Abstract
Leucine-rich repeat kinase 2 (LRRK2) is a promising therapeutic target for some forms of Parkinson’s disease. Here we report the discovery and characterization of 2-arylmethyloxy-5-subtitutent-N-arylbenzamides with potent LRRK2 activities exemplified by GSK2578215A which exhibits biochemical IC50s of around 10 nM against both wild-type LRRK2 and the G2019S mutant. GSK2578215A exhibits exceptionally high selectivity for LRRK2 across the kinome, substantially inhibits Ser910 and Ser935 phosphorylation of both wild-type LRRK2 and G2019S mutant at a concentration of 0.3–1.0 μM in cells and in mouse spleen and kidney, but not in brain, following intraperitoneal injection of 100 mg/kg.
Keywords: LRRK2, Drug discovery, Kinase inhibitors, Parkinson’s disease
Parkinson’s disease (PD) is a debilitating neurodegenerative disease that affects over one million Americans.1,2 Recent genetic studies have revealed an underlying genetic cause in at least 10% of all PD cases,3 which provides new opportunities for discovery of molecularly targeted therapeutics that may ameliorate neurodegeneration. Among the genes associated with PD, leucine-rich repeat kinase 2 (LRRK2) is unique because of a missense mutation, G2019S, that is frequently found not only in familial but also sporadic Parkinson’s disease cases.4,5 The G2019S mutation enhances kinase activity, suggesting that small molecule LRRK2 kinase inhibitors may be able to block aberrant LRRK2-dependent signaling in Parkinson’s disease.6,7
LRRK2 kinase inhibitors are being actively pursued and recently first-generation ‘tool’ inhibitors that exhibit good potency and reasonable selectivity for LRRK2 such as LRRK2-IN-18 and CZC-251469 have been reported. However, off-target activities of these tools may confound interpretation of data in biological systems and neither compound is able to achieve good exposure in mouse brains, which limits their utility in murine PD models and eventual translation into human clinical trials.8,9 Here we report GSK2578215A as an exemplar of a 2-(benzyloxy)-5-(2-fluoropyridin-4-yl)-N-(pyridin-3-yl)benzamide series.10 GSK2578215A is a potent and highly selective LRRK2 kinase inhibitor that possesses good blood-brain barrier (BBB) permeability with a high ratio of brain to plasma distribution in mice (Fig. 1).
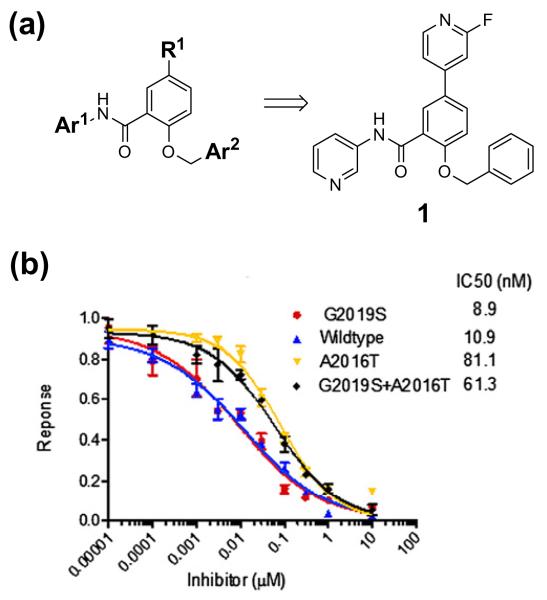
Figure 1. GSK2578215A inhibits LRRK2 in vitro.
(a) Chemical structure of GSK2578215A 1. (b) Enzyme activity of GSK2578215A. GST-LRRK2(1326–2517), GST-LRRK2[G2019S](1326–2517), GST-LRRK2[A2016T](1326–2517) and GST-LRRK2[G2019S + A2016T](1326–2517) were assayed using 20 μM Nictide in the presence of 100 μM ATP. Results are average of duplicate experiments.
