Abstract
Aggregated amyloid-β causes pathological changes in mixed cultures of neurons and astrocytes such as sporadic cytoplasmic intracellular Ca2+-signalling, increase in reactive oxygen species production and cell death. Some of the toxic effects of amyloid-β are mediated through the interaction of the peptide with α7-type nicotinic acetylcholine receptors at the cell surface. Here we demonstrated that affinity purified antibodies to synthetic fragment 173–193 of the α7-subunit of the nAChR are able to protect cells from amyloid-β induced cell death. The antibodies had no effect on the amyloid-β induced calcium signal in astrocytes. However, they significantly reduced amyloid-β induced and NADPH oxidase mediated ROS production. Modulation of the NADPH oxidase activity by either the antibodies, the receptor agonist acetylcholine or the antagonist of the α7-type nicotinic acetylcholine receptors α-bungarotoxin was vital in inhibiting both amyloid-β induced ROS production, caspase 3 cleavage as well as cell death. The uncovered details of the mechanism underlying the action of antibodies to α7-type nicotinic acetylcholine receptors gives additional insight into the involvement of this receptor in Alzheimer’s disease pathology and provides a new approach to anti-Alzheimer’s disease vaccine design.
Keywords: β-Amyloid, NADPH oxidase, Ca2+, α7-Type nicotinic acetylcholine receptors, Reactive oxygen species
1. Introduction
One of the most intensively studied hypotheses of the pathophysiology of Alzheimer’s disease (AD) is that of the mechanism underlying the toxicity of the amyloid-β (Aβ) peptides, which are known to impair neuronal activities, leading to a decline in memory and cognitive function (Hardy and Selkoe, 2002). On a molecular level, the pathology of AD is associated with increased oxidative stress (Hensley et al., 1994; Abramov and Duchen, 2005; Ma et al., 2011), which is regarded as an important factor contributing to the impaired brain metabolism and mitochondrial dysfunction in AD (Abramov et al., 2004; Abramov and Duchen, 2005; Abeti et al., 2011). Aberrant Aβ accumulation along with altered expression and function of nicotinic acetylcholine receptors (nAChRs) feature prominently in the etiology of AD (Court et al., 2001). Since the discovery that Aβ is bound to α7 nAChRs in many experimental settings, including post-mortem AD brain, much effort has been exerted to understand the implications of this interaction in the disease milieu (Wang et al., 2000; Lilja et al., 2011).
Previous studies have shown that Aβ binds to nAChRs and activates signaling cascades that result in the disruption of synaptic functions. It has also been suggested that activation of pathological calcium signaling can be due to binding of Aβ to the nAChRs (Fayuk and Yakel, 2005). However, Aβ is known to induce a calcium signal by other mechanisms, including pore formation in the plasma membrane (Arispe et al., 1993; Abramov et al., 2003; Demuro et al., 2011).
Importantly, stimulation of nAChRs is protective against Aβ-induced neurotoxicity (Moon et al., 2008), as is application of antibodies against nAChRs (Kamynina et al., 2010). Furthermore, vaccination with only the α7-subunit fragment 173–193 was shown to rescue spatial memory, restore the level of α7 nAChR in the cortex, and prevent an increase in the Aβ level in brain tissue in mice with experimentally induced AD.
The cellular mechanism of protection of neurons against Aβ-induced cell toxicity by the activation of nAChRs or using antibodies against the α7-subunit of nAChRs remains unclear. In the present work we have therefore investigated the connection between activation of nAChRs, or application of antibodies to α7 nAChRs, and changes in [Ca2+]c and reactive oxygen species production in the neurotoxicity of Aβ.
2. Material and Methods
2.1. Cell culture
Mixed cultures of hippocampal or cortical neurons and glial cells were prepared as described previously (Abramov et al., 2003) with modifications, from Sprague-Dawley rat pups 2–4 days postpartum (UCL breeding colony). Experimental procedures were performed in full compliance with the United Kingdom Animal (Scientific Procedures) Act of 1986. Hippocampi and cortex were removed into ice-cold PBS (Ca2+, Mg2+-free, Invitrogen, Paisley, UK). The tissue was minced and trypsinised (0.25% for 5 min at 37 °C), triturated and plated on poly-d-lysine-coated coverslips and cultured in Neurobasal A medium (Invitrogen, Paisley, UK) supplemented with B-27 (Invitrogen, Paisley, UK) and 2 mM l-glutamine. Cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air, fed twice a week and maintained for a minimum of 12 days before experimental use to ensure expression of glutamate and other receptors. Neurons were easily distinguishable from glia: they appeared phase bright, had smooth rounded somata and distinct processes, and lay just above the focal plane of the glial layer. Cells were used at 12–15 days in vivo (DIV) unless otherwise stated.
2.2. Peptides and treatments
Aβ 25–35 and Aβ 1–40 (Bachem, St. Helens, UK) were dissolved at 1–5 mM in sterile ultrapure water (Milli-Q standard, Millipore, Watford, UK) and kept frozen until use. The peptides were added to cells during experimental recordings, except for the neurotoxicity measurements, where they were added 24 h before the assays of cell death (see below). Aβ 25–35 was used at concentrations of up to 50 μM and Aβ 1–40 was used at concentration of 10 μM. Acetylcholine (Sigma, Aldrich) and α-bungarotoxin (Tocris, Bioscience, UK) were dissolved in sterile ultrapure water (Milli-Q standard, Millipore, Watford, UK) at concentrations 10 mM and 100 μM, respectively, and kept frozen until use.
For collecting affinity purified antibodies specific for the α7 nAChR, peptide 173–193 173EWDLVGIPGKRSERFYECCKE193 corresponding to the sequence of the human AChR α7-subunit (Swiss-Prot Q5W554) was synthesized manually by solid-phase Fmoc-chemistry (Udenfriend et al., 1987). The homogeneity of the synthesized peptide was estimated by analytical reverse-phase HPLC chromatography on Jupiter columns 5 μ C18 300 A, 250 mm × 4.6 mm (Phenomenex, USA), amino acid analysis on Biotronik LC-3000 (Germany) and MALDI mass spectrometry on a VISION 2000 instrument (Bioanalysis, UK). The synthetic peptide was >98% homogeneous when analyzed by these methods.
