Abstract
Hyperthermia, as an independent modality or in combination with standard cancer treatments such as chemotherapy and radiation, has been established in vitro and in vivo as an effective cancer treatment. However, despite efforts over the past 25 years, such therapies have never been optimized or widely-accepted clinically. Although methods continue to improve, conventionally-delivered heat (RF, ultrasound, microwave etc) can not be delivered in a tumor selective manner. The development of antibody-targeted, or even nontargeted, biocompatible iron oxide nanoparticles (IONP) now allows delivery of cytotoxic heat to individual cancer cells. Using a murine mouse mammary adenocarcinoma (MTGB) and human colon carcinoma (HT29) cells, we studied the biology and treatment of IONP hyperthermia tumor treatment.
Methods
Cancer cells (1 × 106) with or without iron oxide nanoparticles (IONP) were studied in culture or in vivo via implanted subcutaneously in female C3H mice, Tumors were grown to a treatment size of 150 mm3 and tumors volumes were measured using standard 3-D caliper measurement techniques. Mouse tumors were heated via delivery of an alternating magnetic field, which activated the nanoparticles, using a cooled 36 mm diameter square copper tube induction coil which provided optimal heating in 1.5 cm wide region of the coil. The IONPs were dextran coated and had a hydrodynamic radius of approximately 100 nm. For the in vivo studies, intra-tumor, peritumor and rectal (core body) temperatures were continually measured throughout the treatment period.
Results
Although some eddy current heating was generated in non-target tissues at the higher field strengths, our preliminary IONP hyperthermia studies show that whole mouse AMF exposure @160 KHz and 400 or 550 Oe, for a 20 minutes (heat-up and protocol heating), provides a safe and efficacious tumor treatment. Initial electron and light microscopic studies (in vitro and in vivo) showed the 100 nm used in our studies are rapidly taken up and retained by the tumor cells. Additional in vitro studies suggest antibodies can significantly enhance the cellular uptake of IONPs.
Keywords: Iron oxide, nanoparticle, hyperthermia, alternating magnetic field, mouse, MTG-B, efficacy, antibody
2. BACKGROUND
For a number of well published and generally accepted reasons hyperthermia is an excellent adjunct to radiation in cancer treatment. In the appropriate hands hyperthermia has successfully been used in clinical cancer medicine. However, it has not met with the degree of success that experimental studies suggest, and many authorities believe, may be possible. While the general complication of tissue over-heating are reasonable well understood, the effectiveness and safety of specific thermal doses (therapeutic ratio) in various clinical cancer setting is often not well understood or described.
Despite of some level of success, the current techniques used for delivery of local hyperthermia remain inadequate for widespread clinical application. Although significant gains have been made over the past 20 years, hyperthermia techniques including radiofrequency, microwave, laser and ultrasound lack the selective tumor toxicity capability inherently present with radiation and chemotherapy. Similarly, although considerable progress has also been made in the development of hyperthermia treatment planning systems, the ability to significantly improve the hyperthermia therapeutic ratio has not yet materialized. Specifically, it is the inability to obtain homogeneous and precise high intratumoral temperatures, while isolating such temperatures from the surrounding normal tissues, which restricts clinical effectiveness and raises safety concerns.
Intra-tumoral iron oxide nanoparticle thermotherapy represents a recent development in the area of clinical cancer hyperthermia. In this technique, an externally delivered alternating magnetic field (AMF) is coupled magnetically to iron-oxide nanoparticles to create a localized heating effect. Typically, the particles being studied have a core diameter of less than 100 nm. Particle suspension are injected directly into the target region (tumor) and subsequently heated via the previously mentioned externally applied AMF. In contrast to E-field dominant systems used in regional hyperthermia, boundaries of different conductive tissues do not interfere with power absorption in the field containing particles. In vitro experiments with such particles have confirmed the excellent power absorption characteristics present with a heating element present in large number and/or with a large surface area. Although nanoparticle hyperthermia cancer therapy has many variables to consider, particle composition, coating and size remain the key determinants in heating efficacy. Finally, the local and/or intravenous delivery of conjugated tumor-specific antibodies and iron / iron oxide particles should be able to provide a new dimension (selective particle uptake by individual cells / intracellular hyperthermia) in nanoparticle hyperthermia cancer treatment. Although non-targeted local IONP heating has some very attractive features not available from other local heating techniques, antibody- or peptide-targeting of IONPs appears to be the technology with the greatest potential clinical usefulness. Such IONP targeting represents the greatest current challenge for the technology. Even if the appropriate anticancer antibodies are available, it has yet to be determined if such antibodies are capable of delivering sufficient quantities of IONP (to a tumor) for successful treatment alone or in combination with other therapies such as radiation or chemotherapy.
