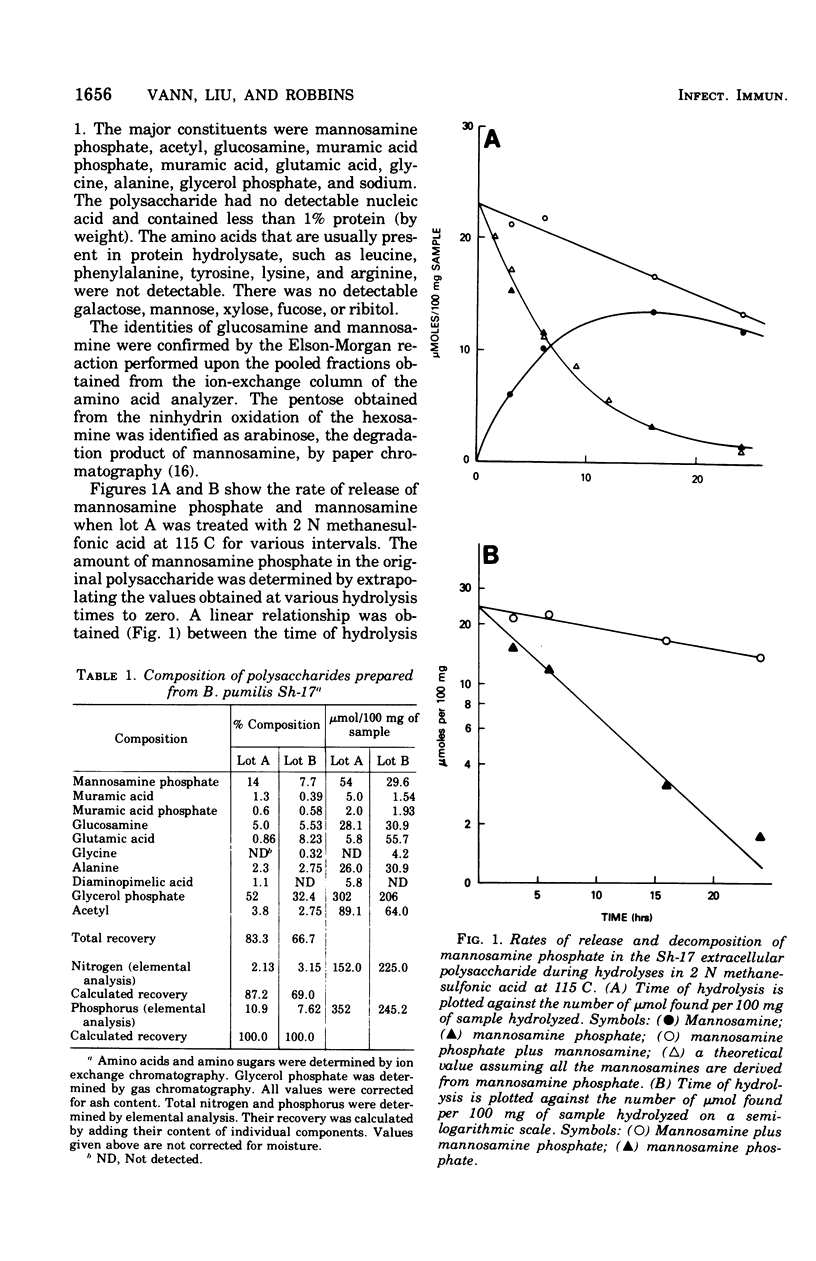
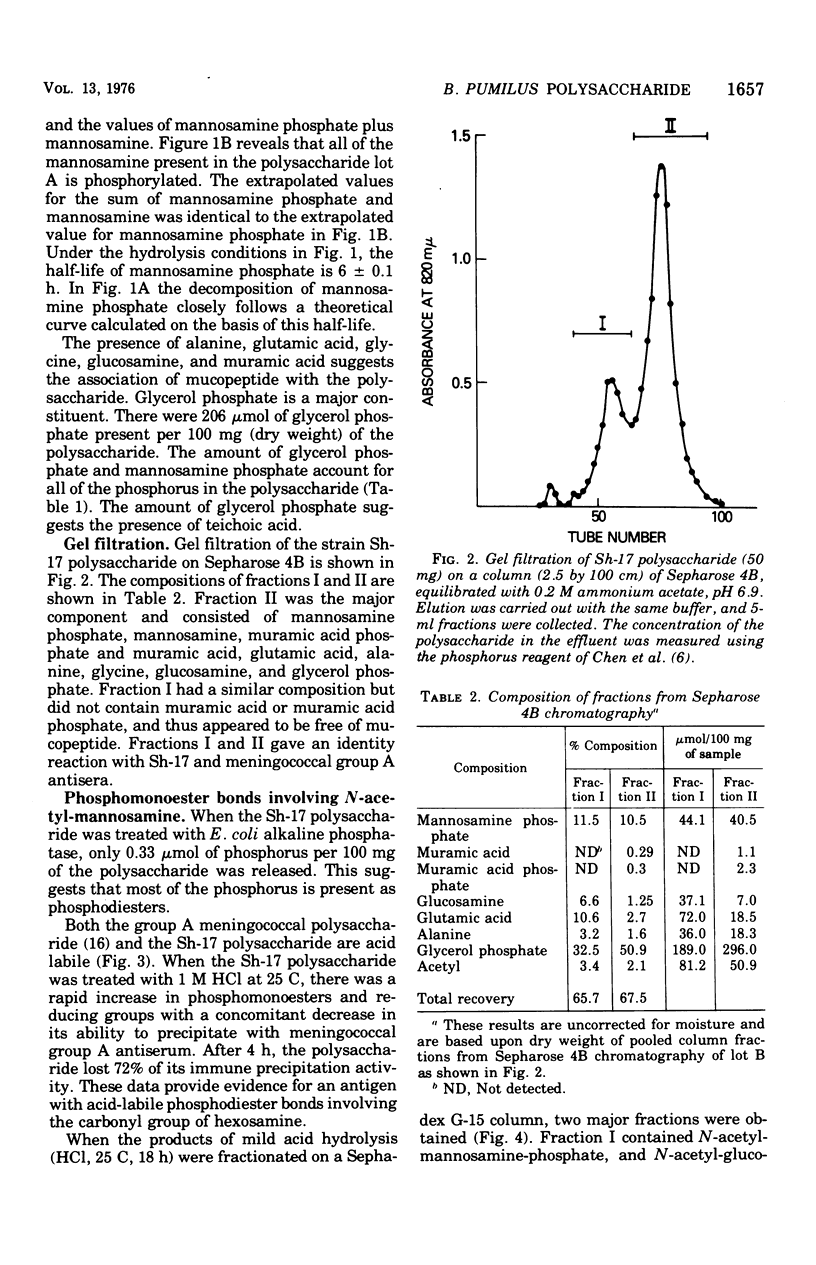
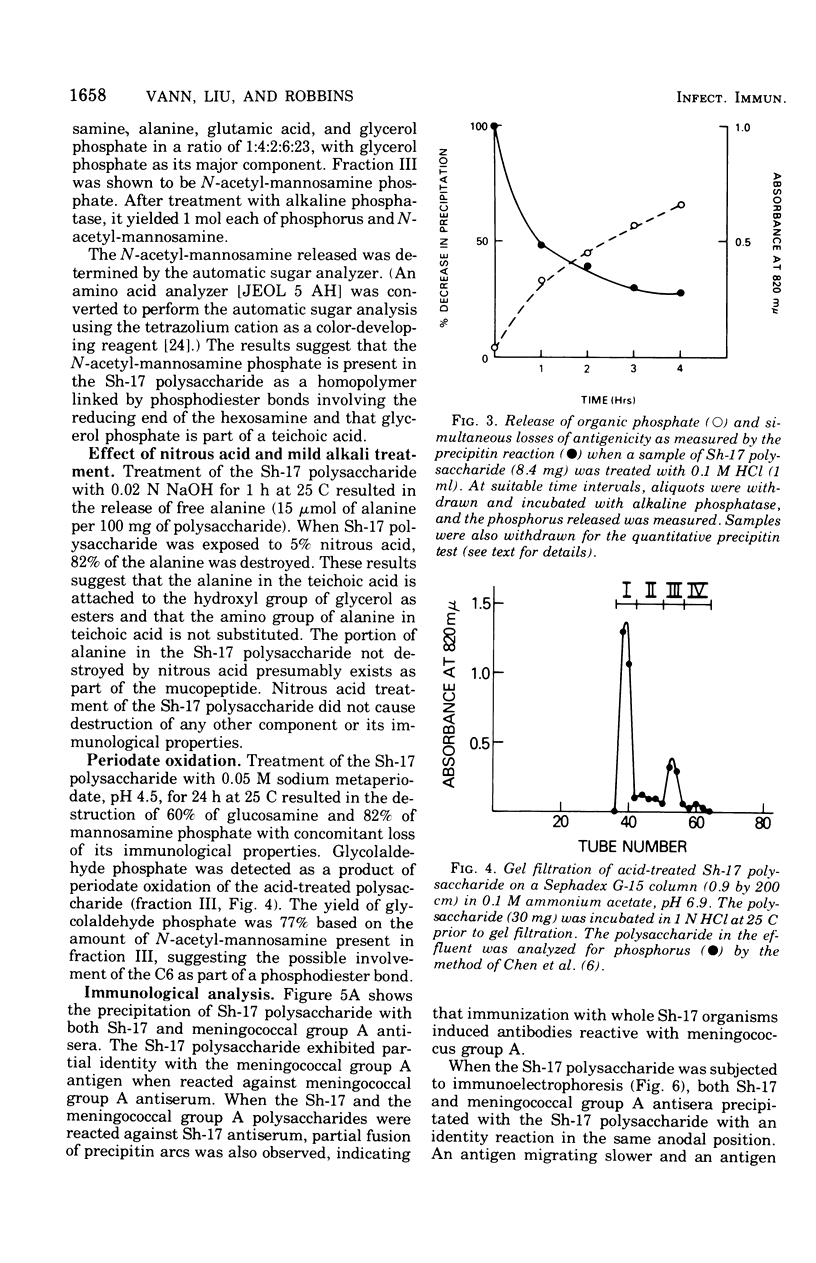
Abstract
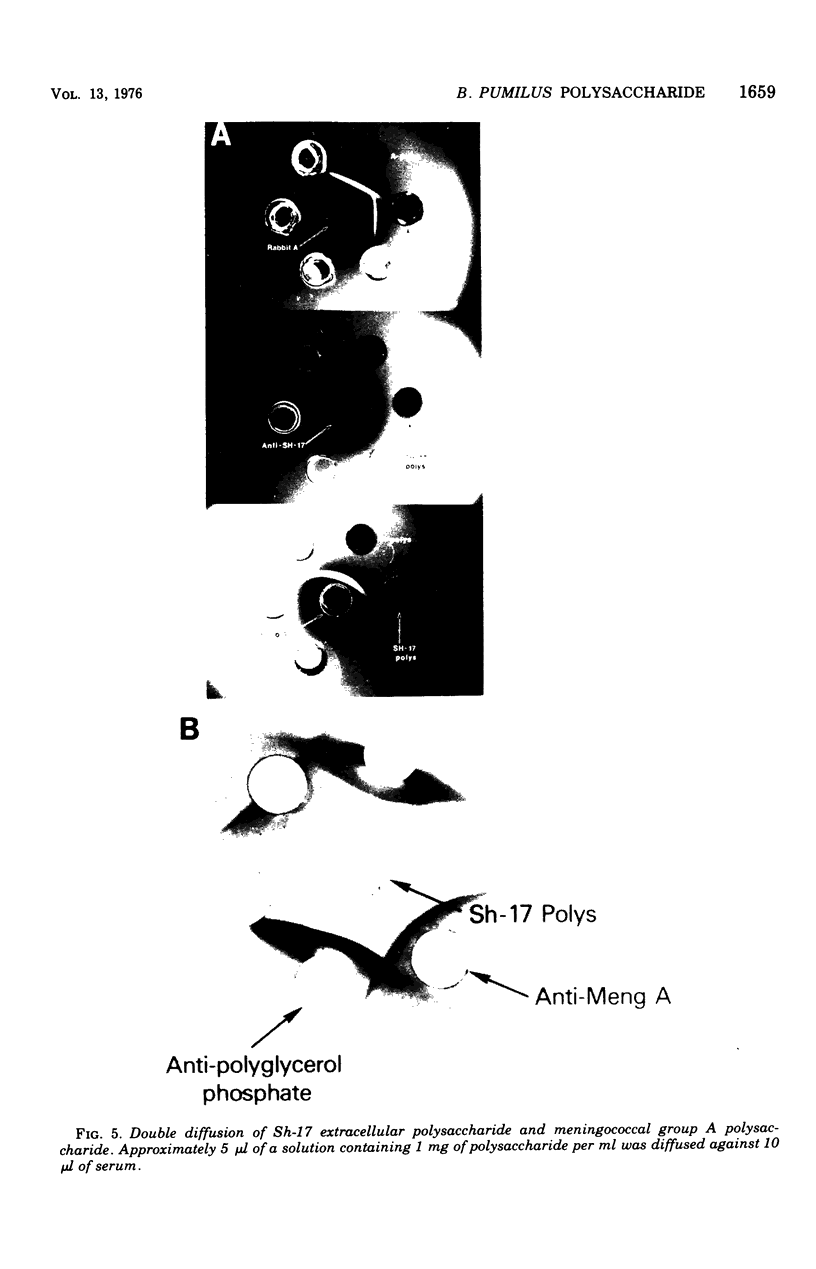
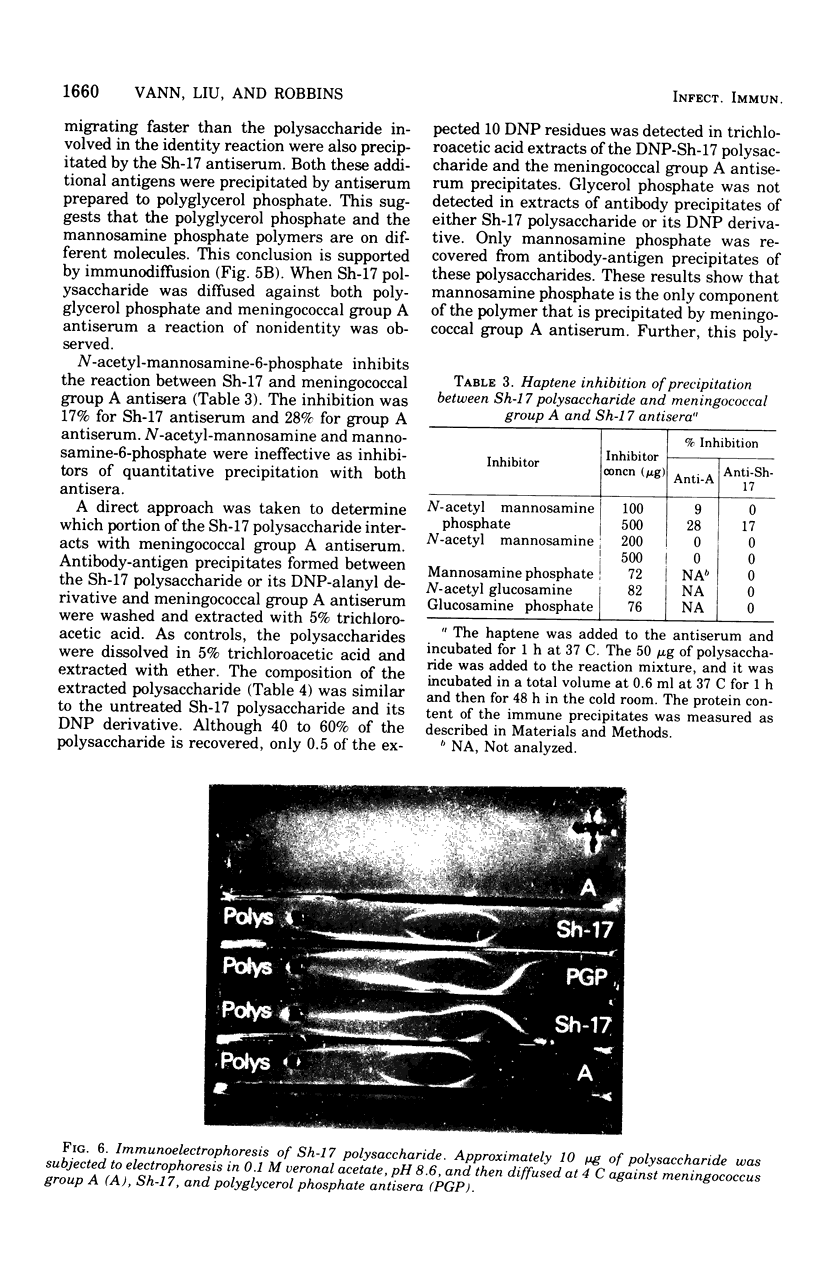
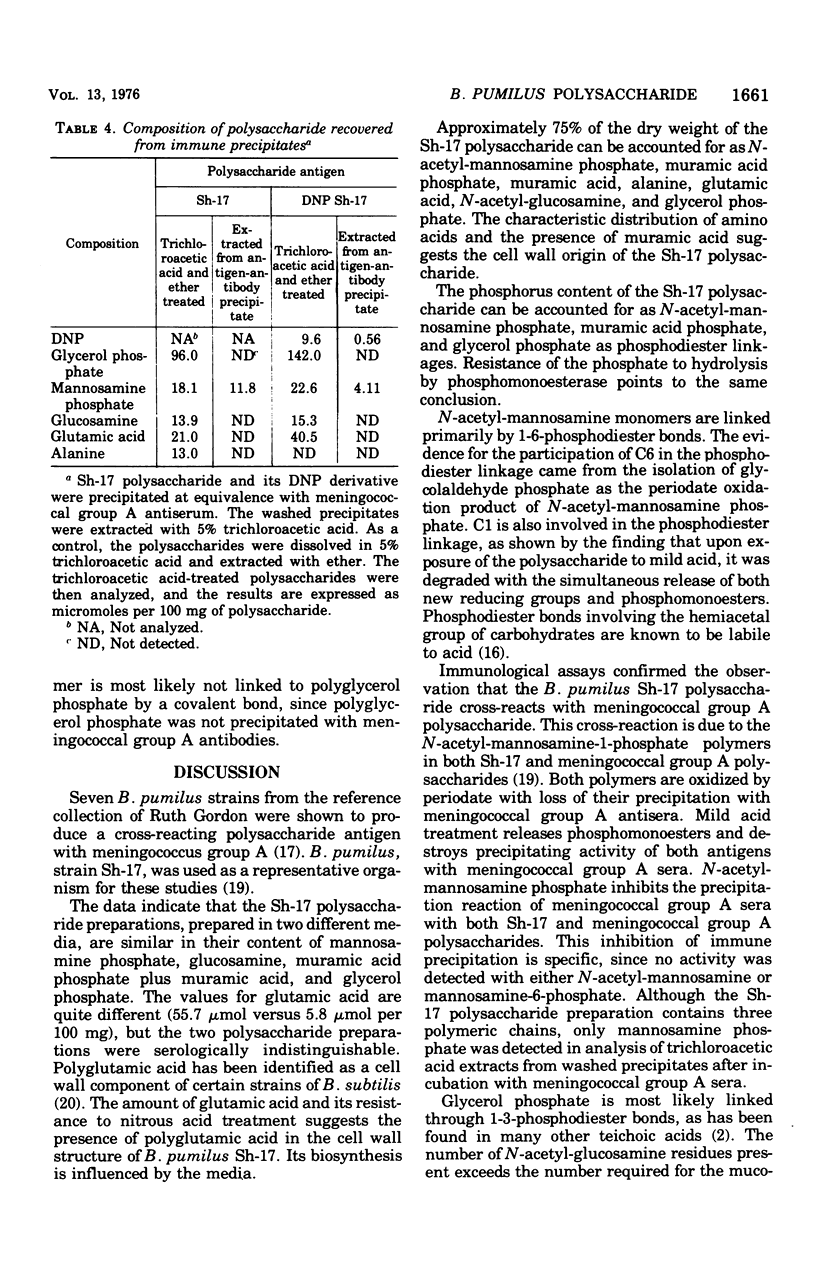
A polysaccharide, antigenically and structurally related to meningococcal group A polysaccharide, was isolated from Bacillus pumilus Sh-17. This enteric bacterium has been implicated as a source of natural meningococcal group A immunity (Myerowitz et al., 1973). The B. pumilus polysaccharide was composed of a homopolymer of (1-6)-N-acetyl-manosamine-1-phosphate, glycerol phosphate teichoic acid-containing N-acetylglucosamine and alkali-labile alanine esters, and a mucopeptide. The cross-reaction was due to the poly-(1-6)-N-acetyl-mannosamine-1-phosphate