may be weakness of the toe flexor and extensor muscles, and subclinical motor involvement can be documented on electrodiagnostic testing. The majority of patients note mild to moderate discomfort associated with the neuropathy, but up to 25% may report a painful diabetic neuropathy. This is typically described as a deep aching pain with a burning or electric/shooting quality that is typically located in the feet. The pain can be exacerbated by activity but is often worst at night.
Small fiber neuropathy is characterized by superficial burning pain in the feet caused by preferential involvement of the small unmyelinated nerve fibers that mediate pain, temperature sensation, and autonomic function. Patients may report deep aching pain, shooting pain in their toes, tingling, and numbness and commonly report that their feet are persistently cold.8 Clinical findings include reduced distal pain and cold perception, sympathetic vasomotor changes (pallor alternating with rubor, cyanosis, and mottling), and, rarely, true allodynia. Strength and reflexes are often normal. Small fiber neuropathies are often seen in patients with impaired glucose tolerance. In one series, 81% of neuropathy patients with impaired glucose tolerance had exclusively sensory concerns, and 92% recognized neuropathic pain as a dominant symptom of their neuropathy.9 Small fiber neuropathies may not have any abnormalities on nerve conduction studies and can be further evaluated with skin biopsy and measurement of the intraepidermal nerve fiber density or sudomotor testing. For more information, refer to the article, “Small Fiber Neuropathies” by Christopher H. Gibbons, MD, FAAN, in this issue of CONTINUUM.
At the other end of the spectrum, some patients with diabetic neuropathy are unaware of their sensory loss and may experience painless injuries. Patients with insensate feet are especially prone to developing foot ulcerations; education regarding proper foot care is especially important in this population. In addition, patients with diabetes mellitus are at a higher risk of falls because of a combination of risk factors including sensory loss and impaired proprioception and spinal reflexes.10
Case 3-1
A 70-year-old man had numbness and tingling in his feet that had been slowly progressive during the past 2 years. The symptoms were described as a burning pain affecting both of his feet that felt like a bee stinging him constantly, and an unpleasant sensation when the bed sheets touched his skin. He had noticed that his feet stayed blue and cold all the time. His medications included olmesartan-hydrochlorothiazide, atorvastatin, aspirin, duloxetine, and gabapentin. He denied any history of tobacco or alcohol abuse. On physical examination, his strength was full throughout, including toe flexors and extensors, and deep tendon reflexes were normal. Sensation was decreased to pinprick in the lower extremities to the midcalves bilaterally and decreased to vibration and proprioception at the big toes bilaterally. Nerve conduction studies demonstrated a mild severity, length-dependent, axonal sensorimotor polyneuropathy. Laboratory studies were significant for a fasting glucose of 93 mg/dL, 2-hour glucose of 227 mg/dL, and glycosylated hemoglobin (HbA1c) of 6.2%.
Comment. A painful sensory neuropathy may be the presenting symptom in a patient with undiagnosed diabetes mellitus. It is important to recognize that neuropathy is not only a late complication of the disease, but can develop at the earliest stages of glucose dysregulation. In patients with a normal or slightly elevated HbA1c, it is important to pursue testing with an oral glucose tolerance test because of its increased sensitivity. In this patient, the HbA1c was in the prediabetic range, but the 2-hour glucose value was diagnostic of diabetes mellitus. Treatment of painful diabetic neuropathies often requires the use of multiple agents, as in this case. This patient was provided with diet and exercise counseling. He was also started on tramadol and experienced improvement of his pain.
AUTONOMIC NEUROPATHY
It is important to recognize the presence of diabetic autonomic neuropathy in patients because of its impact not only on morbidity but also on mortality. Specifically, the presence of cardiac autonomic neuropathy is associated with an increased mortality risk. This may be related to cardiac arrhythmias and silent myocardial ischemia, but the relationship is not fully understood. The symptoms of diabetic autonomic neuropathy depend on which specific component of the autonomic nervous system is affected (Case 3-2) and can include resting tachycardia, exercise intolerance, orthostatic hypotension, abnormal sweat patterns, gastric motor abnormalities, pupillary abnormalities, and erectile dysfunction.11 The incidence of clinical autonomic failure tends to increase with the length of time the patient has had diabetes mellitus and the age of the patient; the majority of diabetic autonomic neuropathies develop after more than 10 years of diabetes mellitus. However, diabetic autonomic neuropathy can also be an isolated finding and precede other complications of diabetes mellitus. The severity of autonomic neuropathy also varies between type 1 diabetes mellitus and type 2 diabetes mellitus. Signs of autonomic dysfunction are present in approximately 16% to 20% of all diabetic subjects and up to 75% of newly diagnosed subjects with type 1 diabetes mellitus.11 A similar pattern of autonomic neuropathy is seen in patients with impaired glucose tolerance.8 Diabetic autonomic neuropathy is infrequently seen in patients with a typical diabetic distal sensory neuropathy. It is more commonly encountered in patients with a predominantly small fiber neuropathy and manifests with vasomotor symptoms (excessive coldness and a blue/white discoloration) and distal hypohidrosis that can be documented with Quantitative Sudomotor Axon Reflex Test (QSART) (Case 3-2). Diabetic autonomic neuropathy can cause disruption of microvascular blood flow to the skin, resulting in dry skin, loss of sweating, and development of fissures and cracks that can lead to skin infections.