3. MATERIALS AND METHODS
3.1 Animal and Tumor Model
C3H/HeJ mice obtained from Jackson Labs, Bar Harbor, ME and Charles River Laboratory were used in this study. Animal care was performed in accordance with all federal and institutional guidelines. Animals received food and water ad libitum. Body weight was monitored three times per week. Tumors were induced by subcutaneous implantation of 1×106 MTG-B mouse mammary carcinoma cells into the shaved right flank of 7 week-old, 18–22 gram mice. Implantation was carried out under general anesthesia following intraperitoneal injection of ketamine (90 mg/ kg) and xylazine (5 mg/kg). Tumors were measured daily in three orthogonal directions (d1, d2, d3) and tumor volumes calculated using the πd1·d2·d3/6 formula. Mice were assigned to random groups once the tumor volume reached a size of 150 ± 40 mm3.
3.2 Iron Oxide Nanoparticles
IONPs were graciously provided by Aduro Biosystems, Berkley, CA. All particles were manufactured by micromod Partikeltechnologie GmbH in Rostock, Germany. The nanoparticles consist of 95–140 nm average diameter dextran coated Fe3O4, having high specific absorption rates (SAR) in applied magnetic fields, and were synthesized by high-pressure homogenization according to a core-shell method.19 The specific absorption rates (SAR) was measured at 150 kHz and various AMF amplitudes.19 The particles possess an iron oxide core (density >5 g/cm3, 10 – 60 nm) that is coated with a layer of dextran. The iron content of the particles is approximately 44% w/w, and they are separable with a permanent magnet. All dilutions made to normalize particle and iron concentration as well as dose volume for different doses were done in sterile phosphate-buffered saline.
3.3 IONP Hyperthermia Treatment Procedure and Techniques
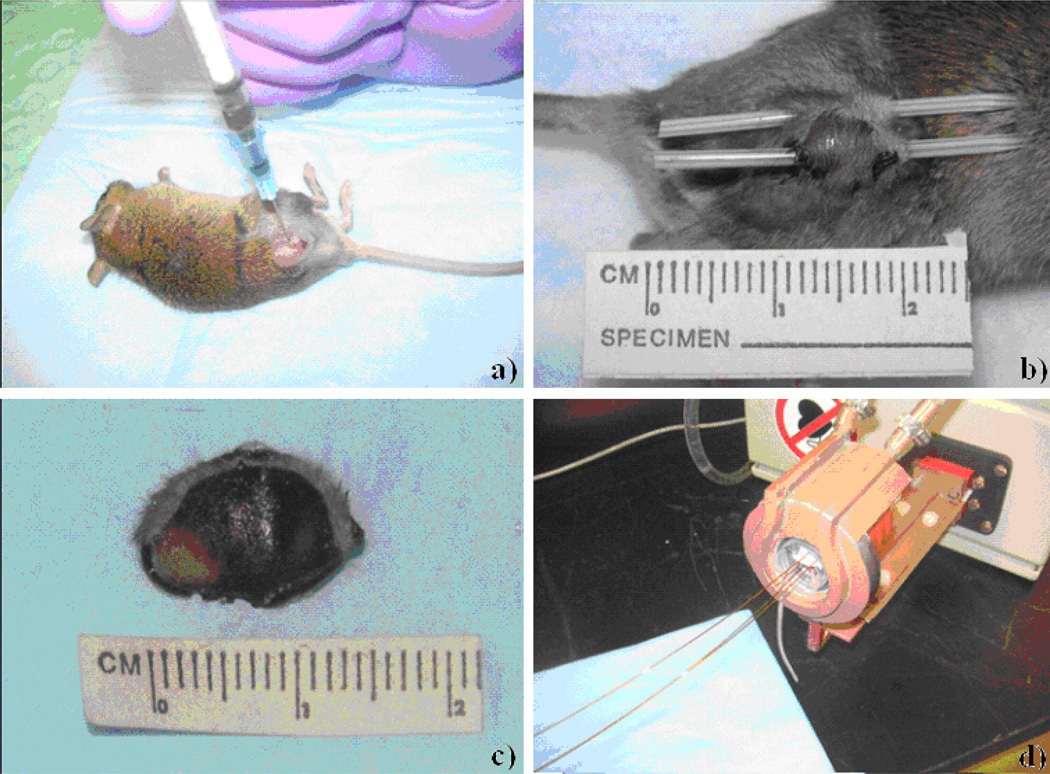
Tumors were injected with a single dose of iron oxide particles in a single central region or multiple regions of the tumor. The dose was calculated according to tumor size with 5 mg iron/cm3 tumor delivered by adjusting for stock particle concentrations of 33–38 mg/ml. Using a 30 gauge needle, particles were injected into each tumor from 15 to 45 minutes prior to AMF exposure (Figure 1a). Each injection required approximately 5 minutes. Gross and histopathologic assessments suggest these incubation periods provide good particle distribution within the tumors.