For ELISA assays the N-terminal extracellular domain of the human α7-subunit nAChR (Sigma, Aldrich) and Aβ 1–42 (Sigma, Aldrich) were dissolved at concentration 5 mg/ml in sterile ultrapure water and kept frozen until use.
2.3. Rabbit blood sera collection for affinity purification of antibodies
To obtain sera with antibodies specific to peptide 173–193 rabbits were double immunized subcutaneously with 1 mg of the peptide in saline solution mixed with equal volume of an adjuvant to obtain emulsion. The first immunization was in Freund’s complete adjuvant, the second immunization was on the 45th day in Freund’s incomplete adjuvant. Blood sera samples were taken from the rabbit ear vessels on the 55th day of the experiment. Sera were prepared from each blood sample and stored at −20 °C until use.
2.4. Purification of monospecific polyclonal antibodies against peptide 173–193 of the α7 nAChR using affinity chromatography
2.4.1. Preparation of affinity adsorbent
One gram of CNBr-activated Sepharose 4B (GE health care, Sweden) was suspended in 3 ml 1 mM NaCl. The adsorbent was washed for 15 min with 1 mM NaCl on a sintered glass filter. Then 1 mg peptide 173–193 was dissolved in 1 ml coupling buffer, 0.1 M NaHCO3 pH 8.3 containing 0.5 M NaCl. The coupling solution was mixed with the prepared adsorbent. The mixture was gently rotated for 1 h at room temperature. Not bound ligand was washed away with 5 medium volumes of coupling buffer. Remaining active groups were blocked in blocking buffer, 0.2 M Gly, 0.1 M NaCl, pH 8.0 for 2 h at room temperature. The adsorbent was then washed with three cycles of 5 medium volumes of buffers with alternating pH: 0.1 M acetic acid/sodium acetate, pH 4.0 containing 0.5 M NaCl followed by a wash with borate buffer, pH 8.0: 0.1 M Na2B4O7 containing 0.1 M NaCl. 0.05% NaN3 was added to the adsorbent and it was stored at 4 °C until use. 1 ml Sepharose conjugated with peptide 173–193 contains 0.4 mg of peptide 173–193 according to amino acid analysis data.
2.4.2. Affinity chromatography
An adsorbent column was prepared by pouring 5 ml of the Sepharose conjugated with peptide 173–193 into a column and settled. The column was equilibrated with 50 ml of PBS, pH 7.4, 10 mM Na2HPO4, 150 mM NaCl. 2 ml of rabbit sera against peptide 173–193 was applied into the column for 1 h. The column was washed with 20 mL of PBS. Elution of affinity antibody against peptide 173–193 was done with 100 mM glycine–HCl, pH 2.2, and the eluate was collected in 1.8 ml fractions in 2 ml Eppendorf tubes containing 50–100 μl (one-tenth volume of glycine) of 1.5 M Tris–HCl, pH 8.7. The affinity purified rabbit antibodies against fragment 173–193 of the α7-subunit nAChRs (AChRabs) were dialyzed against PBS, pH 7.4 for 24 hrs at +4 °C and stored at −20 °C until use. The protein concentration of the samples was determined by UV absorbance (280 nm) and calculated in accordance with c (mg/ml) = D/1.4, D – optical density. Final concentration of AChRabs was 0.279 mg/ml. AChRabs were used in the experiments at the final concentration 13 μg/ml (50 μl/ml).
2.5. Enzyme-linked immunosorbent assay (ELISA)
Rabbit blood sera or affinity purified antibodies were pooled for analysis by ELISA as described in (Udenfriend et al., 1987). Shortly, wells of a 96-well plate Maxisorp (Nunc, Denmark) were coated with 20 μg/ml of either peptide 173–193 or N-terminal extracellular domain of the human α7-subunit nAChR or and Aβ 1–42, incubated with 100 μl prediluted sera or affinity purified antibodies starting from dilution 1:40 or 1:1000, followed by addition of peroxidase-conjugated goat antibody to rabbit IgG (Sigma, USA). Antibody titers of sera were quantified by an end-point dilution with OD >0.1 which three times exceeded the binding with ChromPure rabbit IgG (Johnson ImmunoResearch laboratories, USA).
2.6. Measurements of [Ca2+]c and ROS
For measurements of [Ca2+]c cells were loaded for 30 min at room temperature with 5 μM fura-2 AM and 0.005% pluronic acid in a HEPES-buffered salt solution (HBSS) composed of 156 mM NaCl, 3 mM KCl, 2 mM MgSO4, 1.25 mM KH2PO4, 2 mM CaCl2, 10 mM glucose and 10 mM HEPES; pH adjusted to 7.35 with NaOH.
For measurement of ROS production dihydroethidium (HEt – 2 μM) was present in the solution during the experiment. No pre-incubation (‘loading’) was used for HEt to limit the intracellular accumulation of oxidized products.
To investigate an effect of the antibodies or α-bungarotoxin on Aβ induced Ca2+-signal and on ROS production cells were pre-incubated with 50 μl/ml AChRabs or with 0.5 μM α-bungarotoxin for 20 min in HBSS.
Fluorescence measurements were obtained on an epifluorescence inverted microscope equipped with a 20× (0.5 numerical aperture) fluorite objective. [Ca2+]c was monitored in single cells using excitation light provided by a Xenon arc lamp, the beam passing through a monochromator centred sequentially at 340 (fura-2:Ca2+ wavelength) and 380 (free fura-2 fluorescence) nm (Cairn Research, Kent, UK). Emitted fluorescence light was reflected through a 515 nm long-pass filter to a cooled CCD camera (Retiga, QImaging, Canada). All imaging data were collected and analysed using software from Andor (Belfast, UK). The fura-2 data has not been calibrated in terms of [Ca2+]c because of uncertainty arising from the use of different calibration techniques. For HEt measurements the ratio: 543 nm excitation and 560 nm longpass filter were used for oxidased HEt and excitation 355 nm and measurement at 405–470 was for non-oxidased HEt. All data presented were obtained from at least 5 coverslips and 2–3 different cell preparations.
