Abstract
‘The Big four’ are the most poisonous snakes in India, and especially in Kerala. These include the cobra, the viper, the krait and the sea snake. Most of the poisonous snakebites in India occur in Kerala. We believe there are only a few reports of myocardial infarction after snakebites and most of these are viper bites. We believe this is the second case of primary angioplasty for a snakebite. There are at least a few potential issues in performing a primary angioplasty in a snakebite case, namely (1) Is it a thrombus or a spasm? (2) Are the bleeding parameters deranged? Will the patient tolerate tirofiban and other glycoprotein (GB) 2b3a inhibitors? Will he develop dangerous bleeding due to the high dose of heparin needed? Further, would we save the patient from myocardial infarction only to lose him to renal failure, both due to the nephrotoxicity of the venom, the kidney being further damaged by the contrast media used for the angioplasty? We discuss all these issues as they crossed our mind, and hope it will help further treatment in others. We would like to review the available literature on these points and describe a recent case of ours.
Background
“The Big four” are the most poisonous snakes in India, and especially in Kerala.1 2 These include the cobra, the viper, the krait and the sea snake. Most of the poisonous snakebites in India occur in Kerala.1 2 We believe there are only about five to six reports of myocardial infarction after snakebites and most of these are viper bites.3–8 We believe this is the second case of primary angioplasty for a snakebite (or maybe the third case). Apart from primary care, there are at least a few potential issues in performing a primary angioplasty in a snakebite case, namely (1) Is it a thrombus or a spasm? (2) Are the bleeding parameters deranged?9 Will the patient tolerate tirofiban and other GB 2b3a inhibitors? Will he develop dangerous bleeding due to the high dose heparin needed? Further, would we save the patient from myocardial infarction only to lose him to renal failure, both due to the nephrotoxicity of the venom, the kidney being further damaged by the contrast media used for the angioplasty? We discuss all these issues as they crossed our mind, and hope it will help further treatment in others. We would like to review the available literature on these points and describe a recent case of ours.
Case presentation
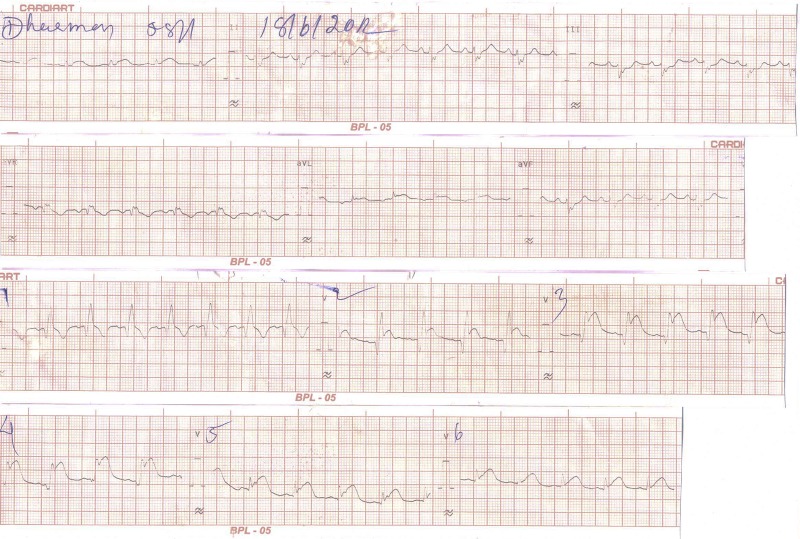
A 60-year-old tribal man was bitten by a snake on his left hand. He was taken to the emergency department of our institution and received initial treatment including antisnake venom. Approximately 5 h later, he developed hypotension and progressively worsening chest pain. The ECG obtained showed sinus rhythm and ST-segment elevation in the anterior leads (figure 1).
Figure 1.
The ECG of a patient who had severe chest pain after a snakebite, probably a viper bite.
He was shifted to the intensive coronary care unit (ICCU) with a heart rate of 100/min and a blood pressure of 70 systolic. His jugular venous pressure was not elevated. On auscultation, his first heart sound was normal, as was his second heart sound, and this was normally split with a normal pulmonary component. His left hand was swollen, but he had no evidence of vascular compromise. He had no evidence of neurotoxicity or fresh bleeding from the wound at the time of admission to the ICCU.
He was started on dopamine infusion and planned for primary angioplasty.
He was a chronic smoker. He had no history of diabetes or hypertension and had no family history of coronary artery disease.
He was taken up for primary angioplasty (figure 2). His right coronary artery was normal. His left main coronary artery was normal, but his left anterior descending coronary artery (LAD) was totally obstructed proximally (figure 3). His left main coronary artery was cannulated with a 6F JL 3.5 Launcher guiding catheter, which showed that the LAD was totally occluded after a short stump. His left circumflex was normal. His lesion in the LAD was crossed with a 0.014 Galeo floppy wire, and this was parked in the distal LAD. The lesion opened up partially. Thrombus aspiration was performed twice with an NIPROTVAC 6F/syringe. The thrombus was cleared partially. A discrete lesion was noted in the proximal LAD, which was directly stented (figures 4 and 5) with a 3.25×15 Prolink (BMS) at 12 atm×10 s. The stent was postdilated at 12 atm for 10 s and Thrombolysis In Myocardial Infarction (TIMI) III flow was attained (figure 4). No dissection or residual stenosis was noted. After the procedure, the patient developed ventricular tachycardia which reverted with 150 mg amiodarone. The patient's blood pressure was 80 systolic at the time. We used intravenous amiodarone as he was restless and we had to hold him down to the table. A DC shock would have affected us. We generally do not use lignocaine in acute myocardial infarction as this can cause asystole. We were hesitant to sedate him with morphine as he was supposed to be a heavy alcoholic.