The discovery and optimization of the 2-(benzyloxy)-5-(2-fluoropyridin-4-yl)-N-(pyridin-3-yl)benzamide series of LRRK2 inhibitors will be described in detail elsewhere.11 Briefly, hits for this series were identified in a screen of GlaxoSmithKline’s KCS (a kinase-focused set of compounds for lead discovery) using a homogeneous time-resolved fluorescence (HTRF) assay that measured the inhibition of phosphorylation of the peptide substrate LRRKtide by baculoviral-derived recombinant 6His-Tev-LRRK2 (1326-2527). SAR and optimization of leads was performed using similar recombinant LRRK2 enzyme assays.
GSK2578215A exhibited biochemical IC50s of 10.9 and 8.9 nM against wild-type LRRK2 and LRRK2[G2019S], respectively (Fig. 1). While the biochemical potency of GSK2578215A for inhibition of wild-type and G2019S LRRK2 is similar to LRRK2-IN-1, the potency of GSK2578215A for inhibition of A2016T mutant LRRK2 was reduced eightfold (Fig. 1). Such sensitivity to A2016T mutation is comparable to that reported previously for sunitinib, Y2763212 and TAE684.13 In contrast, the inhibitory activity of LRRK2-IN-1 was reported to be much more sensitive to the A12016T synthetic mutation, an observation that has been attributed to a steric clash of the anthranilic acid ring of LRRK2-IN-1 with the A2016T residue.8 A2016T-mediated changes in compound sensitivity were not attributable to changes in Km for ATP, since Km was found to be similar for normal and A2016T mutant LRRK2 enzymes (data not shown).
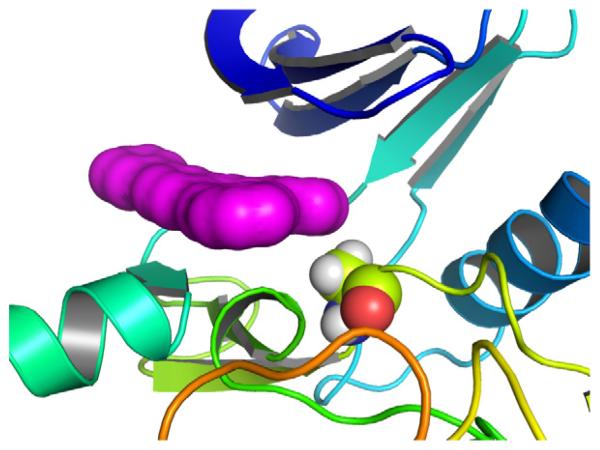
An understanding of the more modest effect of A2016T mutation on the inhibitory activity of GSK2578215A was apparent from modeling studies. Docking of GSK2578215A into a previously described LRRK2 homology model8,12 predicts binding at the hinge of the ATP site and gives essentially the same results whether or not the A2016 residue is mutated to threonine, suggesting that GSK2578215A is able to avoid a steric clash (Fig. 2).
Figure 2. LRRK2 homology model.
GSK2578215A (magenta solvent-accessible surface) binds in the same way to normal and A2016T mutant without a steric clash.
The kinase selectivity of GSK2578215A was assessed using standard radioactivity-based enzymatic assays against a panel of 131 kinases (Dundee profiling)14 and kinase-binding assays against a non-redundant set of 329 additional kinases (KINOMEscan, Ambit Biosciences).15 Analysis of data from these 460 distinct non-LRRK2 kinases revealed that GSK2578215A demonstrated a selectivity profile superior to that of previously reported LRRK2 inhibitors. At a concentration of 10 μM GSK2578215A only one kinase (smMLCK) showed >50% inhibition in the 131 Dundee kinase panel, and only two kinases [ALK and FLT3(D835Y)] exhibited an ambit score of <10 in the KINOMEscan profile (see Supplementary data).