Case 3-2
A 56-year-old man with a history of type 1 diabetes mellitus for the past 34 years presented because of persistent symptoms of previously diagnosed postural hypotension. His past medical history was significant for peripheral neuropathy, proliferative retinopathy, and nephropathy. He reported fatigue, generalized weakness, loss of appetite, and postprandial nausea as well as frequent constipation. He also noticed that for several years his socks have been dry and his hands and feet no longer sweated. However, he did sweat profusely in his face and chest. In addition he experienced difficulty with attaining an erection and had hypersensitivity to bright lights. Figure 3-1 sshows reduced sweat responses, using Quantitative Sudomotor Axon Reflex Test (QSART), in the distal leg and feet with normal sweat responses in the forearm, which is usually spared early in neuropathy. Figure 3-2 shows attenuation in the cardiac response to deep breathing.
Figure 3-1.
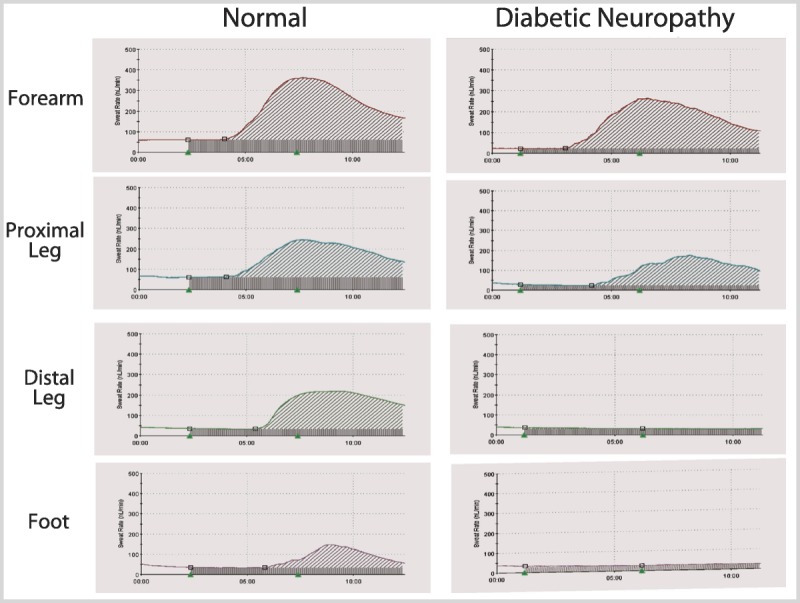
Quantitative Sudomotor Axon Reflex Test (QSART) measures sweat output in the forearm and lower extremity. Compared with the nondiabetic control, there is a decrease in sweating in the distal leg and foot. In contrast, sweat generation in the forearm is normal and marginally decreased in the proximal leg.
Figure 3-2.
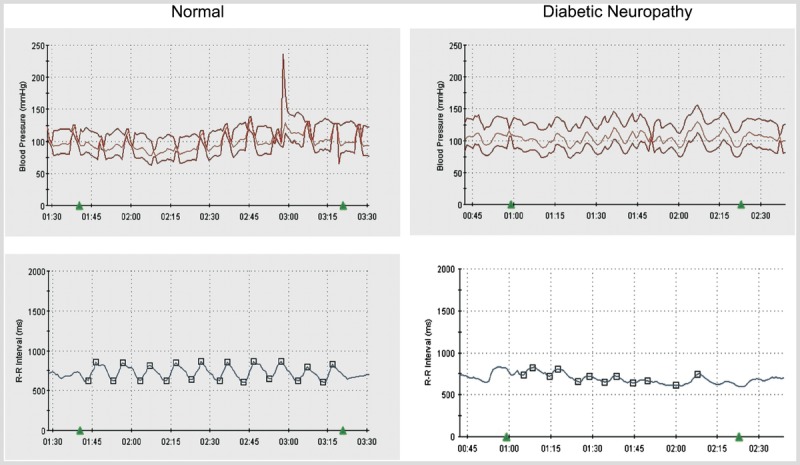
A decrease in heart rate variability (beat-to-beat variation) with deep breathing is present in diabetic autonomic neuropathy.