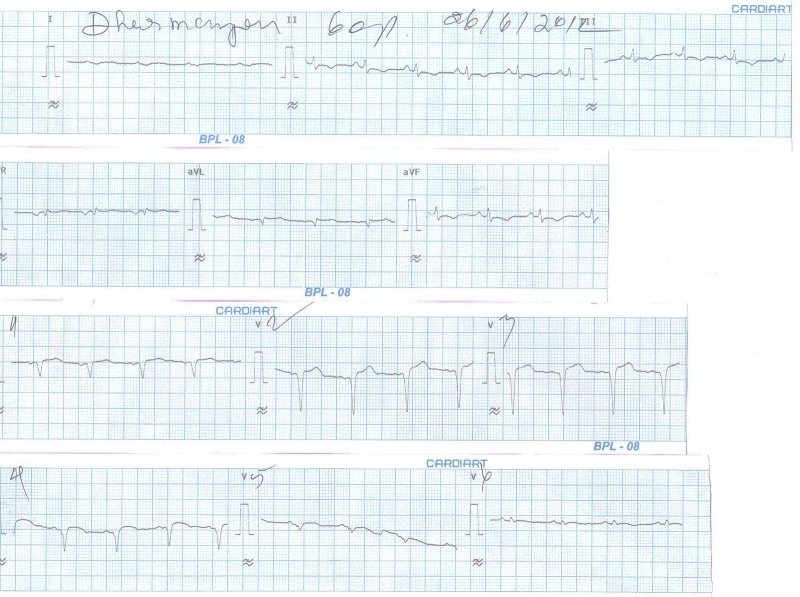
Figure 2.
The ECG of the patient after primary angioplasty and stenting to the LAD.
Figure 3.
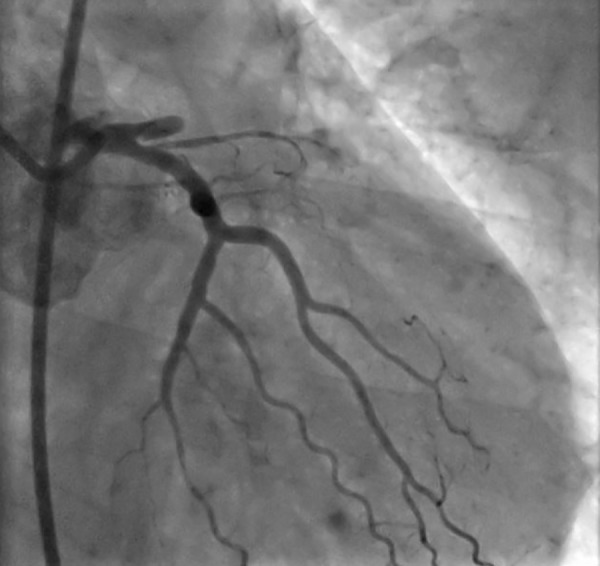
The coronary angiogram of the patient, showing the LAD is blocked totally after a stump.
Figure 4.
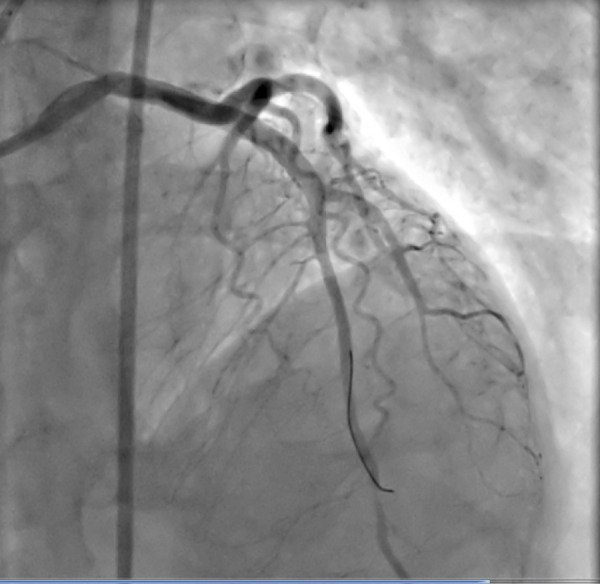
Final view of the LAD after stenting.
Figure 5.
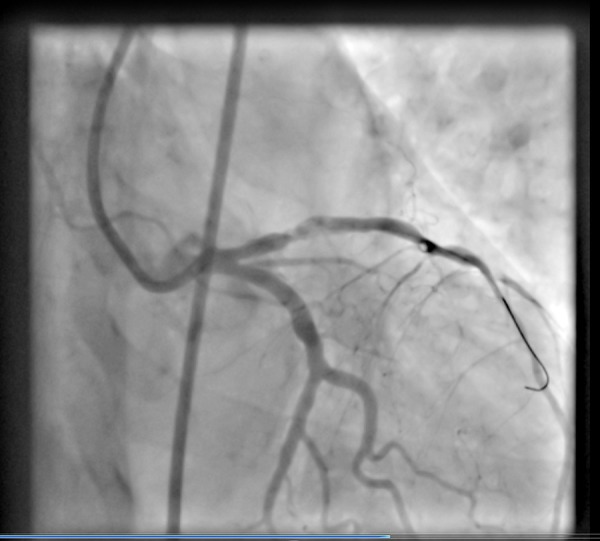
A coronary angiogram showing a residual atherosclerotic lesion in the proximal LAD, after thrombus aspiration and before stenting.