Figure 1.
a–d : This figure demonstrates the iron oxide nanoparticle injection technique: (a). Thermometry catheter placement in the tumor center and peritumor region: (b). Particle distribution via tumor bisection 10 minutes post injection: (c). With the exception of the lower left region, there appears to be excellent nanoparticle coverage of the tumor. A mouse positioned in the heating coil with tumor, peritumor and rectal temperature probes in place: (d).
3.3.1 Thermometry
Three fiber optic temperature probes (FISO, Inc., Quebec, Canada) were positioned to measure temperature in the tumor core, tumor periphery and rectum (core temperature). Temperature measurements were documented at one second intervals by each probe throughout the pretreatment, treatment and post treatment period.
3.3.2 AMF Exposure and Thermal Dose Delivery
AMF exposure (166 @KHz 400 to 550 Oe) of the mice was performed using a Hüttinger generator and a Fluxtrol water-cooled coil, ten minutes following nanoparticle injection and 30 minutes post radiation (when radiation was used). Under general anesthesia, with temperature monitoring probes in place, the mouse was placed inside a 50 ml conical tube and inserted into the circular AMF coil (Figure 1). Although the entire mouse received field exposure, the tumor was positioned in a 1.5 cm central region of the coil where the maximal and most uniform field exposure existed. Temperatures were recorded in all three probes at one second intervals (rectal and peri-tumor) and 10–15 second intervals (tumor) throughout the experimental period (preheat, heat and postheat) and graphed in real-time via software supplied by the temperature monitoring system FISO (Figure 2).
Figure 2.
Example of a temperature change in the tumor (top curve), peri-tumor (middle curve) and rectum (bottom curve) during a typical 550 Oe exposure. As expected the highest temperature (45°C) occurred in the tumor, whereas the peritumor (42.5°C) and rectum / core (37°C) were lower. The significantly steeper heating curves seen in the tumor and peritumor region are reflective of the relative iron oxide content. The relationship of the temperatures measured at the three sites was consistent within an individual treatment group. Although the relative relationship between the temperatures remains similar across treatment groups, when treatment parameters are changed the absolute heating profiles vary significantly.
3.4 IONP uptake by tumors cells (local injection)
MTG-B tumors were implanted in C3H/HeJ mice and tumor size was determined as described above, in section 3.2. When tumors reached volumes of 100 ± 50 mm3, the tumors were considered to be of appropriate size for study. The mice were sedated and nanoparticles were injected into the tumors so that the final concentration of nanoparticles within the tumor was 5 mg Fe per cm3 tumor volume. The nanoparticles were allowed to incubate within the tumors for various lengths of time until the tumors were excised and 1 mm3 samples were taken from the tumors and fixed in 4% glutaraldehyde in 0.1 Molar sodium cacodylate buffer (pH 7.4). The time points considered in this study were 5 and 30 minutes; 1, 2, 3, 4, 9, 13, 18 and 22.5 hours; and 5 days. A tumor without nanoparticle injected was also fixed in glutaraldehyde to serve as a control.
After 12–24 hours of fixation, the tumor samples were washed and transferred to a sodium cacodylate buffer solution. The samples were then transferred to the Dartmouth Electron Microscopy Facility for transmission electron microscopy (TEM) sample preparation and imaging. During the course of sample preparation, OsO4 was used for staining and the samples were en block stained with 2% uranyl acetate for one hour. After further dehydration steps, the samples were embedded in either LR White or Epon resin and 100 nm thick sections were cut. In these preliminary studies, samples from one tumor at each time point were imaged with a FEI Company Tecnai F20 FEG TEM (Figure 3).
Figure 3.