2.7. Caspase 3 activity assay
For measurements of caspase 3 activation cells were loaded for 15 min at room temperature with 10 μM NucView 488 caspase 3 substrate (Biotium, USA) in HBSS. NucView 488 is a novel class of enzyme substrates for real-time detection of caspase-3 activity in live cells. The substrate can rapidly cross cell membrane to enter the cell cytoplasm, where it is cleaved by caspase-3 to release the high-affinity DNA dye. The released DNA dye migrates to the cell nucleus to stain the nucleus brightly green. Cells were then treated with 50 μM Aβ 25–35. In experiments of measurement of caspase 3 inhibition, cells were pre-incubated for 20 min with either 0.5 μM α-bungarotoxin or with 50 μl/ml AChRabs.
Confocal images were obtained using Zeiss (Oberkochen, Germany) 710 confocal laser scanning microscope and a 40× oil immersion objective. The 488 nm argon laser was used to excite NucView 488 fluorescence, which was measured using a bandpass filter from 510 and 560 nm.
2.8. Toxicity experiments
For toxicity assays cells were loaded simultaneously with 20 μM propidium iodide (PI), which is excluded from viable cells but exhibits a red fluorescence following a loss of membrane integrity, and 4.5 μM Hoechst 33342 (Molecular Probes, Eugene, OR), which gives a blue staining to chromatin, to count the total number of cells. Using phase contrast optics, a bright field image allowed identification of neurons, which look quite different to the flatter glial component and also lie in a different focal plane, above the glial layer. A total number of 600–800 neurons or glial cells were counted in 4–5 fields of each coverslip. Each experiment was repeated five or more times using separate cultures.
To investigate an effect of the antibodies or on Aβ induced cell death, primary cultures were pre-incubated with 50 μl/ml AChRabs for 30 min in Neurobasal A medium.
3. Results
Affinity purified monospecific polyclonal antibodies against peptide 173–193 of α7 nAChR bind only to fragment 173–193 and the N-terminal extracellular domain of the human α7-subunit nAChR, but not to Aβ 1–42.
To test the specificity of the raised purified antibodies against peptide 173–193 of the α7-subunit of nAChR (AChRabs) we investigated their binding with the fragment 173–193, N-terminal extracellular domain of the human α7-subunit nAChR and Aβ 1–42 on an ELISA. The results obtained demonstrate high affinity binding of the antibodies to both the peptide 173–193 and the α7-subunit (titers were 1:64,000 and 1:32,000, respectively), rather than with Aβ 1–42 (no binding at AChRabs titers less than 1:40) (Table 1).
Table 1.
Specificity of affinity purified monospecific polyclonal antibodies against peptide 173–193 of α7 nAChRs.
| Antigen | Titers of antibodies |
|---|---|
| Peptide 173–193 | 1:64,000 |
| α7-Domain | 1:32,000 |
| Aβ 1–42 | <1:40 |
3.1. Aβ induced Ca2+-signalling is not affected by antibodies against the acetylcholine receptor
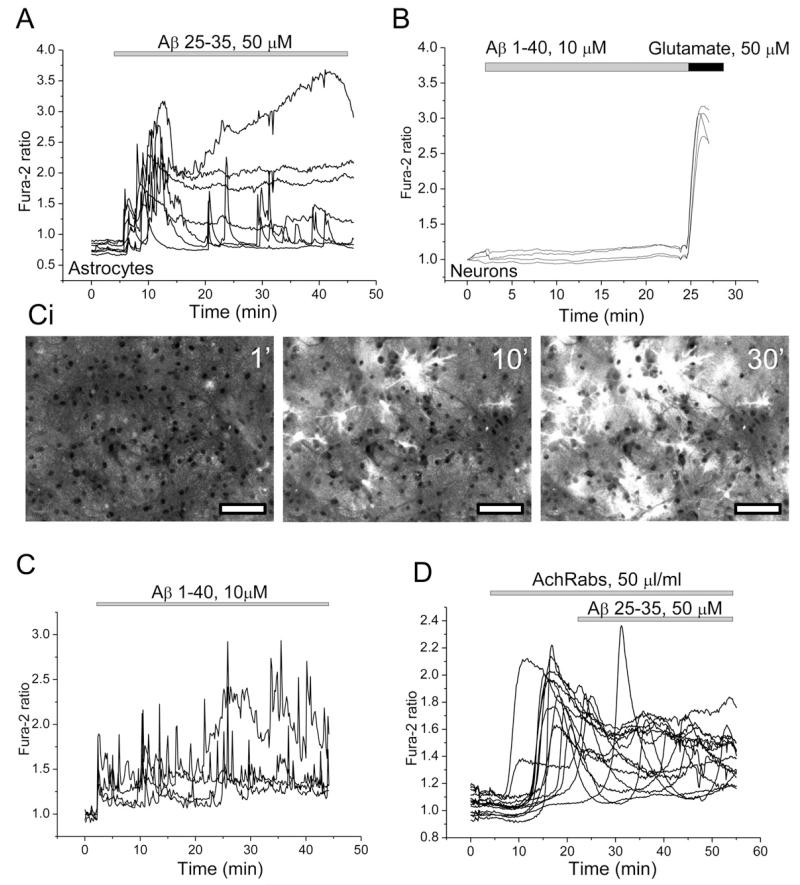
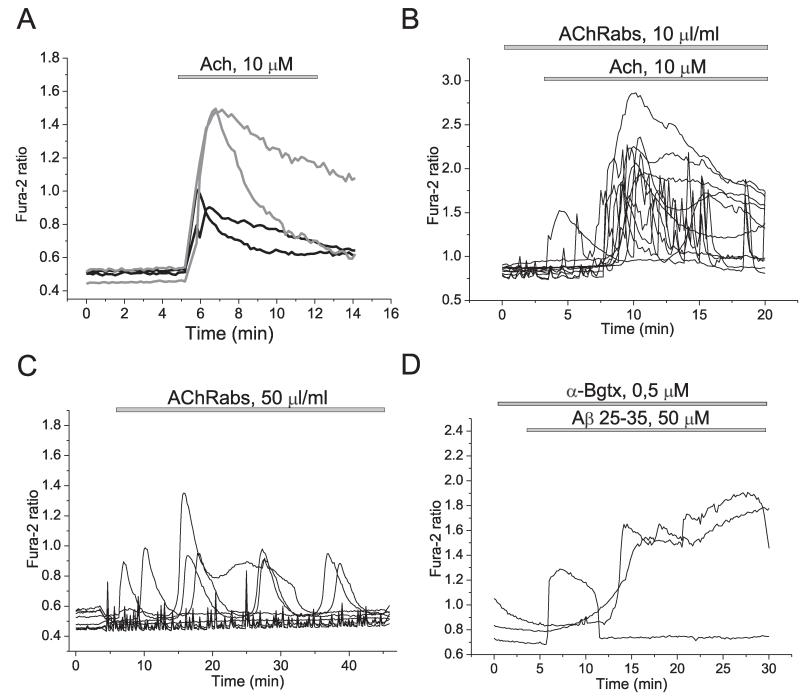