Comment. Orthostatic hypotension is defined as a fall in blood pressure (either greater than 20 mm Hg for systolic or greater than 10 mm Hg for diastolic blood pressure) in response to a change in posture. Common symptoms of orthostatic hypotension include light-headedness, weakness, fatigue, blurry vision, tremulousness or anxiety, nausea, and neck pain. However, many patients, and especially those with diabetes mellitus, may be asymptomatic. Treatment should not only be directed toward increasing blood pressure, but also toward educating patients to avoid situations that may predispose them to develop symptoms. Treatment includes maintaining adequate hydration, elevating the head of the bed, counseling to arise slowly, performing physical counter maneuvers to increase blood flow to the thorax, and avoiding hot showers. In patients with diabetes mellitus, orthostatic hypotension usually results from impairment of efferent sympathetic fibers. Although typically seen in patients with long-standing and poorly controlled diabetes mellitus, autonomic neuropathy may be detected at the time of diagnosis.
DIABETIC LUMBOSACRAL AND CERVICAL RADICULOPLEXUS NEUROPATHY/DIABETIC AMYOTROPHY
Diabetic lumbosacral and cervical radiculoplexus neuropathy,12,13 also referred to as diabetic amyotrophy, is a relatively rare entity, but it causes significant morbidity. It typically affects older patients (older than 50 years) and usually men (Case 3-3). Most patients with diabetic lumbosacral radiculoplexus neuropathy (DLRPN) have type 2 diabetes mellitus, but they may present prior to diagnosis of diabetes mellitus. DLRPN is frequently associated with weight loss, but its occurrence is often not related to glucose control or duration of diabetes mellitus. DLRPN classically starts with severe unilateral pain in the back, hip, or thigh that spreads to involve the entire limb and can involve the other leg within weeks to months. Typically, DLRPN remains asymmetric (Case 3-3). Shortly after the onset of pain, proximal weakness can be detected. Weakness and atrophy may initially be focal, but they can become widespread and bilateral. Physical examination reveals weakness of hip flexors, adductors, and extensors. Profound atrophy of the thigh can be seen. There may also be involvement of the ankle dorsiflexors and plantar flexors. The initial clinical concern may be that the patient has a structural lumbosacral radiculopathy or pelvic tumor; however, it is important to recognize that the weakness involves multiple root levels and peripheral nerves. Patients usually experience distal sensory loss, but this may be indistinguishable from a preexisting distal sensorimotor neuropathy. Usually knee and ankle reflexes are absent. Symptoms can worsen in a stepwise or progressive manner for up to 18 months. Eventually symptoms will stabilize, and the majority of patients will experience gradual improvement, although permanent weakness may result. Footdrop is common, resulting from failure to reinnervate distal segments. In approximately one-third of cases, weakness occurs in arm muscles and is attributed to a cervicobrachial radiculoplexopathy.13 The arm symptoms can begin or progress after the leg symptoms have plateaued or have begun to improve.
Electrodiagnostic studies are useful in diagnosis. Nerve conduction studies frequently cannot differentiate DLRPN from diabetic sensorimotor polyneuropathy, but asymmetries in compound muscle action potential amplitudes may occur. Findings on EMG and nerve conduction studies indicate a multifocal process involving the lumbosacral roots, plexus, and peripheral nerves. There may also be autonomic involvement.13 In addition, a plexus MRI may show nerve root enhancement. CSF analysis may demonstrate an elevated protein level with a normal cell count, which indicates involvement at the root level. Evidence suggests an ischemic injury from microvasculitis as the underlying pathology.13 A recent Cochrane review found no evidence from randomized trials to support the use of immunotherapy treatment in diabetic amyotrophy.14
Case 3-3
A 71-year-old man with type 2 diabetes mellitus for the past 12 years (glycosylated hemoglobin greater than 10% and on insulin for 7 years) presented to clinic because of an inability to walk for the past 6 months. The patient had previously been hospitalized because of a lower gastrointestinal bleed, during which he experienced progressive lower extremity weakness, andwas using a wheelchair at the time of his clinic visit. In addition, he reported shooting-type pains in his legs and back with numbness and tingling in both feet. Over the past year he had lost approximately 110 pounds, which he attributed to a loss of appetite. The left lower extremity showed atrophy of the quadriceps and hamstring muscles. Left leg strength was 2/5 for hip flexion, knee extension, foot dorsiflexion, eversion, and inversion and 3/5 for plantar flexion. The right lower extremity strength was 4/5 throughout. Sensation was decreased to pinprick and temperature to the midcalves bilaterally with distal decreased vibratory sensation. Reflexes were absent at the ankles and knees. Nerve conduction studies of the bilateral lower extremities demonstrated absent sensory and motor responses. EMG demonstrated diffuse subacute neurogenic changes in multiple muscles (tibialis anterior, gastrocnemius, and vastus lateralis), including the thoracic and lumbar paraspinal muscles. The patient was advised to optimize his diabetes mellitus control, and over time his glycosylated hemoglobin improved to 6.1%. He gradually regained strength, but remained weaker in the left leg compared with the right.
