Abstract
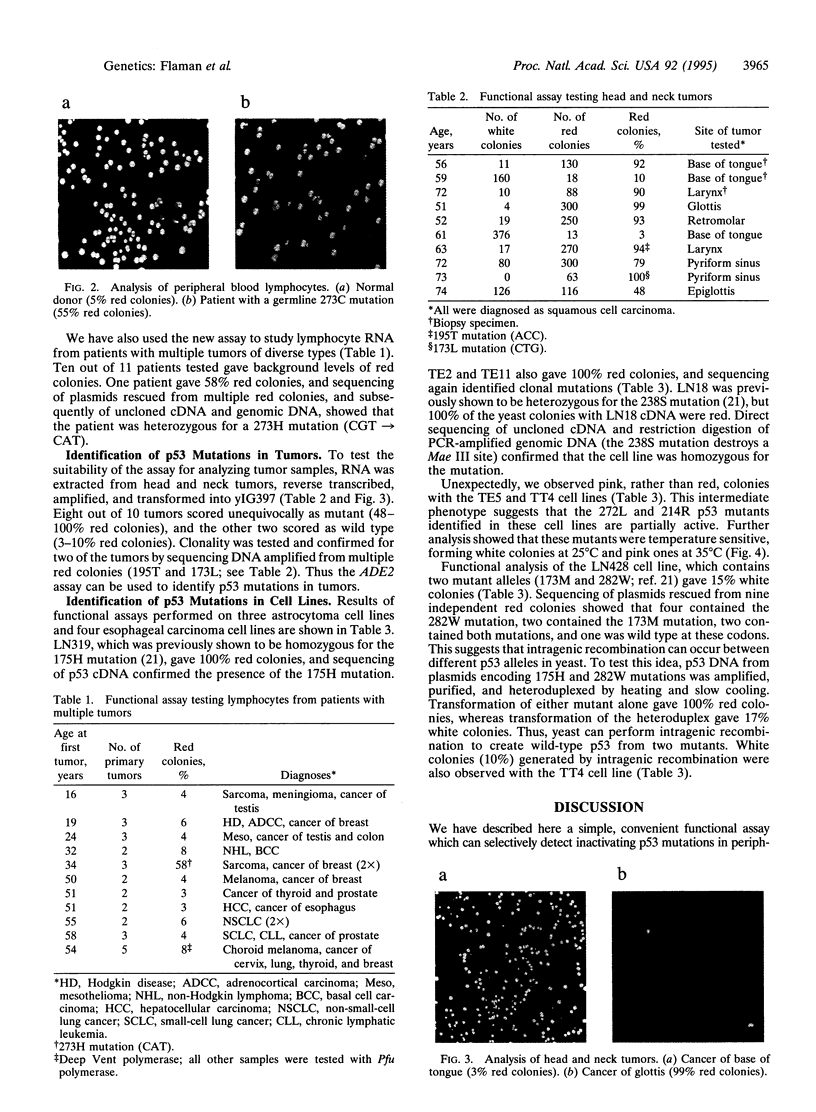
Mutations in the p53 gene are implicated in the pathogenesis of half of all human tumors. We have developed a simple functional assay for p53 mutation in which human p53 expressed in Saccharomyces cerevisiae activates transcription of the ADE2 gene. Consequently, yeast colonies containing wild-type p53 are white and colonies containing mutant p53 are red. Since this assay tests the critical biological function of p53, it can distinguish inactivating mutations from functionally silent mutations. By combining this approach with gap repair techniques in which unpurified p53 reverse transcription-PCR products are cloned by homologous recombination in vivo it is possible to screen large numbers of samples and multiple clones per sample for biologically important mutations. This means that mutations can be detected in tumor specimens contaminated with large amounts of normal tissue. In addition, the assay detects temperature-sensitive mutants, which give pink colonies. We show here that this form of p53 functional assay can be used rapidly to detect germline mutations in blood samples, somatic mutations in tumors, and mutations in cell lines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballester R., Marchuk D., Boguski M., Saulino A., Letcher R., Wigler M., Collins F. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell. 1990 Nov 16;63(4):851–859. doi: 10.1016/0092-8674(90)90151-4. [DOI] [PubMed] [Google Scholar]
- Flaman J. M., Frebourg T., Moreau V., Charbonnier F., Martin C., Ishioka C., Friend S. H., Iggo R. A rapid PCR fidelity assay. Nucleic Acids Res. 1994 Aug 11;22(15):3259–3260. doi: 10.1093/nar/22.15.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frebourg T., Barbier N., Kassel J., Ng Y. S., Romero P., Friend S. H. A functional screen for germ line p53 mutations based on transcriptional activation. Cancer Res. 1992 Dec 15;52(24):6976–6978. [PubMed] [Google Scholar]
- Fridovich-Keil J. L., Jinks-Robertson S. A yeast expression system for human galactose-1-phosphate uridylyltransferase. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):398–402. doi: 10.1073/pnas.90.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend S. p53: a glimpse at the puppet behind the shadow play. Science. 1994 Jul 15;265(5170):334–335. doi: 10.1126/science.8023155. [DOI] [PubMed] [Google Scholar]
- Grompe M. The rapid detection of unknown mutations in nucleic acids. Nat Genet. 1993 Oct;5(2):111–117. doi: 10.1038/ng1093-111. [DOI] [PubMed] [Google Scholar]
- Ishioka C., Frebourg T., Yan Y. X., Vidal M., Friend S. H., Schmidt S., Iggo R. Screening patients for heterozygous p53 mutations using a functional assay in yeast. Nat Genet. 1993 Oct;5(2):124–129. doi: 10.1038/ng1093-124. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Kinzler K. W., Bruskin A., Jarosz D., Friedman P., Prives C., Vogelstein B. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991 Jun 21;252(5013):1708–1711. doi: 10.1126/science.2047879. [DOI] [PubMed] [Google Scholar]
- Kruger W. D., Cox D. R. A yeast system for expression of human cystathionine beta-synthase: structural and functional conservation of the human and yeast genes. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6614–6618. doi: 10.1073/pnas.91.14.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyritsis A. P., Bondy M. L., Xiao M., Berman E. L., Cunningham J. E., Lee P. S., Levin V. A., Saya H. Germline p53 gene mutations in subsets of glioma patients. J Natl Cancer Inst. 1994 Mar 2;86(5):344–349. doi: 10.1093/jnci/86.5.344. [DOI] [PubMed] [Google Scholar]