This ventricular tachycardia was either a reperfusion arrhythmia or due to a transient no reflow phenomenon.
Injection GP IIB/ IIIA infusion (tirofiban) was started.
He was subsequently transferred to the ICCU where he stabilised and was discharged on the sixth day without any further complications or morbidity.
He is a tribal but still comes for follow-up after we contacted his son by telephone (mobile). He is alive in 2014 (2 years follow-up).
About the Gb2b/IIIa inhibitor: In spite of being given 6000 iu of heparin and tirofiban, he had no further bleeding. He had a heavy thrombus burden, so we were compelled to give him a tirofiban infusion at standard doses. His ST segment resolved adequately (figure 2).
Investigations
His haemoglobin was 13.4 g/dL, platelet count was 2.56 lakhs/mm3, prothrombin time/international normalised ratio was 1.56 and activated partial thromboplastin time was 66 msecs. His blood urea was 29 mg/dL and serum creatinine was 1.1 mg/dL.
Differential diagnosis
Acute coronary syndrome secondary to severe vasospasm due to viper bite.
Acute coronary syndrome due to smoking and atherosclerosis worsened by vasospasm due to viper bite.
Acute coronary syndrome after viper bite due to the procoagulant effect of snake venom and hypercoagulable state.
Treatment
Primary angioplasty was performed.
Outcome and follow-up
We believe this is the second case of snakebite where primary angioplasty has been performed and only the first case reported where tirofiban has been given. We hope our experience would be of help to others.
Discussion
The highest number of snakebite deaths occur in India (35 000–50 000).2 In Pakistan, only 8200 of 40 000 die of snakebites. Snakebite has been reported to be a neglected tropical disease. In a recent article, Alirol et al2 have reviewed snakebites in Asia and described a number of complications of snakebite.
The demographics of those who were bitten
Those affected the most were farmers, plantation workers, animal herders and fishermen. Those who slept on the floor tended to be bitten. Alirol et al2 also summarised that the average age of the snakebite victim was a male between 10 and 40 years of age (the male:female ratio was 2:1). More than half of the krait bites occurred at night and viper and cobra bites occurred during the daytime. Almost 60–80% of the bites occurred on the feet or lower extremities. The head, hand and trunk bites occurred at night, especially in those sleeping on the floor.
The bite to treatment delay varied from 30 min to 15 days, with 60% of the victims reaching medical care within 6 h but only a few reaching within 1 h. In 50% of the snakebite victims, the first aid administered was wrong. Death ranged from 5% to 58%.
In 2007, Bangladesh had heavy monsoon floods, and during this time the second most common cause of death after drowning was snakebite.
Venomous snakes: a brief review
In the Himalayas, there are around 300 species of snakes, of which 67 are poisonous (front-fanged snakes).2
The Viperid snakes have 26 species including the true viper (subfamily Viperienae) and pit vipers (Crotalinae). Of the true vipers, the Russell's viper (Dabois reisselens) is associated with the highest morbidity and mortality.
In south India, the Malabar pit viper is supposed to have caused many deaths (Trimeresurus malabaris). The Hump nose pit vipers (Hypunale hypnale and H. nepa) cause renal failure and haemostatic dysfunction.
The Elapidae include 17 species of cobras, kraits and coral snakes (sea) and many of the sea snakes found in India. These spread their hood and raise their heads and anterior body. Naja naja is the common spectacled cobra.
Kraits are nocturnal snakes that enter human dwellings at night in search of prey. Most of the victims are bitten while asleep. A krait bite death rate is 77–100% without treatment. There are eight species of kraits in south India, but the common krait is the Bungarus caeruleus.
Sea snakes are also poisonous. ‘Enhydrina schistose’ is the common sea snake that causes death at sea.
The effects of snakebites
Generally, viperine bites cause a mixture of local reactions, coagulation disorders and haemorrhagic disorders. Traditionally, local hospitals in India study envenomation by doing the clotting time, and by monitoring the urine output and the blood urea. Local swelling, bleeding and blistering, and gum bleeding are all observed with viper bites. Acute renal failure is also reported.
Cobra bites cause local swelling, tissue necrosis and neurotoxaemia. Here the post-synaptic neurons are affected.
Krait venom causes presynaptic neurotoxaemia which damages the nerve endings. Kraits cause extraocular paralysis and droopy eyelids. Patients have difficulty in swallowing and inability to protrude the tongue,2 limb weakness, loss of deep tendon reflexes and diaphragmatic palsy may occur.2 Krait bites cause delayed paralysis and delayed recovery. However, the Cobra and the Krait can paralyse and kill within 30 min.
The toxins of krait venom
The toxins of krait venom are β-burgarotoxin. This destroys the nerve terminals. Krait venom can also cause nephrotoxicity or myotoxicity. Nephrotoxicity is most common with the black krait. Myotoxicity leads to dangerous rhabdomyolysis after a krait bite. Cobra toxins can cause bleeding.
Some relatively rare complications of snakebite (to be looked for) are the following:
Broken neck syndrome
Tetanus
Myocardial infarction
Ventricular tachycardia.
First aid in a snakebite: Should a tourniquet be applied?
After a snakebite–Should a tournique be applied?