Comment. Diabetic lumbosacral radiculoplexus neuropathy (DLRPN) is a relatively uncommon condition that presents acutely or subacutely with significant pain that typically affects one leg. Not uncommonly, symptoms will spread to affect the contralateral leg and can affect the arms as well. Correct diagnosis is important to avoid unnecessary procedures, especially lumbar surgeries. It can be seen after times of stress, for example, during an acute illness or after a surgical procedure. It typically affects patients with type 2 diabetes mellitus, and the risk of developing DLRPN is not associated with the severity or duration of diabetes mellitus. Some evidence exists that DLRPN results from an underlying microvasculitis, but further evidence is needed to support the use of immunosuppressant therapy.
NEUROPATHY ASSOCIATED WITH HYPOGLYCEMIA AND HYPERINSULINEMIA
A polyneuropathy can develop in association with a chronic hyperinsulinemic state with repeated episodes of hypoglycemia, for example, with an insulinoma. Typical cases occur after several episodes of protracted hypoglycemia. A classic patient will present with distal paresthesia and minimal findings on physical examination. A motor-predominant distal symmetric peripheral neuropathy then develops that tends to involve the upper extremities more than the lower extremities. Significant proximal weakness often occurs, but footdrop is also common. Removal of the insulinoma results in some improvement in strength but significant improvement in sensory symptoms.
Treatment-induced neuropathy of diabetes mellitus (also referred to as insulin neuritis) is characterized by the acute onset of severe distal limb pain, peripheral nerve fiber damage (especially of unmyelinated fibers), and autonomic dysfunction that is precipitated by a period of rapid glycemic control. It occurs in both type 1 diabetes mellitus and type 2 diabetes mellitus patients treated with either insulin or oral hypoglycemic agents. The pain, which is often accompanied by hyperalgesia and allodynia,15 is severe and tends to be refractory to medications (Case 3-4). Pain usually improves with ongoing glucose control and typically resolves spontaneously within a year of onset. Autonomic dysfunction is common with this disorder, especially among patients with type 1 diabetes mellitus.16
Case 3-4
A 57-year-old woman with poorly controlled type 2 diabetes mellitus and a glycosylated hemoglobin of 15.2% was started on insulin. Approximately 1 month after starting insulin, she developed tingling and 10/10 burning pain in her feet. Prior to the onset of the pain, she recalled experiencing heart palpitations, nausea, and fatigue. Examination showed reduced sensation to pinprick and temperature to just above her ankles and absent ankle reflexes. Strength was normal. Over the next few weeks, the pain and allodynia progressed to involve her legs and arms. Multiple neuropathic and narcotic medications were unsuccessful in controlling the pain. Over the next 9 months, the pain gradually improved.
Comment. Treatment-induced neuropathy of diabetes mellitus is an acute, painful polyneuropathy associated with rapid correction of hyperglycemia in patients with previously poorly controlled diabetes mellitus. The neuropathic pain is severe and often refractory to medical management. Autonomic dysfunction is also common. Treatment is supportive, and the prognosis is good with eventual resolution of the pain over several months without specific treatment.
DIABETIC NEUROPATHIC CACHEXIA
Another syndrome that is associated with poor glycemic control is diabetic neuropathic cachexia. This condition occurs in type 1 diabetes mellitus and type 2 diabetes mellitus. Most cases have been in older men, but it can occur in adults and children. Patients present with unintentional weight loss and an acute symmetrical painful neuropathy. Response to treatment with neuropathic and opioid pain medications is poor. The pain tends to peak along with weight loss and resolves with weight gain. There can be autonomic involvement. Interestingly, depression is one of the hallmarks of the syndrome. Diabetic neuropathic cachexia is reversible with adequate diabetic control over weeks to months. A clinical distinguishing characteristic is that the pain typically affects the trunk and the presence of proximal or truncal dysesthesia can be a clue to the diagnosis. A residual sensorimotor neuropathy is common.
