Abstract
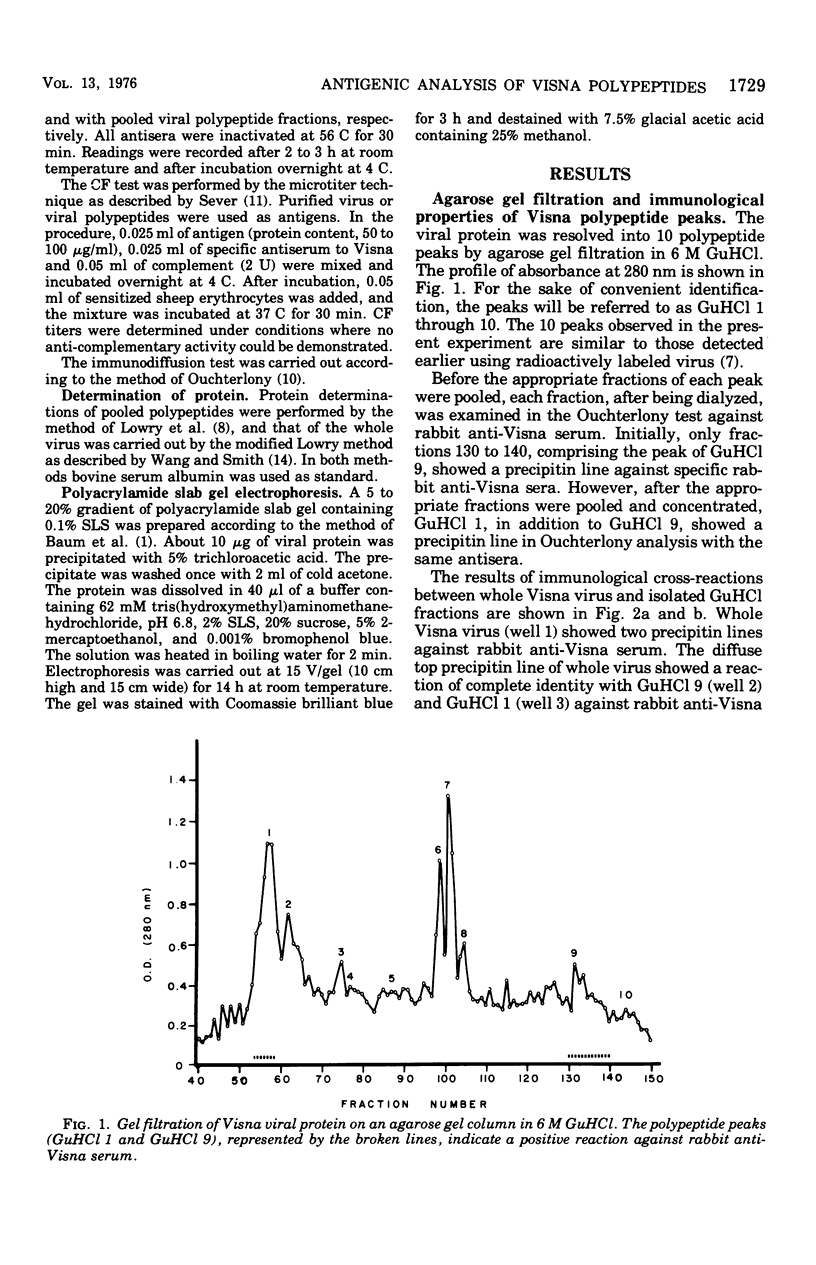
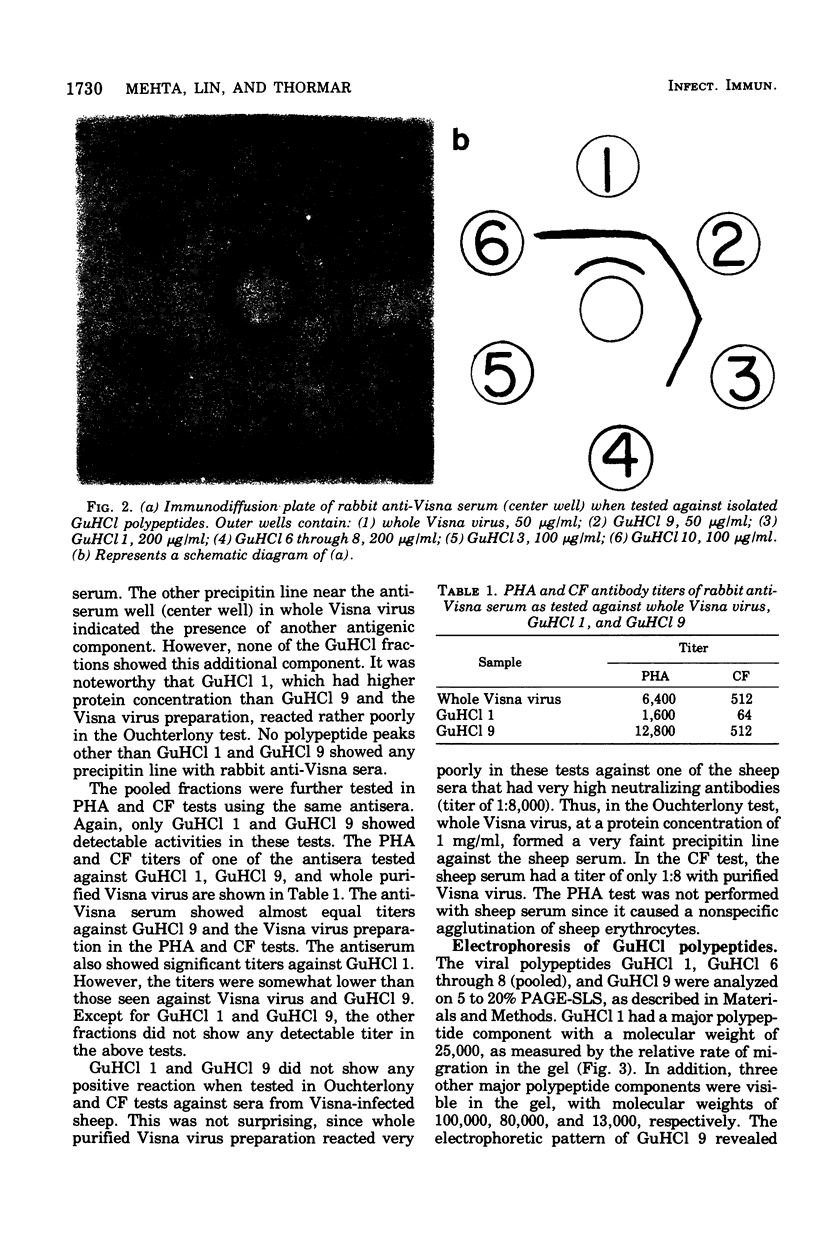
The antigenic activity of 10 Visna polypeptides separated by gel filtration in the presence of 6 M guanidine hydrochloride (GuHCl) was examined with rabbit antisera made specific for Visna virus. The results showed that the first (GuHCl 1) and the ninth (GuHCl 9) polypeptide peak reacted with the antisera when examined in immunodiffusion, passive hemagglutination, and complement fixation tests. Whole virus, GuHCl 1, and GuHCl 9, when tested with the antisera, appeared to be immunologically identical in the immunodiffusion test. However, GuHCl 1 reacted weakly with the antisera by all three techniques as compared with GuHCl 9 and whole virus. GuHCl 9, when subjected to polyacrylamide gel electrophoresis containing 0.1% sodium lauryl sulfate, revealed the presence of one polypeptide with a molecular weight of 25,000. By the same method, GuHCl 1 was found to contain an aggregate of four different polypeptides, the major one having a molecular weight of 25,000. The results indicate that the antigenic activity of both GuHCl 1 and GuHCl 9 was associated with a single polypeptide having a molecular weight of 25,000.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. I. Avian leukemia-sarcoma viruses. J Virol. 1971 Nov;8(5):778–785. doi: 10.1128/jvi.8.5.778-785.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Baringer J. R. The structural polypeptides of RNA slow viruses. Virology. 1974 Jan;57(1):238–250. doi: 10.1016/0042-6822(74)90124-x. [DOI] [PubMed] [Google Scholar]