in the B. pumilus and the meningococcal group A polysaccharides, based on the following evidence. Both polysaccharides contained N-acetyl-mannosamine phosphate. Periodate oxidized the mannosamine phosphate residues of the polysaccharide and destroyed their precipitating activity with meningococcal group A antiserum. Mild acid treatment released phosphomonoesters and destroyed the meningococcal group A precipitating activity of both polysaccharides. N-acetyl-mannosamine-6-phosphate inhibited the precipitation reaction between strain Sh-17 and meningococcal group A antisera. Only mannosamine phosphate was detected in trichloroacetic acid extracts of Sh-17 polysaccharide and meningococcal group A antigen-antibody precipitates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald A. R., Baddiley J. The teichoic acids. Adv Carbohydr Chem Biochem. 1966;21:323–375. doi: 10.1016/s0096-5332(08)60320-3. [DOI] [PubMed] [Google Scholar]
- Argaman M., Liu T. Y., Robbins J. B. Polyribitol-phosphate: an antigen of four gram-positive bacteria cross-reactive with the capsular polysaccharide of haemophilus influenzae type B. J Immunol. 1974 Feb;112(2):649–655. [PubMed] [Google Scholar]
- Brundish D. E., Baddiley J. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. Biochem J. 1968 Dec;110(3):573–582. doi: 10.1042/bj1100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler J., Kaufman B., Roseman S. Enzymic synthesis of a diamino sugar nucleotide by extracts of type XIV Diplococcus pneumoniae. Arch Biochem Biophys. 1966 Sep 26;116(1):466–478. doi: 10.1016/0003-9861(66)90054-3. [DOI] [PubMed] [Google Scholar]