Activation of α7 nAChRs is associated with calcium signalling (Fayuk and Yakel, 2005), while the toxic action of Aβ again has been connected to elevation of [Ca2+]c. We have previously shown that application of the Aβ peptide fragment 25–35 (5–50 μM) or the full length peptide 1–40 (0.5–5 μM) to rat hippocampal neurons and astrocytes in co-culture causes sporadic increases in intracellular calcium ([Ca2+]c) of astrocytes but not in neurons (Abramov et al., 2003; Ionov et al., 2011; Abramov et al., 2011). We therefore investigated whether antibodies to fragment 173–193 of the α7-subunit of nAChR (AChRabs) can modify the Aβ-induced calcium signal. In agreement with our other previous publications Aβ25–35 (50 μM; n = 750 cells) and Aβ1–40 (10 μM, n = 557 astrocytes) induced dramatic [Ca2+]c signals in astrocytes but not in neurons (Fig. 1(A) and (B)). However, 1 h pre-incubation of the co-culture of hippocampal neurons and astrocytes with AChRabs (50 μl/ml, for 45 min) did not alter the ability of Aβ 1–40 (n = 482 cells) or 25–35 (n = 1130 cells) induce calcium signal in astrocytes and the pattern of amyloid induced [Ca2+]c traces (Fig. 1(C)). Importantly, acethylcholine (Ach) by itself induced elevation of [Ca2+]c in both neurons and astrocytes (n = 1225 cells; with a smaller signal in astrocytes – see black traces in Fig. 2(A) and the presence of the AChRabs did not significantly change the value and shape of the signal (n = 1152 cells; Fig. 2(B). It should be noted that application of AChRabs to astrocytes and neurons stimulated a calcium signal in both cell types (n = 1286 cells), suggesting that antibodies act more like an agonist rather than antagonist on the receptor (Fig. 2(C)). It has been shown that the α7-type AChR calcium channel antagonist α-bungarotoxin inhibits the Ach-induced calcium signal (Sekiguchi-Tonosaki et al., 2009). However, pre-treatment of neurons and astrocytes with α-bungarotoxin did not induce any significant changes to the Aβ-induced calcium signal in astrocytes (n = 164 cells; Fig. 2D). Thus, incubation of the neurons and astrocytes with AChRabs did not alter effect of Aβ 25–35 and 1–40 on [Ca2+]c.
Fig. 1.
Aβ raises [Ca2+]c in astrocytes, effects of the antibodies on α7-type nAChRs. (A, C and D) Shows representative recordings of fura-2 ratio from astrocytes in hippocampal co-cultures following exposure to Aβ 25–35 (A) and Aβ 1–40 (C) peptides. Ci-representative images of fura-2 ratio under exposure to 10 μM Aβ 1–40. Bars on the images are 50 μm. The neurons showed no change in signal at all under application of amyloid (B). Their identity was confirmed by their response to glutamate (50 μM) at the end of the experiment. AchRabs, 50 μl/ml did not affect the calcium response to Aβ 25–35 in astrocytes (D). Each trace indicates [Ca2+]c measurement from a single cell.
Fig. 2.
Modulation of [Ca2+]c of primary co-cultures of neurons and astrocytes. Acetylcholine induced calcium signal in neurons (gray traces) and astrocytes (black traces) (A) independently of antibodies to α7-type nAChRs (B). Antibodies induced changes in [Ca2+]c of astrocytes (C). α-bungarotoxin did not block the Aβ-induced calcium signal (D). Each trace indicates [Ca2+]c measurement from a single cell.