A–D. These in vivo transmission electron microscopy images were take from a murine MTG-B tumors. Fig A is a control with no nanoparticles. Fig B was injected with nanoparticles 5 minutes prior to removal and fixed in glutaraldehyde (all nanoparticles are interstitial). Fig C was injected with nanoparticles 2 hours before the excision and fixation. At two hours post injection most nanoparticles seen are still interstitial, but many are associated with the cell plasma membrane. By four hours, (Fig D), the tumor cells appear to have taken up most of the visible nanoparticles. The nanoparticles appear to be contained within endosomes within the cells (arrow). Tumors imaged more than four hours after injection of nanoparticles appeared similar to the tumor imaged at four hours.
4. IN VIVO STUDY PARAMETERS AND RESULTS
4.1 Phase I study
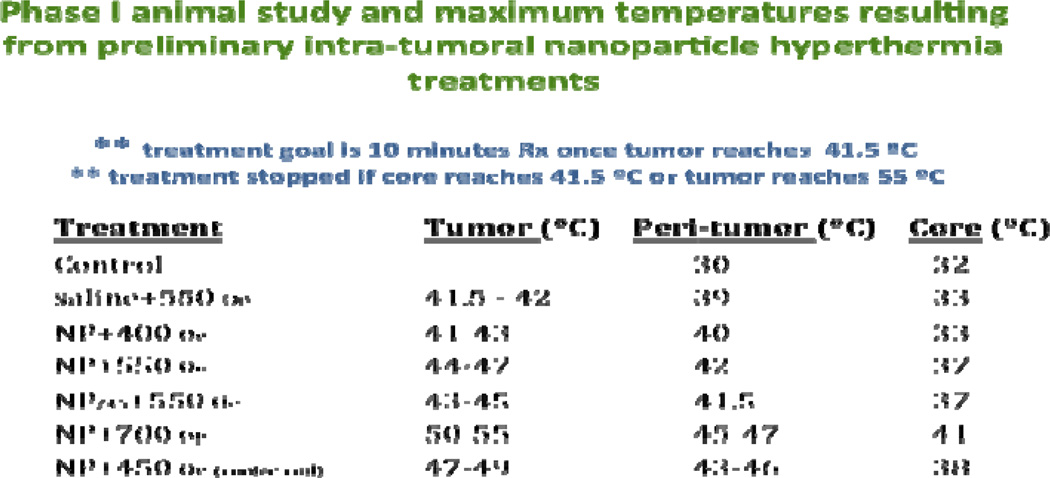
Animals were randomly allocated to eight different groups. Five mice were included in each group. Treatments were carried out under ketamine and xylazine anesthesia (as described previously) when a tumor volume reached 150 mm3 +/− 40 mm3. AMF treatment consisted of thermocouple implantation, a pretreatment heating period (29.0 – 41.5°C) which typically ranged from 3– 9 minutes and a 10 minute treatment period (initiated when the tumor reached 41.5°C). The variation in pretreatment length varied depending on the AMF strength and nanoparticle parameters (single injection vs. multiple injection). Pretreatment core/rectal, peritumor and tumor temperatures averaged 32°C, 28°C and 27 °C, respectively (Figure 4). Although the situation did not occur in these experiments, study guidelines called for immediate stoppage of AMF exposure if temperatures of 41.5° or 55° C were achieved in the rectum/core or tumor respectively.
Figure 4.
Maximum temperatures allowed/reached in various nanoparticle treatment groups. For all treatment groups, core temperature was not allowed to exceed 41.5°C.
4.2 Statistical Analysis
Due to the ongoing nature of these studies, statistical determination of variance between groups has not been performed. At the conclusion of the study such calculations will be performed using a one-way analysis of variance (ANOVA).