DEMYELINATING NEUROPATHY
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) and other demyelinating neuropathies may occur in patients with diabetes mellitus and may represent a diagnostic challenge (Case 3-5). Furthermore, diabetic neuropathy may be associated with demyelination, and both diabetes mellitus and CIDP may have elevated CSF protein. The importance lies in recognizing that the demyelinating neuropathy may be treatable with IV immunoglobulin or immunomodulatory therapy that minimizes the impact on the concomitant diabetes mellitus. It is not known if diabetes mellitus predisposes to CIDP as no systematic large prospective epidemiologic study has been performed.
Case 3-5
A 62-year-old man had bilateral, left greater than right, upper extremity weakness that started 10 years ago and had been slowly progressive. He previously worked as a plumber, but had not been able to use a hammer for the past 5 years. Occasionally, his left second and third digits felt numb upon awakening in the morning, but otherwise he denied any sensory symptoms. On examination, mild atrophy of the left biceps and deltoid muscles was seen. Strength was 4/5 in the bilateral deltoids, 4/5 in the right biceps and 3/5 in the left biceps, 2/5 interosseous bilaterally, grip 4/5 on the right, 4/5 bilateral opponens pollicis, wrist extension 3/5 on the right and 4/5 on the left, finger extensors 4/5, flexors 5/5, abductor pollicis brevis 5/5, and legs 5/5. Sensation was intact except for minimally decreased vibratory sensation at the toes. Laboratory testing was significant for a fasting glucose of 149 mg/dL and glycosylated hemoglobin of 7.3%. Cervical spine MRI demonstrated moderate left cervical neural foraminal stenosis. CSF glucose was 71 mg/dL, protein 71 mg/dL, white blood cell count 4/mm3, and red blood cell count 64/mm3. Nerve conduction study demonstrated a diffuse neuropathy that was more severe in the upper extremities and associated with partial conduction block of the bilateral median nerves between the wrist and the elbow. Figure 3-3 shows evidence of partial conduction block in the left median nerve. EMG demonstrated diffuse neurogenic changes throughout the left arm and leg. The patient was treated with monthly infusions of IV immunoglobulin, and his strength improved to normal, with noticeable improvement after the first infusion.
Figure 3-3.
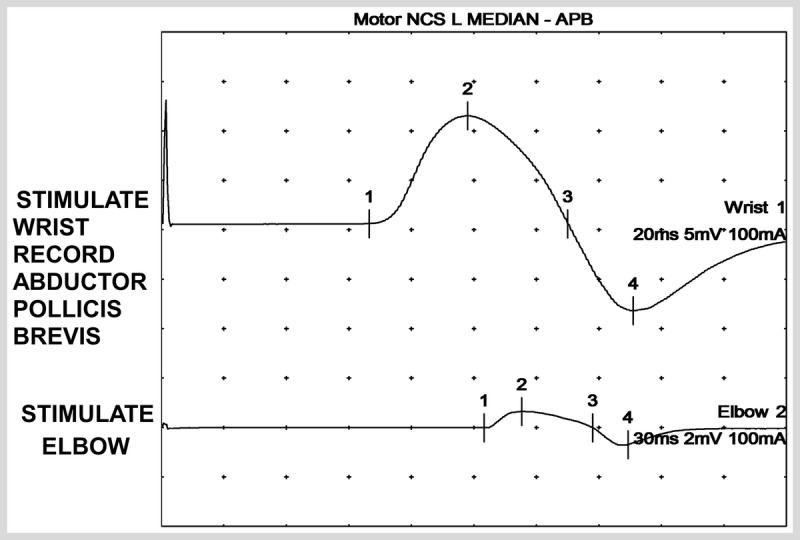
Partial conduction block, with proximal stimulation, in a patient with diabetes mellitus. The primary cause of the neuropathy in this patient is multifocal neuropathy with conduction block.
NCS = nerve conduction study; L = left; APB = abductor pollicis brevis.
Comment. This patient developed an asymmetric, upper limb predominant neuropathy with conduction block on nerve conduction studies consistent with a diagnosis of multifocal motor neuropathy. While peripheral neuropathy is the most common complication of diabetesmellitus, it is important to consider all possible causes of neuropathy in order to properly treat the patient.
Diabetic neuropathy can cause demyelinating features on nerve conduction studies and an elevation in CSF protein levels, but multifocal motor neuropathy is a treatable condition with a rapid response to IV immunoglobulin.