3.2. Modulation of the acetylcholine receptor alters Aβ induced ROS production
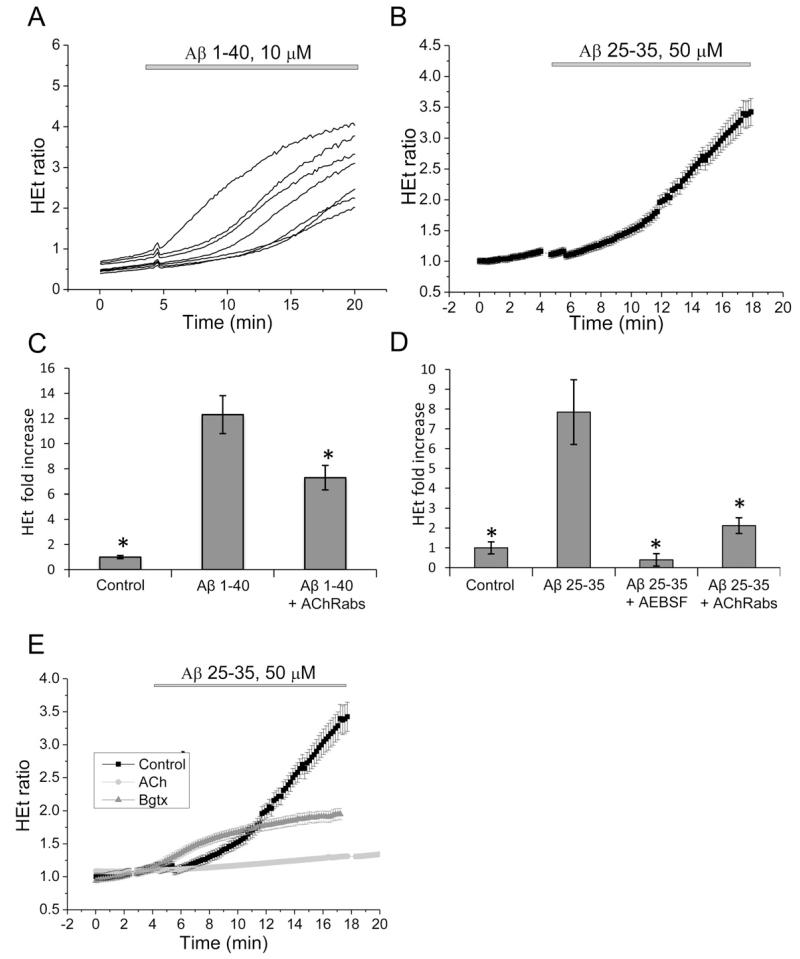
One of the most profound effects of Aβ on brain cells is the action of the peptide on production of ROS. We have previously shown that Aβ induces excessive ROS production via the NADPH oxidase, which is activated by the amyloid-induced calcium signal (Abramov et al., 2004; Abramov and Duchen, 2005). In agreement with our previous work we have found that application of the full length (1–40, 10 μM) (n = 526 cells) and the 25–35 fragment (50 μM) (n = 1208 cells) of Aβ significantly enhanced the rate of ROS production in hippocampal astrocytes (Fig. 3(A)–(D)). Pre-incubation of neurons and astrocytes with 20 μM inhibitor of NADPH oxidase, AEBSF, almost completely blocked the effect of Aβ on ROS production (n = 423 cells) (Fig. 3(D)), suggesting that most of the Aβ-induced free radicals are generated by the NADPH oxidase. Interestingly, pre-incubation of cortical co-culture of neurons and astrocytes for 20 min with 50 μl/ml AChRabs also reduced the rate of Aβ-induced ROS production (n = 2368 cells) (Fig. 3(C) and (D)). In our experiments the AChRabs had an agonistic effect on [Ca2+]c (Fig. 2), therefore we investigated whether Ach had a similar effect to the antibodies. We found that pre-incubation of cells with Ach also decreased the effect of Aβ on the rate of ROS production (n = 398 cells) (by 23%; Fig. 3(E)). Interestingly, α-bungarotoxin also effectively inhibited superoxide production (n = 210 cells) (Fig. 3(E)). This would suggest that application of antibodies modulates activity of the NADPH oxidase in astrocytes. This effect cannot be explained solely by the induction of a calcium signal because antibodies did not change Aβ-induced calcium signal.
Fig. 3.
Aβ increases generation of reactive oxygen species (ROS) in astrocytes, effect of antibodies and Ach. Addition of Aβ 1–40, 10 μM (A) Aβ 25–35, 50 μM (B) caused a clear increase in the rate of ROS generation (representing the traces from single cellos. The rate of increase of production was significantly reduced in the presence of antibodies to α7-type nAChRs or the NADPH oxidase inhibitor AEBSF (20 μM) ((C) and (D); signal is averaged), Ach and α-bungarotoxin also suppresses the effect of Aβ on the rate of ROS production (E). Histograms (C) and (D) show the fold increase in rate of HEt fluorescence as compared to the basal rate.
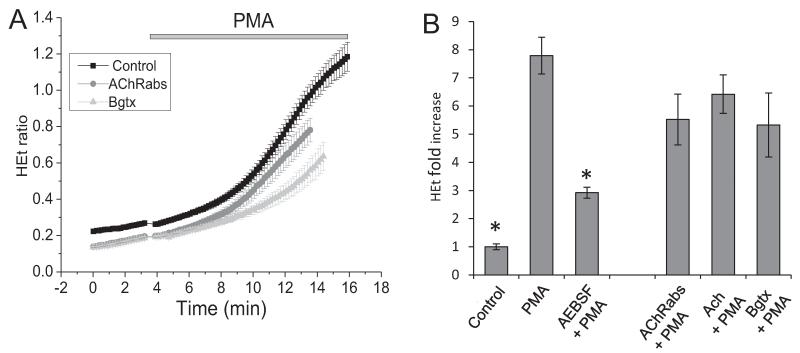
To investigate the mechanism of action of the antibodies on NADPH oxidase, we stimulated the cortical astrocytes with an activator of NOX2 (1 mg/ml phorbol 12-myristate 13-acetate, PMA) which induced a 8 -fold increase in the rate of HEt fluorescence in the cells (n = 732 cells) (Fig. 4(A)). This effect was significantly blocked in the presence of the inhibitor of NADPH oxidase AEBSF (20 μM) (n = 563 cells) (Fig. 4(B)). PMA-induced ROS production in astrocytes was also partially inhibited by AChRabs (n = 687 cells) (Fig. 4(A) and (B)). Thus, pre-incubation of the primary co-cultures with the antibodies affected NADPH oxidase activation. Again, pre-incubation of the cells with Ach significantly reduced ROS production by NADPH oxidase in cortical astrocytes (n = 421 cells) (Fig. 4(A) and (B)). Furthermore, the inhibitor of α7 nAChRs α-bungarotoxin inhibited the effect of PMA on ROS production (n = 360) (Fig. 4(A) and (B)) which suggests a role for this channel in activation of the NADPH oxidase.
Fig. 4.
Effect of acetylcholine and antibodies against the α7-type nAChRs on ROS production by NADPH oxidase. Ach, α-bungarotoxin and the antibodies suppress the effect of 1 mg/ml PMA on ROS production. The histogram (B) shows the fold increase of the rate of HEt fluorescence as compared to the basal rate.