- Lane D. P. On the expression of the p53 protein in human cancer. Mol Biol Rep. 1994 Jan;19(1):23–29. doi: 10.1007/BF00987319. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Bodis S., McClatchey A., Remington L., Ruley H. E., Fisher D. E., Housman D. E., Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994 Nov 4;266(5186):807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- Malkin D., Jolly K. W., Barbier N., Look A. T., Friend S. H., Gebhardt M. C., Andersen T. I., Børresen A. L., Li F. P., Garber J. Germline mutations of the p53 tumor-suppressor gene in children and young adults with second malignant neoplasms. N Engl J Med. 1992 May 14;326(20):1309–1315. doi: 10.1056/NEJM199205143262002. [DOI] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Muhlrad D., Parker R. Premature translational termination triggers mRNA decapping. Nature. 1994 Aug 18;370(6490):578–581. doi: 10.1038/370578a0. [DOI] [PubMed] [Google Scholar]
- Pavletich N. P., Chambers K. A., Pabo C. O. The DNA-binding domain of p53 contains the four conserved regions and the major mutation hot spots. Genes Dev. 1993 Dec;7(12B):2556–2564. doi: 10.1101/gad.7.12b.2556. [DOI] [PubMed] [Google Scholar]
- Pietenpol J. A., Tokino T., Thiagalingam S., el-Deiry W. S., Kinzler K. W., Vogelstein B. Sequence-specific transcriptional activation is essential for growth suppression by p53. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):1998–2002. doi: 10.1073/pnas.91.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S. M., Petersen G. M., Krush A. J., Booker S., Jen J., Giardiello F. M., Hamilton S. R., Vogelstein B., Kinzler K. W. Molecular diagnosis of familial adenomatous polyposis. N Engl J Med. 1993 Dec 30;329(27):1982–1987. doi: 10.1056/NEJM199312303292702. [DOI] [PubMed] [Google Scholar]
- Sameshima Y., Tsunematsu Y., Watanabe S., Tsukamoto T., Kawa-ha K., Hirata Y., Mizoguchi H., Sugimura T., Terada M., Yokota J. Detection of novel germ-line p53 mutations in diverse-cancer-prone families identified by selecting patients with childhood adrenocortical carcinoma. J Natl Cancer Inst. 1992 May 6;84(9):703–707. doi: 10.1093/jnci/84.9.703. [DOI] [PubMed] [Google Scholar]
- Schärer E., Iggo R. Mammalian p53 can function as a transcription factor in yeast. Nucleic Acids Res. 1992 Apr 11;20(7):1539–1545. doi: 10.1093/nar/20.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerra A. Phosphorothioate primers improve the amplification of DNA sequences by DNA polymerases with proofreading activity. Nucleic Acids Res. 1992 Jul 25;20(14):3551–3554. doi: 10.1093/nar/20.14.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Tong Y. A., Devadas K., Zou Z. Q., Sykes V. W., Chen Y., Blattner W. A., Pirollo K., Chang E. H. Detection of both mutant and wild-type p53 protein in normal skin fibroblasts and demonstration of a shared 'second hit' on p53 in diverse tumors from a cancer-prone family with Li-Fraumeni syndrome. Oncogene. 1992 May;7(5):987–991. [PubMed] [Google Scholar]
- Stotz A., Linder P. The ADE2 gene from Saccharomyces cerevisiae: sequence and new vectors. Gene. 1990 Oct 30;95(1):91–98. doi: 10.1016/0378-1119(90)90418-q. [DOI] [PubMed] [Google Scholar]
- Toguchida J., Yamaguchi T., Dayton S. H., Beauchamp R. L., Herrera G. E., Ishizaki K., Yamamuro T., Meyers P. A., Little J. B., Sasaki M. S. Prevalence and spectrum of germline mutations of the p53 gene among patients with sarcoma. N Engl J Med. 1992 May 14;326(20):1301–1308. doi: 10.1056/NEJM199205143262001. [DOI] [PubMed] [Google Scholar]
- Van Meir E. G., Kikuchi T., Tada M., Li H., Diserens A. C., Wojcik B. E., Huang H. J., Friedmann T., de Tribolet N., Cavenee W. K. Analysis of the p53 gene and its expression in human glioblastoma cells. Cancer Res. 1994 Feb 1;54(3):649–652. [PubMed] [Google Scholar]