OTHER DIABETIC NEUROPATHIES
Diabetic oculomotor palsy presents with acute onset of pain behind or above the eye, paresis of the oculomotor innervated muscles, and ptosis. Presumed microvascular ischemia associated with diabetes mellitus accounted for 11% of subjects in one large cohort with third nerve palsies.17 Interestingly, 53% of subjects with diabetes mellitus had pupillary involvement, often bilateral, suggesting that there was concomitant autonomic nerve involvement.17 Other cranial neuropathies that occur with diabetes mellitus are fourth, sixth, and seventh cranial nerve palsies.18 Diabetes mellitus is the most common cause of isolated fourth nerve palsies and has a presumed microvascular etiology.19 Although seventh nerve palsies and diabetes mellitus are reported concurrently in numerous case reports, no strong evidence that diabetes mellitus is pathogenic in facial palsy exists. Cranial neuropathies in diabetes mellitus tend to improve and may resolve over time.
A higher prevalence of compressive neuropathies, including carpal tunnel syndrome and ulnar neuropathy at the elbow, exist in patients with diabetes mellitus compared with the general population. Upper limb compressive neuropathies should be evaluated and treated as with nondiabetic cases of carpal tunnel syndrome or compressive ulnar neuropathy. Treatment may include splinting or decompression of the nerve where appropriate. In diabetes mellitus, lower limb compressive neuropathy of the common peroneal, deep peroneal, or branches of the tibial nerve may be observed, particularly on nerve conduction studies. The value of decompression in these circumstances is less clear.20 Mononeuropathy multiplex or multifocal neuropathy can occur with diabetes mellitus; however, other causes of this condition, such as nerve vasculitis, should be considered and a nerve biopsy may be required as part of the diagnostic evaluation. It is important to diagnose nerve vasculitis when present because the condition is usually treatable with immunosuppressive medications. In diabetic multifocal motor neuropathy, mild inflammatory infiltrates and loss of axons may be observed. Diabetic thoracic radiculopathy is a rare, usually unilateral radiculopathy that presents with severe pain along the trunk, chest, or abdomen.
The pathogenesis of cranial and focal mononeuropathies has not been systematically studied. Although many of the same general mechanisms described in the pathogenesis section apply, microvascular and inflammatory causes may be more frequent in focal diabetic neuropathies. The value of immunosuppressive medications is unclear in this instance.
DIFFERENTIAL DIAGNOSIS OF DIABETIC NEUROPATHY
The differential diagnosis of diabetic neuropathy is broad because of the wide variety of presentations of the disease. Some diseases that mimic diabetic neuropathy are listed in Table 3-3. It is important to recognize that diabetes mellitus is a common disease and may occur concurrently with other diseases. In these situations, diabetes mellitus may not be the primary pathogenesis for the neuropathy, and a more treatable and reversible cause for the neuropathy may be found.
Table 3-3.
Some Mimickers of Diabetic Neuropathy

PATHOGENESIS OF DIABETIC NEUROPATHY
The pathogenesis of diabetic neuropathies in general is complex. Several trials have established a clear link between impaired glycemic control, neuropathy, and retinopathy.21 Furthermore, it is clear from these data that any increase in glucose above normal is associated with an increased risk of end organ injury, including neuropathy. However, recent data indicate that hyperlipidemia in addition to hyperglycemia may be important in the pathogenesis of diabetic neuropathy. There are common complex biochemical and signaling pathways that are implicated in the pathogenesis of diabetic neuropathy, which are reviewed in detail elsewhere.22,23
MANAGEMENT OF DIABETIC NEUROPATHY
Currently, no treatments exist that convincingly reverse diabetic neuropathies. However, the severity of diabetic neuropathy may be reduced. It is especially important to identify patients with prediabetes and neuropathy since interventions can be most effective in this population. However, management of diabetic neuropathy should address: (1) treatment of risk factors; (2) a diet and exercise lifestyle intervention; and (3) considering administration of α-lipoic acid. Abnormal glucose metabolism can be aggravated by thiazide diuretics in both diabetic and nondiabetic patients. Therefore, in diabetic patients with hypertension, an alternative antihypertensive should be considered and may include an angiotensin-converting enzyme inhibitor or an angiotensin-receptor blocker that may reduce the risk of diabetes mellitus and complications.24 Improved glycemic control can reduce the progression of diabetes mellitus and complications that include neuropathy. This is certainly true for type 1 diabetes mellitus but may also be true for type 2 diabetes mellitus.21 Another risk factor that should be treated is hyperlipidemia because of the association with neuroaxonal injury.23 Currently, no evidence from a randomized study exists showing that a lifestyle intervention can reverse somatic neuropathy (efferent and afferent nerves of the voluntary nervous system). However, studies have shown that an intensive diet and exercise intervention can delay the onset of type 2 diabetes mellitus5 and may reduce progression of small fiber neuropathy.4 These results need to be replicated in a prospective randomized study. Multiple clinical trials with α-lipoic acid have been completed using a variety of study designs and routes of administration. The results have not been definitive; however, oral treatment with 600 mg of α-lipoic acid once daily was shown to improve neuropathic symptoms and deficits in patients with diabetic sensory neuropathy when treated for 4 years.25