3.3. Aβ induces caspase 3 activation
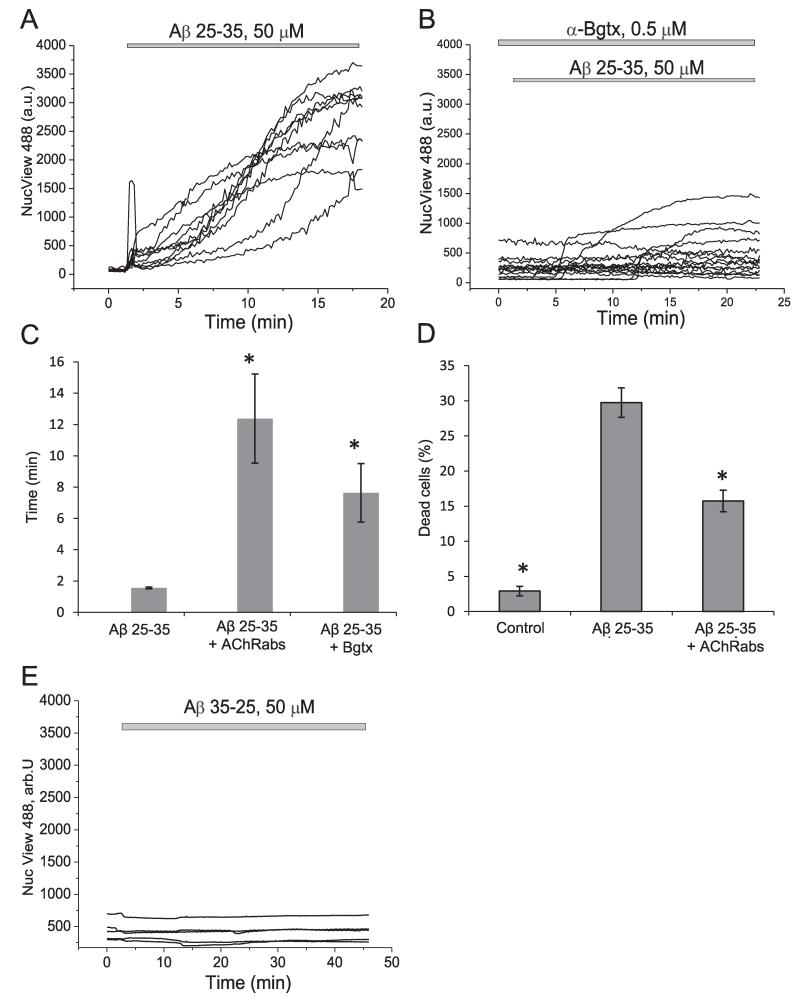
Aβ can trigger the cell death cascade by activation of caspase 3 (Harada and Sugimoto, 1999). To investigate the effect of antibodies on Aβ-induced caspase 3 activation we used NucView 488 caspase 3 substrate which allows the visualization of the activation of this enzyme in real time. Application of Aβ 25–35 induced a rapid activation of caspase 3 in neurons and astrocytes (n = 330 cells) (Fig. 5(A) and (C)). Pre-incubation of the cells with AChRabs significantly reduced the rate of appearance of caspase 3 activation and the number of cells with green nuclei (n = 295 cells) (Fig. 5(C)). Importantly, α-bungarotoxin, which is toxic by itself, protected cells significantly against Aβ-induced caspase 3 activation (n = 287 cells) (Fig. 5(B) and (C)).
Fig. 5.
Protective effect of antibodies against Aβ-induced caspase 3 activation and cell death. 50 μM Aβ 25–35 significantly activates the NucView 488 caspase 3 substrate in neurons and astrocytes. Antibodies against the α7-type nAChRs and α-bungarotoxin significantly reduce (B) and delay caspase 3 activation (C), presented as time from addition of the peptide to activation of the substrate, seen as increase of fluorescence at the nucleus. (D) Cell death in the presence of amyloid fragments, with and without treatment with antibodies. Cell death was assessed using propidium iodide (PI) to label dead cells and Hoechst to label all cells. (E) The reverse peptide (35–25, 50 μM) was used as a control for caspase 3 activation.
3.4. Antibodies against the acetylcholine receptor are protective against Aβ induced cell death
We examined the effect of a 24 h exposure of hippocampal cultures of neurons and astrocytes to Aβ 25–35 on cell viability and found that, remarkably, 30.2 ± 2.1% of cells (n = 9 experiments) died during this period (Fig. 5(D)). 20 min pre-incubation of primary co-culture with 50 μl/ml of AChRabs significantly reduced cell death of hippocampal neurons and astrocytes (from 30.2 ± 7.5% to 1575 ± 1.53% dead cells, p < 0.05, n = 9 experiments) (Fig. 5(D)).
4. Discussion
α7 nAChRs is known to be involved in AD pathology (Wang et al., 2000; Lilja et al., 2011). Preventing the receptor from binding with Aβ seems to be a promising approach for AD treatment. We have previously shown that antibodies to the α7 nAChRs improved memory conditions and other pathological features of AD in a mice model of sporadic form of AD (Kamynina et al., 2010). In this study we demonstrated that pre-incubation of primary cultures with antibodies against synthetic fragment 173–193 of α7-subunit of the AChR significantly protected neurons and astrocytes against Aβ-induced cell death. This suggests that the effect of the vaccination with the fragment on the rescue of spatial memory, and on the restoration of levels of α7 nAChRs in the cortex of mice with experimentally induced AD (Kamynina et al., 2010), is most likely explained by the protective effect of the antibodies against neuronal loss.
The protective effect of the antibodies is unlikely to be mediated through the modulation [Ca2+]c as they do not alter the Aβ-induced Ca2+-signal. In agreement with our previous data (Abramov et al., 2003; Ionov et al., 2011; Abramov et al., 2011) we found that Aβ 25–35 or 1–40 induced a Ca2+-signalling in hippocampal astrocytes but not in neurons. However, pre treatment of cells with neither antibodies to the AChR α7-subunit nor with α-bungarotoxin altered Aβ-induced Ca2+-signaling suggesting that the receptor does not play a crucial role in Aβ-induced Ca2+-signal. Aβ is known to become inserted into the plasma membrane where it acts as a pore permeable for Ca2+ (Kawahara, 2010) most likely abolishing the effect of other Ca2+-channels such as the α7 nAChR on [Ca2+]c increase after receptor activation. This may explain why we do not see any influence of the antibodies or α-bungarotoxin on Aβ-induced Ca2+-signalling.