- Karl S. C., Thormar H. Antibodies produced by rabbits immunized with visna virus. Infect Immun. 1971 Dec;4(6):715–719. doi: 10.1128/iai.4.6.715-719.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Characterization of ribonucleic acid from visna virus. J Virol. 1971 May;7(5):582–587. doi: 10.1128/jvi.7.5.582-587.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Ribonucleic acid-dependent deoxyribonucleic acid polymerase in visna virus. J Virol. 1970 Nov;6(5):702–704. doi: 10.1128/jvi.6.5.702-704.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Substructures and polypeptides of Visna virus. J Virol. 1974 Oct;14(4):782–790. doi: 10.1128/jvi.14.4.782-790.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P. D., Thormar H. Comparative studies of Visna and Maedi viruses as antigens. Infect Immun. 1975 Apr;11(4):829–834. doi: 10.1128/iai.11.4.829-834.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- SIGURDSSON B., PALSSON P., GRIMSSON H. Visna, a demyelinating transmissible disease of sheep. J Neuropathol Exp Neurol. 1957 Jul;16(3):389–403. doi: 10.1097/00005072-195707000-00010. [DOI] [PubMed] [Google Scholar]
- STAVITSKY A. B. Micromethods for the study of proteins and antibodies. I. Procedure and general applications of hemagglutination and hemagglutination-inhibition reactions with tannic acid and protein-treated red blood cells. J Immunol. 1954 May;72(5):360–367. [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]