First aid is ideally not using a tourniquet and immobilising the limb, but this should not result in delay in sending the patient to the hospital. The earlier the patient reaches the hospital, the better.10
A recent study by Inamdar et al11 has thrown some light on the traditional practices of first aid. In their study from Maharashtra, they report that snakebite mortality was higher in the rural areas than in the urban areas (6.3% vs 3.4%). In this study, those who received an incision and a tourniquet before reaching the hospital had lower mortality than those who did not receive first aid (3.1% vs 9.2%; p<0.01). Further, mortality was higher when the time to treatment was more than 6 h (8.4% vs 4.4%). It was less when the patient reached the hospital in less than 6 h from the onset of the bite. The first aid given in this study was immobilising the bite area, applying a bandage firmly, and incising to draw blood.
These practices have been discouraged by previous studies as incising can increase the chances of bleeding and sucking the wound can cause envenomation for the person who sucks it. Further, it is believed that the venom spreads very fast. In our centre, we do not recommend either sucking the wound or incising it or putting a tourniquet.
The controversy about the tourniquet
Tourniquets probably help only when the distance to the treating hospital is very far. Another set of workers have shown that tourniquets delay the time to respiratory paralysis, and that removing the tourniquet caused early paralysis. We do not advocate tourniquets before the antivenom is given; rather, we recommend immediate transportation to a major hospital. All government hospitals and many big private hospitals have antivenom, the needed ventilators and manpower.
The Pakistani Medical Association12 does not advocate incision or the use of suction devices or the use of tourniquets as this has been shown to increase tissue necrosis. They believe that tourniquets applied to the lower limb usually cause gangrene while upper limb tourniquets do not cause gangrene as the upper limbs have a blood supply via osseous arteries. However, most people are untrained and tourniquets cause more harm than good. The tourniquet should just block the venous return but not be so tight as to occlude the arteries of the limb. Further, ICE or electric shock is not recommended as a local treatment.
Karalliedde13 has commented that when extremely neurotoxic animals are the cause of bites from elapid snakes like black mamba, taipan, cobra or krait, a tourniquet delays the onset of respiratory failure until the victim reaches the hospital. However, these should not be used if the venom is known to cause necrosis. Tourniquets applied in 94% of 36 patients bitten by the Philippine cobra produced a delay in the onset of respiratory paralysis. Four were asymptomatic before release, and in 11 symptoms worsened after release. Four patients developed severe respiratory paralysis on release of the tourniquet. So tourniquets should be released only when ventilatory help is available.
Workers have described two useful signs of snake bite. The upper level of the swelling on the limb should be marked with an indelible marker. If the swelling progresses then it means envenomation. A progressive increase in the size of the cellulitis is consistent with envenomation. Further if the patient has progressive oedema or compartment syndrome, it is suggestive of envenomationn. Sometimes, features of limb ischaemia may be present and help to point to envenomation. Chugh and others also have described renal failure after viperine bites.14 15
Treatment
Ideally, the snake should be identified. However, most bites occur even without the person seeing the snake. So the snake is identified by “the syndrome” it causes.
Simple pointers to poisonous bites would be—prolonged bleeding time, decreased haemoglobin or haematocrit, a sharply decreased platelet count or haematuria. As some snakes’ mouths contain gram-negative bacteria, an acute increase in the size of local lymph nodes or an increase in the WBC count also helps denote poisonous bites.
Some advanced centres perform an Elisa test to diagnose which snake has bitten the victim. Swabs from the bite site are used. Further, PCR amplification and sequencing of snakebite site swabs has worked in experimental situations and animal models in Bangladesh and Nepal.
Kumar and Basheer17 have beautifully described the biochemical changes in snakebite cases. They have reported that the clotting time increased to 55.83±38.5 min in cases of viper bites, but did not increase in cases of cobra bites. We believe that our patient had a viper bite. They also describe the time course of elevation of the blood urea in a viper bite. Briefly, the blood urea rises significantly after the sixth hour and some cases even need a dialysis. The antivenom does not decrease the blood urea. Patients with viper bite and severe envenomation also had elevated serum Na and serum K values. They also describe the content of snake venom, this contains sodium, calcium, magnesium, zinc and small amounts of iron. Some venoms contain glycoproteins, lipids and around 26 enzymes. Elapid venoms are rich in acetyl cholinesterase, and crotalid or viperoid venoms are rich in endopeptidases. Maybe this is why viper bites cause a heart attack. The other components of snake venom are thrombin/thrombin-like enzymes, proteolytic enzymes (that may cause tissue damage) collagenase (promotes spreading of the venom) hyaluronidase, phospholipase A2, phospholipase B, and C, lactic dehydrogenase, RNase, and DNase.
Jensen18 has described in detail the haematological effects of snake venom.19 Of course, this is based in Papua New Guinea. However, the poisonous snakes are from the same orders the world over. So to highlight some of the haematological effects of snake venom: The snake venoms contain coagulants and procoagulants. Haemolysis occurs after some bites as does impairment of platelet function. Collapse a few hours after a snakebite is believed to be due to myocardial ischaemia due to these procoagulants. Some patients may even have a stroke or cerebral thrombosis. Superficial bleeding, venous thrombosis, gum bleeding, haemoptysis and bleeding from venepuncture sites have all been described. The author recommends using only one cannula in such cases. Other sites of bleeding are nasal bleeding, oral bleeding, subconjunctival bleeding, subglottic haematoma, under the tongue, (such patients need intubation), microscopic or macroscopic haematuria, retroperitoneal bleeding, or placental bleeding in pregnant women and intracranial bleeding have been described. We were worried that our patient would bleed. We were worried that after the primary angioplasty, our patient could have bloody diarrhea, or malena or rectal bleeding.