4.3 Phase I In Vivo Results
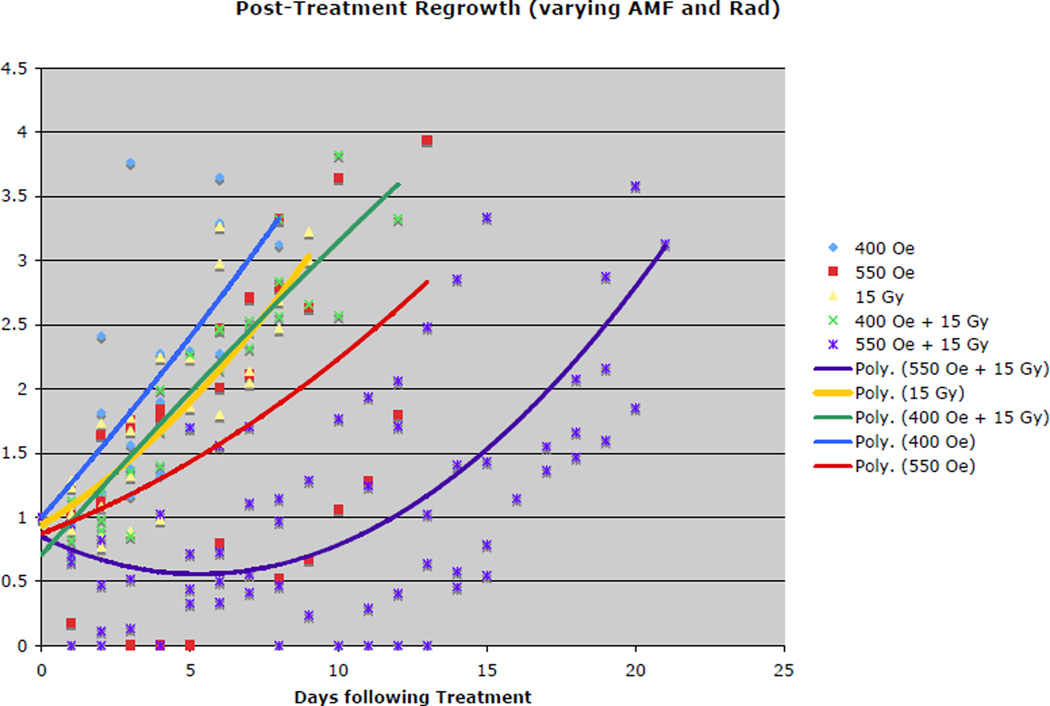
Our phase I in vivo studies are ongoing, however, preliminary information suggests the possibility of a high therapeutic ratio (Figure 5). A 400 Oe AMF nanoparticle treatment resulted in no significant regrowth delay, whereas the 550 Oe group achieved a delay of approximately 9 days (5 mice). When improved particles and heating coil upgrades instituted, significant gains were made in tumor treatment efficacy (tumor regrowth delay). An example of such an effective treatment can be seen in Figure 6. Virtually all regrowth tumor contain a significant region of peri-tumoral edema and numerous iron pigment containing macrophages (Figures 7a and b). The peri-tumoral iron containing macrophages suggest iron from necrotic tumor cells makes its way out of the tumor into residing peri-tumoral scavenger cells.
Figure 5.
This tumor regrowth delay curve compares the relative treatment efficacy of a conventional nonhyperthermia treatment, 15 Gy radiation alone, with iron oxide nanoparticle hyperthermia treatment alone (400 or 550 Oe) and in combination (15 Gy + nanoparticle hyperthermia) in syngeneic MTGB (mouse mammary) tumors.
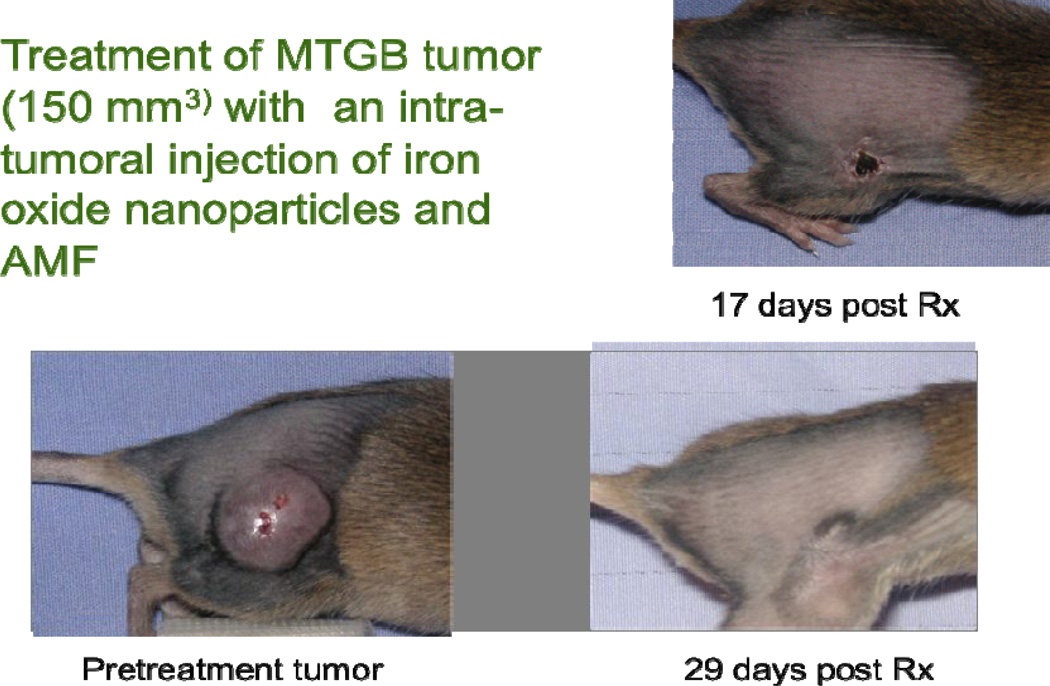
Figure 6.