Although neurons and glia was prepared from 2 to 3 days old rat, this cell co-culture reflects the adult-like cells because they express all receptors by the days 12–14 in vitro. Considering this, the co-culture of neurons and astrocytes isolated from postnatal rats can be used for study of Aβ-induced neurotoxicity. However, we understand that effects of α7 nAChRs on Aβ-induced cell death in neurons and astrocytes may vary in brain of old rats, considering age dependent toxicity of βA in the culture of postnatal and adult glial cell (Floden and Combs, 2006).
It is more likely that the protective action of the antibodies is mediated through modulation of NADPH oxidase ROS production in astrocytes. AD pathology is known to be associated with a selective increase of α7 on astrocytes (Teaktong et al., 2003; Xiu et al., 2005; Yu et al., 2005) In accordance with our previous publications (Abramov et al., 2004; Abramov and Duchen, 2005) we have shown that Aβ induces excessive ROS production via NADPH oxidase. Pre-incubation of the cortical co-culture of neurons and astrocytes with AChRabs significantly reduced the rate of either Aβ- or PMA-induced ROS production. Modulation of the α7 AChR decreased the rate of PMA-induced ROS production, suggesting a crucial role for this channel in activation of the NADPH oxidase. Importantly, both AChRabs and α-bungarotoxin decreased the rate of Aβ-induced caspase 3 activation, the initial step in Aβ-induced cell death.
Our experimental results have allowed us to uncover a novel pathway of Aβ toxicity involving the α7-type AChR. We suggest that Aβ interacts with the α7 AChR and thus modulates Ca2+-independent ROS production via NADPH-oxidase in glial cells. AChRabs act more like agonists of the α7 nAChRs, inducing a Ca2+-influx, which seems to prevent this interaction from inhibiting ROS production in glial cells. This results in blocking of caspase 3 activation and cell death. Moreover, agonists and modulators of the α7 nAChR are currently being developed to ameliorate cognitive deficits in diseases such as AD (Faghih et al., 2008; Haydar and Dunlop, 2010). It is therefore conceivable that Ach and specific antibodies that target α7-type nAChRs may have significant therapeutic potential in neuroinflammatory diseases in the brain.
Acknowledgements
Anna Kamynina was supported by FEBS Collaborative Experimental Scholarship for Central and Eastern Europe and RAS program FSN-2012 and RFBR grant 10-04-01256.
Abbreviations
- Aβ
amyloid β peptide
- AD
Alzheimer’s disease
- HBSS
HEPES-buffered salt solution
- ROS
reactive oxygen species
- α7 nAChRs
α7-type nicotinic acetylcholine receptors
- AchRabs
affinity purified antibodies to synthetic fragment 173–193 of the α7-subunit of the nAChR.
References
- Abeti R, Abramov AY, Duchen MR. β-Amyloid activates PARP causing astrocytic metabolic failure and neuronal death. Brain. 2011;134:1658–72. doi: 10.1093/brain/awr104. http://brain.oxfordjournals.org/content/134/6/1658.abstract. [DOI] [PubMed] [Google Scholar]
- Abramov AY, Canevari L, Duchen MR. Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. Journal of Neuroscience. 2003;23:5088–95. doi: 10.1523/JNEUROSCI.23-12-05088.2003. ISI:000183891700035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. Journal of Neuroscience. 2004;24:565–75. doi: 10.1523/JNEUROSCI.4042-03.2004. http://dx.doi.org/10.1523/JNEUROSCI.4042-03.2004. ISI: 000188098000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramov AY, Duchen MR. The role of an astrocytic NADPH oxidase in the neurotoxicity of amyloid beta peptides. Philosophical Transactions of the Royal Society B-Biological Sciences. 2005;360:2309–14. doi: 10.1098/rstb.2005.1766. http://dx.doi.org/10.1098/rstb.2005.1766. ISI:000233992100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramov AY, Ionov M, Pavlov E, Duchen MR. Membrane cholesterol content plays a key role in the neurotoxicity of beta-amyloid: implications for Alzheimer’s disease. Aging Cell. 2011;10:595–603. doi: 10.1111/j.1474-9726.2011.00685.x. http://dx.doi.org/10.1111/j.1474-9726.2011.00685.x. [DOI] [PubMed] [Google Scholar]
- Arispe N, Pollard HB, Rojas E. Giant multilevel cation channels formed by Alzheimer disease amyloid beta-protein [A beta P-(1–40)] in bilayer membranes. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10573–7. doi: 10.1073/pnas.90.22.10573. http://www.pnas.org/content/90/22/10573.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court J, Martin-Ruiz C, Piggott M, Spurden D, Griffiths M, Perry E. Nicotinic receptor abnormalities in AlzheimerΓÇÖs disease. Biological Psychiatry. 2001;49:175–84. doi: 10.1016/s0006-3223(00)01116-1. http://dx.doi.org/10.1016/S0006-3223(00)01116-1. http://www.sciencedirect.com/science/article/pii/S0006322300011161. [DOI] [PubMed] [Google Scholar]
- Demuro A, Smith M, Parker I. Single-channel Ca2+ imaging implicates Aβ1ΓÇô42 amyloid pores in AlzheimerΓÇÖs disease pathology. The Journal of Cell Biology. 2011;195:515–24. doi: 10.1083/jcb.201104133. http://jcb.rupress.org/content/195/3/515.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghih R, Gopalakrishnan M, Briggs CA. Allosteric modulators of the alpha7 nicotinic acetylcholine receptor. Journal of Medical Chemistry. 2008;51:701–12. doi: 10.1021/jm070256g. http://dx.doi.org/10.1021/jm070256g. [DOI] [PubMed] [Google Scholar]
