Figure 5.
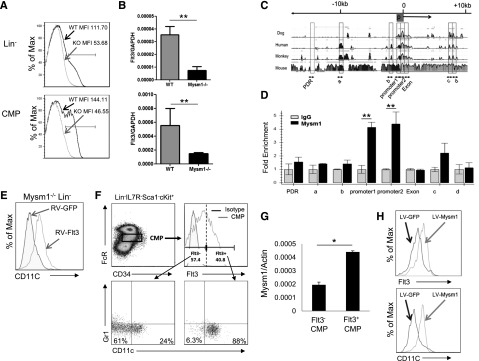
Mysm1 drives CMP toward DC lineage specification by upregulating Flt3 expression. Expression of Flt3 in Lin− BM cells and CMP population was analyzed by (A) FACS and (B) qPCR; the messenger RNA (mRNA) expression was normalized to glyceraldehyde 3-phosphate dehydrogenase messenger RNA amount. (C) Sequence alignment of dog, human, monkey, and mouse Flt3 genes; transcription start site (0); and promoter site (P). (D) ChIP assay analyzed recruitment of Mysm1 to Flt3 gene locus in WT Lin− BM cells. The prepared chromatin was immunoprecipitated with either an anti-Mysm1 antibody or control IgG, and pulled-down DNA was eluted and subjected to qPCR using the primers designed from conserved region (boxed) among species. (E) Lin− cells were sorted from Mysm1−/− BM and transduced with retrovirus-expressing GFP (RV-GFP) or Flt3 (RV-Flt3) for 12 hours. Cells were cultured in DC culture media supplemented with 100 ng/mL of Flt3L (at a density of 1 × 105 cells/100 uL) for 8 days. FACS was used to analyze the differentiation of CD11c+ DCs. (F-H) Mysm1 drives CMPs to DC lineage specification. CMPs from WT BM was sorted and divided into 2 populations by Flt3 expression: Flt3− CMPs and Flt3+ CMPs. The sorted cells were cultured in Flt3L-supplemented media for 8 days and surface markers CD11c and Gr1 was used to analyze DC differentiation by FACS (F), and Mysm1 levels in these 2 populations were measured by qPCR (G). Flt3− CMP population was infected with lentivirus expressing either GFP or Mysm1, and was cultured in Flt3L-supplemented media for 8 days. Surface expression of CD11c and Flt3 was analyzed by FACS (H). *P < .05; **P < .01.