Although eddy currents from the AMF field can result in core body temperature elevation (depending on frequency and field strength), our studies show that whole mouse AMF exposure at160 KHz and 400 or 550 Oe, for a 20 minutes (heat-up and protocol heating), is safe. This figure demonstrates complete tumor regression (no regrowth) at 29 days. The tumor had not recurred by the 60 day sacrifice date.
Figure 7.
a & b. Figures 7a and 7b are H&E stained low and high magnification photomicrographs, respectively, of a representative subcutaneous MTGB tumor 12 days following iron oxide nanoparticle/550 Oe AMF treatments. Central necrosis of the tumor can be seen at the left hand margin in Figure 7a. Also visible in Figure 7a is a rim of tumor regrowth and fibroplasia surrounding the necrosis and invading adjacent muscle tissue. A zone of edema, with many iron containing macrophages exists between the tumor reaction and normal muscle tissue. Figure 7b is a higher magnification photomicrograph of the tumor regrowth/fibroplasia rim and the bordering edema and iron containing macrophages (dark brown cells).
4.4 Phase II In Vivo Results
With the addition of the chiller to cool the AMF coil, it became possible to achieve high intratumoral temperatures at 450 Oe of AMF (Figure 4). To determine the effect of nanoparticle concentration on efficacy, a six-group study was undertaken. For treatment groups, the mice were anesthetized and IONP solution was injected into tumors to achieve a final iron concentration of 2.5, 5, or 7.5 mg Fe per cm3 of tumor tissue. As described in section 3.3.2, mice were put into the AMF field at a field strength of 450 Oe. Treatment was deemed to have occurred if either the mouse received 10 minutes of additional AMF after the peritumor region reached a temperature of 41.5 degrees C, or if the mice were subjected to AMF for 20 minutes without reaching a peritumor temperature of 41.5 degrees C.
There were three control groups used in this study: some mice were anesthetized and their tumors were injected with IONP so that a final iron concentration of 7.5 mg Fe per cm3 of tumor tissue was obtained. Another group of mice was anesthetized, their tumors injected with Phosphate Buffered Saline at an equivalent volume to a nanoparticle injection, and they were exposed to 20 minutes of AMF at a field strength of 450 Oe. The last control group consisted of mice that were anesthetized and exposed to 20 minutes of AMF at a field strength of 450 Oe.
The parameter being measured was the time delay between when the tumor was treated and its growth to triple its original volume. This was recorded as regrowth delay. Alternatively, in the rare case that a mouse died more than a week after receiving treatment, the time of death was recorded as the regrowth delay if the tumor had not yet regrown to triple its original volume. The data accrued during this study are shown in Figure 8.
Figure 8.
Regrowth delay in days from preliminary studies using 450 Oe AMF treatments. For mice receiving particles, the amount injected is indicated in mg/g. Regrowth delay for mice receiving higher doses of particles with AMF treatment is generally higher than mice receiving lower doses.