Cases of ecchymosis and DIC have also been described. All of the above are caused by Papuan taipans.19 Another rare occurrence after a snakebite is a bilateral parotid swelling.20
Meenatchisundaram and Michael21 have described the lethal dose of each snake venom.
Frangides et al22 have described that viperine bites are the most common in Greece. However, they have not described any myocardial infarctions. In their series, 13% of viperine bites had hypotension and shock. It is also worth mentioning how they decide on envenomation. They use the Downey system.23 In this system, grade 0 means no envenomation but swelling and erythema is found around the fang marks for an area of less than 2.5 cm. grade 1 is swelling and erythema from the fang marks, 2.5–15 cm but no systemic signs. Grade 2 is swelling and erythema of 15–40 cm from the fang marks with mild systemic signs, and grade 3 is swelling and erythema of more than 40 cm with systemic symptoms. Grade 4 is severe systemic signs including coma and shock. In their series, only 2 patients (of 147) had grade 4 envenomation.
Irrigation of the eyes
Venomous snakes in mountainous areas include the spitting cobras that are toxic. If they spit into the eyes of a victim, the eye should be irrigated with water for about 20 min.
Myocardial infarction and snakebite
There are about 13 reports of myocardial infarction and snakebite and 4 reports of ventricular tachycardia due to snakebite (3, 4, 5, 6, 7, 8, 16, 24 and 25, pure myocardial infarctions, and 26–29 are reports with ventricular tachycardia, and 30–34 are myocardial infarctions without ventricular tachycardia; table 1). The mechanisms and causes of myocardial infarction after snakebite have been attributed to
Extreme coronary spasm: Intense coronary spasm can lead to myocardial infarction after snakebite.
Toxins: Saadeh et al7 have described a case of snakebite who developed a myocardial infarction after a possible viper bite. After receiving the antivenom, he developed ST elevation suggestive of acute inferior wall myocardial infarction, which responded only to an injection of morphine. The local necrosis subsided gradually, and one month later he had a coronary angiogram that was normal. The authors thought that arterial thrombi caused by the venom or prolonged vasoconstriction, caused by the endothelins or sarafatoxins in the venom, had caused the myocardial infarction. Focal myocardial haemorrhage and microvascular fibrin deposition might have also caused the myocardial infarction in the case reported. Heamorrhagins have also been described that lead to vasoconstriction.
A myocarditis: apart from viper bites, krait bites have also been shown to cause toxic myocarditis. Agarwal et al24 describe acute pulmonary oedema in a patient with a krait bite (B. caeruleus). This occurred when the patient went into shock, and he was given rapid intravenous fluids that precipitated the pulmonary oedema. The patient survived after mechanical ventilation, supportive treatment and antivenom. Their patient was a 26-year-old farmer who had been bitten 6 h previously in a field. This patient developed desaturation on ventilator and was found to be Troponin T positive and had ST depression and T inversion in AVL, L1 and V1 to V6. His echocardiogram revealed an EF of 40%. He later improved and on follow-up at 6 weeks had a normal EF (62%).24
Owing to shock: Maheshwari et al25 have described a type of hypovolaemic shock after viper bite. This can lead to severe myocardial ischaemia and myocardial infarction.
Owing to hypercoagulability: snake venom contains arginine esterase hydrolase.25 This, plus the haemoconcentration due to volume loss, can lead to myocardial infarction.
Owing to bradykinin production: Brown and Dewar3 have postulated that snake venom when in contact with the plasma globulins produces bradykinin, which can in turn cause vasospasm and blood pressure fall.
Koumis syndrome: MI due to anaphylaxis to the antivenom, causing myocardial infarction, has also been described.
The effect of Taicatoxin: The Papuan taipan snake has a myocardial toxin in its venom that is believed to be a direct myocardial calcium channel blocker. It causes extreme vasospasm (Aaron) and suppression of myocardial function.18 19
There are around three reports of ventricular tachycardia after snakebite, similar to that of our patient.26 27 Francis28 also reported a case of ventricular tachycardia after a snakebite. In all three cases, the patients seemed not to have any residual myocardial infarctions. The other common complications of snakebite have been described by another report from India.29
Table 1.
Snakebite reports causing myocardial infarction
| Author | Snake | Timing | Type MI | Coronaries | References |
|---|---|---|---|---|---|
| Brown | Viper/adder | Delayed 30 h | Lateral wall MI | Normal | 3 |
| Aravanis | Viper | Immediate | With CVA | 4 | |
| Tony | Viper | 29 h | MI | 5 | |
| Blondheim | Viper | Immediate | Collapse | 6 | |
| Saadeh | Viper | 4 h | Acute inferior wall MI | CAG-N (CAG after 1 month was normal) | 7 |
| Silva | Viper | Immediate | Inferior wall | 8 | |
| Sundaraperumal | Viper | 3 cases | 9 | ||
| 1–11 h 2–2 h 3 h–immediate |
Anterior wall Anterior wall STEMI |
||||
| Gupta | Viper | 5 h | Anterior wall | LAD was blocked | (present) |
| LAD total | |||||
| Jang | Unknown | 24 h | Ischaemia | 16 | |
| Agarwal | Krait | 8 h after bite | Acute pulmonary oedema | 24 | |
| ST depression V1 to V6 | |||||
| Maheshwari | Viper | Within minutes | Inferior wall | 25 | |
| Niraj | Viper | Immediate | 30 | ||
| Menon | Viper | 15 h | Inferior | 31 | |
| Al Durihim | Possibly viper | 18 h | Inferior (collapse, rhabdomyolysis, needed haemodialysis, and had DIC, died) | 32 | |
| Gaballa | Viper | Immediate | Anterior wall dyskinesis | Had thrombus; aspiration, normal coronaries, no stenting. Tissue Doppler imaging reverted | 33 |
| Satish | Viper | Immediate | Inferior | 34 | |
| Karlson-Stiber | Adder | 8 days after bite | 35 | ||
| Luksic | Viper | Immediate | Shock | 36 | |
| Naidoo | Black mamba | 2.5 h | ST elevation with q waves | 39 |
CAG, coronary angiography; DIC, disseminated intravascular coagulation; MI, myocardial infarction; STEMI, ST-elevation MI.