Symptomatic treatment is often the focus of therapy for diabetic neuropathic pain, but it is important to point out that lifestyle interventions may also reduce the severity of neuropathic pain.4 The American Academy of Neurology has recently published guidelines for the treatment of painful diabetic neuropathy providing an extensive review of the subject.26 For patients who require pharmacologic treatment, first-line therapies include tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and anticonvulsants. Amitriptyline is often recommended as the first-line treatment for painful diabetic neuropathy because of its efficacy and low cost. However, its clinical use is often limited by side effects of sedation, hypotension, dry mouth, cardiovascular abnormalities, and urinary retention. Nortriptyline is often used because of its improved side effect profile in older adults. The serotonin-norepinephrine reuptake inhibitors used to treat painful diabetic neuropathy include duloxetine and venlafaxine. Compared with tricyclic antidepressants, duloxetine has fewer side effects. For the anticonvulsants, gabapentin is generally well tolerated with a low side effect profile and low cost. Pregabalin is structurally related to gabapentin, but it has linear pharmacokinetics across different doses and improved absorption. Narcotic (morphine, oxycodone) and non-narcotic (tramadol) pain medications are used when first-line neuropathic agents are ineffective. They should be closely monitored because of the development of tolerance and potential for physical and psychological dependency.
CONCLUSION
Diabetic neuropathy is a common disorder with a diverse presentation. Improvement in glycemic control, blood pressure control, and earlier diagnosis and intervention have all helped to reduce the severity and slow the progression of diabetic neuropathy. Evidence from natural history studies also suggests that diet and exercise interventions may reduce the progression of neuropathy or possibly even result in the regrowth of the epidermal nerve fibers.4 Other clinical intervention studies have thus far not shown that a specific pharmacologic approach can reverse or prevent diabetic neuropathy. However, in the treatment of neuropathic pain, there has been greater treatment success.26
KEY POINTS
Neuropathy is not only a late complication of diabetes mellitus, but also can develop at any time during the course of the disease. It is increasingly recognized in patients with prediabetes who are at high risk of developing diabetes mellitus.
Two-hour glucose tolerance testing should be ordered in patients with an otherwise idiopathic neuropathy to examine for the presence of prediabetes.
The most common complication in diabetes mellitus is peripheral neuropathy.
Sympathetic vasomotor changes often seen with small fiber neuropathies include pallor alternating with rubor, cyanosis, and mottling.
Small fiber neuropathies may not have any abnormalities on nerve conduction studies and can be further evaluated with skin biopsy and measurement of the intraepidermal nerve fiber density or sudomotor testing.
The presence of cardiac autonomic neuropathy is associated with a two to five times increased risk of all-cause mortality.
Symptoms of resting tachycardia, palpitations, exercise intolerance, or orthostatic hypotension may indicate cardiac autonomic neuropathy.
Diabetic lumbosacral radiculoplexus neuropathy should be suspected in a patient with diabetes mellitus who reports acute, unilateral pain in the hip or thigh.
Treatment-induced neuropathy of diabetes mellitus is an acute painful neuropathy. It is a reversible disorder that is seen in patients with previously uncontrolled diabetes mellitus who rapidly improve their glycemic control.
Diabetic neuropathic cachexia is a partially reversible disorder that presents with unintentional weight loss and an acute painful neuropathy in patients with poorly controlled diabetes mellitus. Depression is very common.
The presence of proximal or truncal dysesthesia may be a clue to the diagnosis of diabetic neuropathic cachexia.
Severe diabetic neuropathy can mimic chronic inflammatory demyelinating polyradiculoneuropathy on nerve conduction studies, and the diagnostic distinction is important for determining treatment options.
If possible, patients with hypertension and a diabetic neuropathy should be taken off thiazide diuretics and placed on an angiotensin-converting enzyme inhibitor or an angiotensin-receptor blocker if needed.
Strict glucose control can delay the onset or slow the progression of diabetic neuropathy, but there is an increased risk of hypoglycemic events.
Patients with prediabetes or early diabetes mellitus and neuropathy should be offered dietary and exercise counseling.
First-line agents for the treatment of painful diabetic neuropathy include amitriptyline, venlafaxine, duloxetine, gabapentin, and pregabalin.