5. IN VITRO ANTIBODY DIRECTED IONP STUDIES
Preliminary in vitro antibody-directed IONP studies were conducted with 80–100 nm IONP conjugated to ING-1 antibody. The antibody-conjugated particles had a final hydrodynamic diameter of 155–160 nm. ING-1 antibody was thiolated using 2-iminothiolane (Pierce Chemical Co.), resulting in 1 to 2 mol thiol per mol antibody. A total of 1 to 2 ml of ~ 2 × 10−5 M thiolated antibody in degassed in phosphate buffered saline (PBS) was added to 20 ml (400 mg) of BNF-maleimide nanoparticles in degassed PBS. The reaction mixture was shaken for one hour at room temperature. N-ethylmaleimide (NEM) was added to a concentration of 10 mM and the mixture shaken for 40 minutes followed by magnetic separation for 30 minutes. The supernatant was removed and fresh PBS added for an additional magnetic separation step. Washing was repeated two more times and the nanoparticle conjugate resuspended in 2 mM mercaptoethanol. The suspension was shaken for 30 minutes and the conjugate washed three times with PBS. The antibody-functionalized conjugate was resuspended and stored in PBS at 4°C. Both mouse (MTG-B) and human (HT-29) cancer cells were incubated with the conjugated particles, then cells were washed three times with PBS and trypsin digest-harvested at 0, 1 and 4 hours post-particle addition. Harvested cells were dissolved and sent out for iron quantification using inductively coupled plasma mass spectrometry (ICP-MS) methods. The MTG-B cell line does not express significant ING-1 antigen, whereas HT-29 cells do have significant antigen expression. Results demonstrated this parameter in a dramatic fashion (Figure 9a) with HT-29 cells retaining 12.5 times more iron than the minimally ING-1 expressing MTG-B cells. To compare conjugated targeted nanoparticles (TNT) with unconjugated nontargeted (NT) particles, HT-29 cells were further tested with both particle types and iron quantified using the methods described above for 1 and 4 hour incubation times. Results showed much greater iron association in the TNT groups than in the NT groups over the given incubation times (Figure 9b).
Figure 9.
Preliminary data for targeted nanotherapy cellular association. A) Comparison of non-ING-1 antigen-expressing MTG-B cell and ING-1 antigen-expressing HT-29 iron association with ING-1-conjugated iron oxide particle (TNT) incubation. B) Comparison of iron association between HT-29 cells incubated with ING-1-conjugated and non-conjugated particles (NT). Figures demonstrate increased association of TNT particles compared to NT particles on antigen-expressing cells.
6. DISCUSSION
In these preliminary studies we have shown that local hyperthermia, using AMF activated magnetic nanoparticles, in a variety of situations and independent of other treatment modalities, can result in significant local tumor treatment efficacy (mouse breast cancer model) at an AMF level that does not result in systemic thermal morbidity. These studies demonstrated a positive correlation between tumor thermal dose and tumor regrowth delay. Although field strength and heating time also correlate with increases in core temperature, the relationship suggesting IONP hyperthermia alone may be able to generate a therapeutic ratio that is greater (more effective) than non-particle tumor heating techniques. Although it is too early to judge conclusively, our data also suggest nanoparticle hyperthermia has the potential for and effective stand alone treatment and for improving the therapeutic ratio of conventional tumor treatments such as radiation and chemotherapy.
Preliminary in vivo transmission electron microscopy studies indicate that up to three hours post-injection of IONPs, the nanoparticles are predominantly found within the interstitial space of the tumors and adjacent to cell plasma membranes. After approximately three hours post-injection nanoparticles are almost exclusively found within intracellular endosomes. The IONPs are heating foci when exposed to AMF so it is possible that different cytotoxic mechanisms are present during exposure of IONP-bearing tumors to AMF depending on the morphologic location of the INOPs.
Preliminary in vitro antibody directed IONP studies show dramatic enhancement of TNT-cell association compared to NT association when the antigenic determinates of the target cells match the antibody conjugated to the IONP.
With respect to treatment optimization, our studies also point out the need to examine the relationship of intratumoral nanoparticle hypethermia / radiation treatment efficacy with particle post-injection incubation times, particle composition, size and coating, treatment fractionation and scheduling, AMF frequency and field strength and delivery method.
Acknowledgements
The authors would like to thank Aduro Biotech, Berkley, CA (formerly Triton Biosystems, Chelmsford, MA) for graciously providing the particles used in this experiment.