The pattern of myocardial infarction seen after snakebite
History: Probably the first-ever report of myocardial infarction after a snakebite was described by Brown and Dewar3 in 1965. They themselves describe an earlier case of posterior wall myocardial infarction described by Askanas in 1959 in Polish. We do not have access to this article. However, in the case of Brown et al, a young 22-year-old paratrooper was bitten by an adder on the finger. His wound swelled within minutes and within 15 min he was staggering. Within 1 h of the bite, he had severe hypotension and was put on intravenous fluids and norepinephrine. Thirty hours after admission, he had a sudden blood pressure fall and chest pain, and was found to have T inversion in AVL and V5 and V6. He gradually recovered and went home, but 4.5 months later he developed recurrent chest pain. At this time, he had a coronary angiogram that was normal and still later returned to work, lifting 10.5 tons of coal per day.
In this case, it was believed that bradykinin caused severe spasm of the coronaries or that the snake venom caused thrombosis or haemorrhage of the coronary arteries.3 The bradykinin that was produced by the action of the snake venom on the plasma globulins might have produced the shock, or the direct action of the venom caused direct myocardial parenchymal damage.
As can be seen in table 1, two common modes of onset of myocardial infarction after snakebite have been observed. One onset is immediate, where the patient is profoundly shocked and develops a myocardial infarction within minutes of the snakebite. This type of myocardial infarction may be due to the low blood pressure causing coronary perfusion to suffer.
The second mode of onset of myocardial infarction after snakebite is with a gap, maybe 30 min to 1 h or even later after a snakebite. This type of myocardial infarction is probably due to extreme coronary spasm, maybe due to the endothelins in the snake venom.
As can be noted, no obvious case of cobra bite and myocardial infarction and only one case of krait bite have been described after envenomation. This may be because the cobra venom does not cause a heart attack or that the patients could not reach the hospital alive.
Management of the cardiovascular effects of snakebite
Most patients would be in profound shock, so an intravenous line, vasopressors and intravenous fluid replacement have to be given. Polyvalent antivenom should be given. Steroids to prevent any anaphylaxis should be given. Numerous reports of snakebite survivors having been ventilated for prolonged periods are available. So facilities for endotracheal intubation and mechanical ventilation should be provided. Many snakebite victims have arrhythmias. So advanced life support equipment, defibrillators and other drugs should be available. Biochemical tests for serum Na, serum k blood urea, serum creatinine and clotting factor testing should be available and be used round the clock.
All the survivors of myocardial infarction were given antivenom whether they were in shock or not, and if they were in shock they were given inotropes. So this should form an important part of their management. The other cases of snakebite and myocardial infarction are described in table 1.30–34
Some authors advocate intravenous H1 and H2 receptor blockers. Some advocate epinephrine to prevent antivenom anaphylaxis.35
Anticholinesterase drugs for neurotoxicity: those who show ptosis or other signs of neurotoxicity should be tested for the need of anticholinesterase drugs (first intravenous atropine 5 mg should be administered in adults). Then edrophonium should be given (0.25 mg/kg intravenously until a maximum dose of 10 mg). If no improvement is seen, then neostigmine should be tried within 5 min. The dose—neostigmine 0.01 mg/kg up to 0.5 mg every 30 min, until full recovery occurs. Atropine should be repeated when bradycardia returns. This may have to be given repeatedly.
Specific heart attack treatment
We have not come across reports where thrombolysis with streptokinase has been tried. However, we have come across two cases (one is our case) where primary angioplasty after thrombus aspiration was performed. The coronary arteries appear to be filled with thrombus, so thrombus aspiration devices should be used. Further, if there is a residual coronary stenosis, a stent should be deployed. In one case, this was not required. We were forced to use the Gb2b/IIIa inhibitor tirofiban infusion and intravenous heparin for our case. We had loaded the patient with 325 mg of aspirin and 300 mg of clopidogrel, and followed with 325 mg daily aspirin and 75 mg daily clopidogrel. Our patient did not have significant bleeding. However, we do have 24 months follow-up. He was discharged and after follow-up is fine 2 years later.