- Fayuk D, Yakel JL. Ca2+ permeability of nicotinic acetylcholine receptors in rat hippocampal CA1 interneurones. The Journal of Physiology. 2005;566:759–68. doi: 10.1113/jphysiol.2005.089789. http://jp.physoc.org/content/566/3/759.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden AM, Combs CK. Beta-amyloid stimulates murine postnatal and adult microglia cultures in a unique manner. Journal of Neuroscience. 2006;26:4644–8. doi: 10.1523/JNEUROSCI.4822-05.2006. 10.1523/JNEUROSCI.4822-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada J, Sugimoto M. Activation of caspase-3 in β-amyloid-induced apoptosis of cultured rat cortical neurons. Brain Research. 1999;842:311–23. doi: 10.1016/s0006-8993(99)01808-9. http://dx.doi.org/10.1016/S0006-8993(99)01808-9. http://www.sciencedirect.com/science/article/pii/S0006899399018089. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. http://www.sciencemag.org/cgi/content/abstract/297/5580/353. [DOI] [PubMed] [Google Scholar]
- Haydar SN, Dunlop J. Neuronal nicotinic acetylcholine receptors - targets for the development of drugs to treat cognitive impairment associated with schizophrenia and Alzheimer’s disease. Current Topics in Medical Chemistry. 2010;10:144–52. doi: 10.2174/156802610790410983. CTMC-Abs-0012-10-2. [DOI] [PubMed] [Google Scholar]
- Hensley K, Carney JM, Mattson MP, Aksenova M, Harris M, Wu JF, et al. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:3270–4. doi: 10.1073/pnas.91.8.3270. http://www.pnas.org/content/91/8/3270.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionov M, Burchell V, Klajnert B, Bryszewska M, Abramov AY. Mechanism of neuroprotection of melatonin against beta-amyloid neurotoxicity. Neuroscience. 2011;180:229–37. doi: 10.1016/j.neuroscience.2011.02.045. 10.1016/j.neuroscience.2011.02.045. [DOI] [PubMed] [Google Scholar]
- Kamynina AV, Volpina OM, Medvinskaya NI, Aleksandrova IJ, Volkova TD, Koroev DO, et al. Vaccination with peptide 173-193 of acetylcholine receptor α7-subunit prevents memory loss in olfactory bulbectomized mice. Journal of Alzheimer’s Disease. 2010;21:249–61. doi: 10.3233/JAD-2010-091474. 10.3233/JAD-2010-091474. [DOI] [PubMed] [Google Scholar]
- Kawahara M. Neurotoxicity of beta-amyloid protein: oligomerization, channel formation, and calcium dyshomeostasis. Current Pharmaceutical Design. 2010;16:2779–89. doi: 10.2174/138161210793176545. BSP/CPD/E-Pub/000195. [DOI] [PubMed] [Google Scholar]
- Lilja AM, Porras O, Storelli E, Nordberg A, Marutle A. Functional interactions of fibrillar and oligomeric amyloid-beta with alpha7 nicotinic receptors in Alzheimer’s disease. Journal of Alzheimer’s Disease. 2011;23:335–47. doi: 10.3233/JAD-2010-101242. 10.3233/JAD-2010-101242. [DOI] [PubMed] [Google Scholar]
- Ma T, Hoeffer CA, Wong H, Massaad CA, Zhou P, Iadecola C, et al. Amyloid-β induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial superoxide. The Journal of Neuroscience. 2011;31:5589–95. doi: 10.1523/JNEUROSCI.6566-10.2011. http://www.jneurosci.org/content/31/15/5589.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JH, Kim SY, Lee HG, Kim SU, Lee YB. Activation of nicotinic acetylcholine receptor prevents the production of reactive oxygen species in fibrillar beta amyloid peptide (1–42)-stimulated microglia. Experimental and Molecular Medicine. 2008;40:11–8. doi: 10.3858/emm.2008.40.1.11. 200802292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi-Tonosaki M, Obata M, Haruki A, Himi T, Kosaka J. Acetylcholine induces Ca2+ signaling in chicken retinal pigmented epithelial cells during dedifferentiation. American Journal of Physiology. Cell Physiology. 2009;296:C1195–206. doi: 10.1152/ajpcell.00423.2008. 10.1152/ajpcell.00423.2008. [DOI] [PubMed] [Google Scholar]
- Teaktong T, Graham A, Court J, Perry R, Jaros E, Johnson M, et al. Alzheimer’s disease is associated with a selective increase in alpha7 nicotinic acetylcholine receptor immunoreactivity in astrocytes. Glia. 2003;41:207–11. doi: 10.1002/glia.10132. 10.1002/glia.10132. [DOI] [PubMed] [Google Scholar]
- Udenfriend S, Gerber L, Nelson N. Scintillation proximity assay: a sensitive and continuous isotopic method for monitoring ligand/receptor and antigen/antibody interactions. Analytical Biochemistry. 1987;161:494–500. doi: 10.1016/0003-2697(87)90479-9. [DOI] [PubMed] [Google Scholar]
- Wang HY, Lee DHS, D’Andrea MR, Peterson PA, Shank RP, Reitz AB. β-Amyloid1ΓÇô42 binds to α7 nicotinic acetylcholine receptor with high affinity. Journal of Biological Chemistry. 2000;275:5626–32. doi: 10.1074/jbc.275.8.5626. http://www.jbc.org/content/275/8/5626.abstract. [DOI] [PubMed] [Google Scholar]
- Xiu J, Nordberg A, Zhang JT, Guan ZZ. Expression of nicotinic receptors on primary cultures of rat astrocytes and up-regulation of the alpha7, alpha4 and beta2 subunits in response to nanomolar concentrations of the beta-amyloid peptide(1–42) Neurochemistry International. 2005;47:281–90. doi: 10.1016/j.neuint.2005.04.023. 10.1016/j.neuint.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Yu WF, Guan ZZ, Bogdanovic N, Nordberg A. High selective expression of alpha7 nicotinic receptors on astrocytes in the brains of patients with sporadic Alzheimer’s disease and patients carrying Swedish APP 670/671 mutation: a possible association with neuritic plaques. Experimental Neurology. 2005;192:215–25. doi: 10.1016/j.expneurol.2004.12.015. 10.1016/j.expneurol.2004.12.015. [DOI] [PubMed] [Google Scholar]