REFERENCES
- 1. Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27 (5): 1047– 1053 [DOI] [PubMed] [Google Scholar]
- 2. Zilliox L, Russell JW. Treatment of diabetic sensory polyneuropathy. Curr Treat Options Neurol 2011; 13 (2): 143– 159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albers JW, Herman WH, Pop-Busui R, et al. Subclinical neuropathy among Diabetes Control and Complications Trial participants without diagnosable neuropathy at trial completion: possible predictors of incident neuropathy? Diabetes Care 2007; 30 (10): 2613– 2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith AG, Russell JW, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care 2006; 29 (6): 1294– 1299 [DOI] [PubMed] [Google Scholar]
- 5. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346 (6): 393– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dyck PJ, Albers JW, Andersen H, et al. Toronto Expert Panel on Diabetic Neuropathy. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity [published online ahead of print June 21, 2011]. Diabetes Metab Res Rev 2011. doi:10.1002/dmrr.1226. [DOI] [PubMed] [Google Scholar]
- 7. Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33 (10): 2285– 2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zilliox L, Peltier AC, Wren PA, et al. Assessing autonomic dysfunction in early diabetic neuropathy: the Survey of Autonomic Symptoms. Neurology 2011; 76 (12): 1099– 1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singleton JR, Smith AG, Russell JW, Feldman EL. Microvascular complications of impaired glucose tolerance. Diabetes 2003; 52 (12): 2867– 2876 [DOI] [PubMed] [Google Scholar]
- 10. Goldberg A, Russell JW, Alexander NB. Standing balance and trunk position sense in impaired glucose tolerance (IGT)-related peripheral neuropathy. J Neurol Sci 2008; 270 (1–2): 165– 171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spallone V, Ziegler D, Freeman R, et al. Toronto Consensus Panel on Diabetic Neuropathy. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management [published online ahead of print June 22, 2011]. Diabetes Metab Res Rev 2011. doi:10.1002/dmrr.1239. [DOI] [PubMed] [Google Scholar]
- 12. Dyck PJ, Windebank AJ. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve 2002; 25 (4): 477– 491 [DOI] [PubMed] [Google Scholar]
- 13. Massie R, Mauermann ML, Staff NP, et al. Diabetic cervical radiculoplexus neuropathy: a distinct syndrome expanding the spectrum of diabetic radiculoplexus neuropathies. Brain 2012; 135 (pt 10): 3074– 3088 [DOI] [PubMed] [Google Scholar]
- 14. Chan YC, Lo YL, Chan ES. Immunotherapy for diabetic amyotrophy. Cochrane Database Syst Rev 2012; 6: CD006521. [DOI] [PubMed] [Google Scholar]
- 15. Gibbons CH, Adler GK, Bonyhay I, Freeman R. Experimental hypoglycemia is a human model of stress-induced hyperalgesia. Pain 2012; 153 (11): 2204– 2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gibbons CH, Freeman R. Treatment-induced diabetic neuropathy: a reversible painful autonomic neuropathy. Ann Neurol 2010; 67 (4): 534– 541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Keane JR. Third nerve palsy: analysis of 1400 personally-examined inpatients. Can J Neurol Sci 2010; 37 (5): 662– 670 [DOI] [PubMed] [Google Scholar]
- 18. Said G. Focal and multifocal diabetic neuropathies. Arq Neuropsiquiatr 2007; 65 (4B): 1272– 1278 [DOI] [PubMed] [Google Scholar]
- 19. Khaier A, Dawson E, Lee J. Clinical course and characteristics of acute presentation of fourth nerve paresis. J Pediatr Ophthalmol Strabismus 2012; 49 (6): 366– 369 [DOI] [PubMed] [Google Scholar]
- 20. Chaudhry V, Russell J, Belzberg A. Decompressive surgery of lower limbs for symmetrical diabetic peripheral neuropathy. Cochrane Database Syst Rev 2008; (3): CD006152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev 2012; 6: CD007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russell JW, Smith AG, Singleton JR. Impaired glucose regulation and neuropathy. In: Gilman S, ed. Neurobiology of diseases. San Diego: Elsevier, 2006: 849– 869 [Google Scholar]
- 23. Callaghan BC, Cheng HT, Stables CL, et al. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol 2012; 11 (6): 521– 534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NAVIGATOR Study Group, McMurray JJ, Holman RR, Haffner SM, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 2010; 362 (16): 1477– 1490 [DOI] [PubMed] [Google Scholar]
- 25. Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care 2011; 34 (9): 2054– 2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2011; 76 (20): 1758– 1765 [DOI] [PMC free article] [PubMed] [Google Scholar]