Both Luksic et al36 and Onyiruika et al37 describe snakebites in children. Onyiruika has in fact reviewed the different toxins in snake venom, with special reference to species in Nigeria. He has described that the genes encoding body proteins are responsible for modifying the components of the venom. The Echis ocellatus venom contains a zinc metalloproteinase called Ecarin, this activates prothrombin to produce Ecarin thrombin. This is a strong coagulant that cannot be neutralised by natural antithrombin III or heparin. This can cause clotting. This venom also contains Echistatin – this is a fibrinogen receptor antagonist that inhibits platelet aggregation. This can cause bleeding.37 The component Echis also activated factors V, X and pro-thrombin in the snake bite victim and caused further clotting. This can also lead to disseminated intravascular coagulopathy (DIC).37 Neurotoxins paralyse the victims and cardiotoxins cause cardiac damage.
Another inhibitor of platelet aggregation, called echistatin, is a fibrinogen receptor antagonist. Further, biochemical analysis of snake venom can be reviewed in the article written by Wickramaratna et al.38
Most of the myocardial infarctions after snakebite have been described after viper bites. However, there is a report of a myocardial infarction after a black mamba bite, possibly the 14th report.39 It has been described that there are three different fractions in the black mamba venom. Fraction I is a neurotoxin, fraction II is a histamine-liberating agent and fraction III is an anticoagulant factor.
Naidoo et al39 describe that only fraction I is lethal. It causes haemorrhages, oedema of the myocardial cells and hydropic degeneration in the cardiac muscle cells when injected into albino rats (in an experiment by Zaki et al).40 The rats also showed lymphocytic infiltration and perivascular oedema. So the authors concluded that fraction I had a direct toxic effect on the myocardium, possibly causing myocardial infarction. This would have been the first case of an Elapid bite causing myocardial infarction.
It would be incomplete not to mention two excellent reviews of snakebites.41 42 Those in the mountainous regions should read the article by Boyd et al.43 He deals with the issue of irrigation of the eyes when spitting snakes cause envenomation. The issue of overdosing with antivenom has been dealt with by a recent editorial.44 These would also guide a physician in managing snakebites.
Learning points.
Myocardial infarction can occur after viper bite either because of severe vasospasm due to the endothelins in the snake venom, or due to severe hypotension causes by the extreme platelet permeability caused by the venom.
From region to region the composition of the viper venom varies mildly, some causing more local necrotic effects and some causing bleeding, while some types of viperine snakes cause increased coagulation.
The treatment of heart attack after viper bite is probably by thrombus aspiration and stenting if there is any residual lesion.
Tirofiban can be given safely if the clotting time is normal and there is no active bleeding after the viper bite, provided the antivenom has already been given.
If the patient is a smoker, then atherosclerotic narrowing of the coronary artery can occur and this has to be stented.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mathew JL, Gera T. Ophitoxeamia (venomous snakebites.). Priory.com, Medicine online
- 2.Alirol E, Sharma SK, Bawaskar HS, et al. Snake bite in South Asia: a review. PLoS Negl Trop Dis 2010;4:e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown R, Dewar HA. Heart damage following adder bite in England. Br Heart J 1965;27:144–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aravanis C, Ionnides PJ, Krevas J. Acute myocardial infarction and cerebrovascular accident in a young girl after viper bite. Br Heart J 1982;47:500–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tony JC, Bhat R. Acute myocardial infarction following snake bite. Trop Doct 1995;25:137. [DOI] [PubMed] [Google Scholar]
- 6.Blondheim DS, Pleich M, Berman M, et al. Acute myocardial infarction complicating viper bite. Am J Cardiol 1996;78:492–3 [DOI] [PubMed] [Google Scholar]
- 7.Saadeh AM. Case report: acute myocardial infarction complicating a viper bite. Am J Trop Med Hyg 2001;64:280–2 [DOI] [PubMed] [Google Scholar]
- 8.Silva A, Plaptiya S, Siriboddana S, et al. Acute myocardial infarction following a possible intravenous bite of Russell's Viper (Daboisa russelli). BMC Res Notes 2012;5:500. http://www.biomedcentral.com/1751–0500/5/500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundaraperumal R, Mohanasundaran K, Kumaraswamy S. Acute coronary syndrome following snake bite. A report of 3 cases from a tertiary care hospital in rural South India. Trop Doct 2012;42:171–3. http://1 tdo.sagepub.com content/42/3/171 [DOI] [PubMed] [Google Scholar]
- 10.Cribari C. The management of Poisonous Snakebites. Guidelines American College of Surgeons 2004. Commitee on Trauma 1–4
- 11.Inamdar IF, Aswar NR, Ubaidulla M, et al. Snakebite: admissions at a tertiary health care center in Maharashtra, India. S Afr Med J 2010;100:465–8 [DOI] [PubMed] [Google Scholar]