- Feldman H. A. Meningococcal infections. Adv Intern Med. 1972;18:117–140. [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y., Artenstein M. S. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J Exp Med. 1969 Jun 1;129(6):1349–1365. doi: 10.1084/jem.129.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYNDRICKX A. Paper chromatography of choline and the vitamins B1, B2, niacin and niacinamide; preparation of radioactive choline acetate and study of its hydrolysis. J Am Pharm Assoc Am Pharm Assoc. 1953 Nov;42(11):680–681. doi: 10.1002/jps.3030421111. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C., Jonssen E. K., Wysocki J. R. Studies on the meningococcal polysaccharides. I. Composition and chemical properties of the group A polysaccharide. J Biol Chem. 1971 May 10;246(9):2849–2858. [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C. Muramic acid phosphate as a component of the mucopeptide of Gram-positive bacteria. J Biol Chem. 1967 Feb 10;242(3):471–476. [PubMed] [Google Scholar]
- Myerowitz R. L., Gordon R. E., Robbins J. B. Polysaccharides of the genus Bacillus cross-reactive with the capsular polysaccharides of Diplococcus pneumoniae type 3, Haemophilus influenzae type b, and Neisseria meningitidis group A. Infect Immun. 1973 Dec;8(6):896–900. doi: 10.1128/iai.8.6.896-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Robbins J. B., Myerowitz L., Whisnant J. K., Argaman M., Schneerson R., Handzel Z. T., Gotschlich E. C. Enteric bacteria cross-reactive with Neisseria meningitidis groups A and C and Diplococcus pneumoniae types I and 3. Infect Immun. 1972 Nov;6(5):651–656. doi: 10.1128/iai.6.5.651-656.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARON N., JEANLOZ R. W. The diaminohexose component of a polysaccharide isolated from Bacillus subtilis. J Biol Chem. 1960 Jan;235:1–5. [PubMed] [Google Scholar]
- TORII M., KABAT E. A., BEZER A. E. SEPARATION OF TEICHOIC ACID OF STAPHYLOCOCCUS AUREUS INTO TWO IMMUNOLOGICALLY DISTINCT SPECIFIC POLYSACCHARIDES WITH ALPHA- AND BETA-N-ACETYLGLUCOSAMINYL LINKAGES RESPECTIVELY. ANTIGENICITY OF THEICHOIC ACIDS IN MAN. J Exp Med. 1964 Jul 1;120:13–29. doi: 10.1084/jem.120.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Choline in the cell wall of a bacterium: novel type of polymer-linked choline in Pneumococcus. Science. 1967 Aug 11;157(3789):694–697. doi: 10.1126/science.157.3789.694. [DOI] [PubMed] [Google Scholar]