REFERENCES
- 1.Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3:487–497. doi: 10.1016/s1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
- 2.Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyper- thermia. Crit Rev Oncol Hematol. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 3.Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD, Gonzalez -Gonzalez D, Liu FF, Goodman P, Sherar M. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys. 1996;35:731–744. doi: 10.1016/0360-3016(96)00154-x. [DOI] [PubMed] [Google Scholar]
- 4.Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys. 1994;28:163–169. doi: 10.1016/0360-3016(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 5.Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, Bentzen SM. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. Int J Hyperthermia. 1996;12:3–20. doi: 10.3109/02656739609023685. [DOI] [PubMed] [Google Scholar]
- 6.Sneed PK, Stauffer PR, McDermott MW, Diederich CJ, Lamborn KR, Prados MD, Chang S, Weaver KA, Spry L, Malec MK, Lamb SA, Voss B, Davis RL, Wara WM, Larson DA, Phillips TL, Gutin PH. Survival benefit of hyperthermia in a prospective rando- mized trial of brachytherapy boost hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40:287–295. doi: 10.1016/s0360-3016(97)00731-1. [DOI] [PubMed] [Google Scholar]
- 7.Anscher MS, Samulski TV, Dodge R, Prosnitz LR, Dewhirst MW. Combined external beam irradiation and external regional hyperthermia for locally advanced adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1997;37:1059–1065. doi: 10.1016/s0360-3016(97)00109-0. [DOI] [PubMed] [Google Scholar]
- 8.Algan O, Fosmire H, Hynynen K, Dalkin B, Cui H, Drach G, Stea B, Cassady JR. External beam radiotherapy and hyperthermia in the treatment of patients with locally advanced prostate carcinoma. Cancer. 2000;89:399–403. [PubMed] [Google Scholar]
- 9.Van Vulpen M, De Leeuw AA, Raaymakers BW, Van Moorselaar RJ, Hofman P, Lagendijk JJ, Battermann JJ. Radiotherapy and hyperthermia in the treatment of patients with locally advanced prostate cancer: Preliminary results. BJU Int. 2004;93:36–41. doi: 10.1111/j.1464-410x.2004.04551.x. [DOI] [PubMed] [Google Scholar]
- 10.Kalapurakal JA, Pierce M, Chen A, Sathiaseelan V. Efficacy of irradiation and external hyperthermia in locally advanced, hormone-refractory or radiation recurrent prostate cancer: A preliminary report. Int J Radiat Oncol Biol Phys. 2003;57:654–664. doi: 10.1016/s0360-3016(03)00625-4. [DOI] [PubMed] [Google Scholar]
- 11.Coughlin CT. Interstitial thermobrachytherapy. In: Nag S, editor. Principles and practice of brachytherapy. NY: Futura publishing; 1997. pp. 639–647. [Google Scholar]
- 12.Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys D: Appl Phys. 2003;36:167–181. [Google Scholar]
- 13.Moroz P, Jones SK, Gray BN. Magnetically mediated hyperthermia: Current status and future directions. Int J Hyperthermia. 2002;18:267–284. doi: 10.1080/02656730110108785. [DOI] [PubMed] [Google Scholar]
- 14.Jordan A, Wust P, Fahling H, John W, Hinz A, Felix R. Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int J Hyperthermia. 1993;9(1):51–68. doi: 10.3109/02656739309061478. [DOI] [PubMed] [Google Scholar]
- 15.Jordan A, Wust P, Scholz R, Tesche B, Fahling H, Mitrovics T, Vogl T, Cervos Navarro J, Felix R. Cellular uptake of magnetic fluid particles and their effects in AC magnetic fields on human. doi: 10.3109/02656739609027678. [DOI] [PubMed] [Google Scholar]
- 16.Johannson M, Jordan A, et al. Thermotherapy using magnetic nanoparticles combined with external radiation in an orthotopic prostate model. The Prostate. 2006;66:97–104. doi: 10.1002/pros.20324. [DOI] [PubMed] [Google Scholar]
- 17.DeNardo SJ, DeNardo GL, Forman AR, Ivkov R, et al. Development of tumor targeting bioprobes (111In-Chimeric L6 monclonal antibody nanoparticles) for alternating magnetic field cancer therapy. Clin Cancer Res. 2005;11(19 Suppl):7087s–7092s. doi: 10.1158/1078-0432.CCR-1004-0022. [DOI] [PubMed] [Google Scholar]
- 18.Ivkov R, Forman AR, DeNardo SJ, DeNardo GL, et al. Appplication of high amplitude alternating magnetic fields for heat induction of nanoparticles localized in cancer therapy. Clin Cancer Res. 2005;11(19 Suppl):7093s–7103s. doi: 10.1158/1078-0432.CCR-1004-0016. [DOI] [PubMed] [Google Scholar]
- 19.Gruettner C, Mueller K, Teller J, Westphal F, Foreman A, Ivkov R. J. Magn. Magn. Mater. 2007;311:181. [Google Scholar]