- 12.Snakebite Management in Asia and Africa. A2, Guidelines produced by the Pakistan Medical Research Council. 1–162. by Huma Qureshi. email of adviser Ian D Simpson. (iandsimpson@gmail.com)
- 13.Karalliedde L. Animal toxins. Br J Anaesth 1995;74:319–27 [DOI] [PubMed] [Google Scholar]
- 14.Chugh KS. Snake bite induced acute renal failure in India. Kidney Int 1989;3:891–907 [DOI] [PubMed] [Google Scholar]
- 15.Sitprija V. Snake bite nephropathy. Nephrology 2006;11:442–8 [DOI] [PubMed] [Google Scholar]
- 16.Jang TC, Seo YW, Lee KW. A case of a acute myocardial infarction complicated by a snake bite. J Korean Soc Emerg Med 2011;22:760–3 [Google Scholar]
- 17.Kumar PKM, Basheer MP. Snake bite: biochemical changes in blood after envenomation by viper and cobra. J Med Allied Sci 2011;1:36–41 [Google Scholar]
- 18.Jensen S. Symptoms and signs of snakebite in Papua New Guinea. In Clinical Management of Snake bite in Papua New Guinea, Chapter 4, pages 4.1–4.13
- 19.Aaron KL, Williams D, Jensen S. Treating other effects of envenomation in Clinical Management of Snake bite in Papua New Ginea, Chapter 10. (10.1–10.11)
- 20.Deepak M, Basavaprabhu A, Ramapuram JT, et al. Bilateral parotid enlargement following snake bite: a rare sign. Asian Pac J Trop Biomed 2013;3:154–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meenatchisundaram S, Michael A. Snake bite and therapeutic measures: Indian scenario. Indian J Sci Technol 2009;2:69–73. ISSN: 0974-6846 [Google Scholar]
- 22.Frangides CY, Koulouras V, Kouni SN, et al. Snake venom poisoning in Greece. Experiences with 147 cases. Eur J Intern Med 2006;17:24–7 [DOI] [PubMed] [Google Scholar]
- 23.Downey DJ, Omer GE, Moncim MS. New Mexico rattlesnake bites; demographic review and guidelines for treatment. J Trauma 1991;31:1380–6 [PubMed] [Google Scholar]
- 24.Agarwal R, Singh AP, Agarwal AN. Pulmonary oedema complicating snake bite due to Bungarus caeruleus. Singapore Med J 2007;48:e277–30 [PubMed] [Google Scholar]
- 25.Maheshwari M, Mittal SR. Acute myocardial infarction complicating snake bite. JAPI 2004;52:62–4 [PubMed] [Google Scholar]
- 26.Thewjitcharoen Y, Poopitaya S. Ventricular tachycardia, a rare manifestation of Russell's viper bite :case report. J Med Assoc Thai 2005;88:1931–3 [PubMed] [Google Scholar]
- 27.Suchitra N, Pappachan JM, Sujathan S. Snake bite envenomation in Kerala, South India, a clinical profile and factors involved in adverse outcomes. Emerg Med J 2008;25:200–4 [DOI] [PubMed] [Google Scholar]
- 28.Francis J. Ventricular tachycardia following snakebite envenomation. Emerg Med J 2009;26:151. [PubMed] [Google Scholar]
- 29.Nayak KC, Jain AK, Sharda DP, et al. Profile of cardiac complications of snake bite. Indian Heart J 1990;42:185–8 [PubMed] [Google Scholar]
- 30.Niraj M, Jayaweera JL, Kumar IWGD, et al. Acute myocardial infarction following a Russell's viper bite: a case report. Int Arch Med 2013;6:7. http://www.intarchmed.com/content/6/1/7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menon JC, Joseph JK, Madhukar M, et al. Double trouble-a case report of myocardial infarction complicating hemotoxic snake bite. JAPI 2000;48: 1109–1011310393 [Google Scholar]
- 32.Al Durihim H, Al-Hussaini M, Bin Salih S, et al. Snake bite envenomation: experience at King Abdul Aziz medical city, Riyadh. East Mediterr Health J 2010;16: 438–41 [PubMed] [Google Scholar]
- 33.Gaballa M, Taher T, Brodin LA, et al. Myocardial infarction as a rare consequence of snake bite. Diagnosis with novel echocardiographic tissue Doppler techniques. Circulation 2005;112:e140–2 [DOI] [PubMed] [Google Scholar]
- 34.Satish R, Kanchan R, Yashawant R, et al. Acute MI in a stented patient–possibility of stent thrombosis–a case report. Indian Heart J 2013;65:327–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlson-Stiber C, Salmonson H, Perssoun H. A nationwide study of Vipera berus bites during 1 year—epidemiology and morbidity of 231 cases. Clin Toxicol 2006;44:25–30 [DOI] [PubMed] [Google Scholar]
- 36.Luksic B, Culic V, Sticevic L, et al. Infant death after nose-horned viper (Vipera ammodytes) bite in croatia: a case report. Toxicon 2010;56:1506–9 [DOI] [PubMed] [Google Scholar]
- 37.Onyiriuka AN. Snake bite poisoning in childhood: approach to diagnosis and management. In Paediatrics Today 2012;8:11–21. http://www.paediatricstoday.com [Google Scholar]
- 38.Wickramaratna JC, Fry BG, Auilar M-I, et al. Isolation and pharmacological characterization of a phospholipase A2 myotoxin from the venom of Irian Jayan death adder. (Acanthophis rugosus). Br J Pharmacol 2003;138: 333–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naidoo DP, Lockhat HS, Naiker IP. Myocardial infarction after probable black mamba envenomation. SAMJ 1987;7:1388–9 [PubMed] [Google Scholar]
- 40.Zaki OA, Kbogali A, Zak F. Black mamba venom and its fractions. Arch Pathol 1970;89:30–7 [PubMed] [Google Scholar]
- 41.Warrell DA. Snake bite. Lancet 2010;375:77–86 [DOI] [PubMed] [Google Scholar]
- 42.Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med 2002;347:347–56 [DOI] [PubMed] [Google Scholar]
- 43.Boyd JJ, Agazzi G, Svajda D, et al. Venomous snakebite in mountainous terrain: prevention and management. Wilderness Environ Med 2007;18:190–202 [DOI] [PubMed] [Google Scholar]
- 44.Patil TB. Snake bite envenomation: a neglected public health problem in India. Med J DY Patil Univ 2013;6:123–5 [Google Scholar]