We next examined the ability of GSK2578215A to inhibit LRRK2 in a cellular context in comparison to LRRK2-IN-1. As there are no validated direct phosphorylation substrates of LRRK2, we monitored phosphorylation of Ser910 and Ser935, two residues whose phosphorylation is known to be dependent upon LRRK2 kinase activity16 (Fig. 3). GSK2578215A induced a dose-dependent inhibition of Ser910 and Ser935 phosphorylation in both wild-type LRRK2 and LRRK2[G2019S] stably transfected into HEK293 cells (Fig. 3a). Significant dephosphorylation of Ser910 and Ser935 was observed at 0.3–1.0 μM of GSK2578215A for wild-type LRRK2 and at slightly higher doses for LRRK2[G2019S] (Fig. 3a), which is almost equivalent to that observed using LRRK2-IN-1 (compare Fig. 3a and 3b). Consistent with the biochemical results, GSK2578215A also induced dephosphorylation of Ser910 and 935 at a concentration of 1–3 μM in the inhibitor-resistant LRRK2[A2016T + G2019S] and LRRK2[A2016T] mutants (Fig. 3a), suggesting that GSK2578215A binds to LRRK2 differently relative to LRRK2-IN-1 (compare Fig. 3a and 3b).
Figure 3. GSK2578215A inhibits LRRK2 in cells.
(a) HEK 293 cells stably expressing wild-type GFP-LRRK2, GFP-LRRK2[G2019S], GFP-LRRK2[G2019S + A2016T], and GFP-LRRK2[A2016T] were treated with DMSO or increasing concentrations of GSK2578215A for 90 min. Cell lysates were subjected to immunoblotting for detection of LRRK2 phosphorylated at Ser910 and Ser935 and for total LRRK2. (b) As in (a) except LRRK2-IN-1 was used.
We next examined the effect of GSK2578215A on endogenously expressed LRRK2 in human lymphoblastoid cells derived from a control and Parkinson’s disease patient homozygous for the LRRK2[G2019S] mutation (Fig. 4a). We found that increasing doses of GSK2578215A led to similar dephosphorylation of endogenous LRRK2 at Ser910 and Ser935, as was observed in HEK293 cells stably expressing wild-type LRRK2 or LRRK2[G2019S] (compare Fig. 3a to Fig. 4a). Moreover, endogenous LRRK2 was equally sensitive to GSK2578215A and LRRK2-IN-1, which is consistent with the trend we observed in HEK293 cells. We also found that GSK2578215A induced similar dose-dependent Ser910 and Ser935 dephosphorylation of endogenous LRRK2 in mouse Swiss 3T3 cells (Fig. 4b).
Figure 4. GSK2578215A inhibits endogenously expressed LRRK2.
(a) Endogenous LRRK2 from EBV immortalized human lymphoblastoid cells from a control subject and a Parkinson’s disease patient homozygous for the LRRK2[G2019S] mutation. After treatment of the cells with DMSO or the indicated concentration of GSK2578215A (or LRRK2-IN-1) for 90 min, cell lysates were subjected to immunoblot analysis with the purified indicated antibody for western analysis. Immunoblots were performed in duplicate, and results were representative of at least two independent experiments. (b) As in (a) except mouse Swiss 3T3 cells were used.
Evaluation of the pharmacokinetic profile of GSK2578215A in normal mice demonstrated that the compound achieves exposure in the brain with a brain to plasma ratio of 1.9. GSK2578215A exhibits low oral bioavailability (12.2%F), a half-life of 1.14 h and plasma exposure (635.3 h ng/mL, AUClast) (Table 1). Based on these pharmacokinetic properties, pharmacodynamic experiments examining inhibition of LRRK2 Ser910/Ser935 phosphorylation were conducted after intraperitoneal injection with 100 mg/kg of GSK2578215A to normal mice. We observed complete Ser910 and Ser935 dephosphorylation of LRRK2 in the kidney and spleen, which also demonstrated similar potency relative to LRRK2-IN-1 (Fig. 5).8 Again, despite the significant exposure of GSK2578215A in the brain, no inhibition of LRRK2 Ser910 or Ser935 phosphorylation was observed in the brain, a finding similar to that observed for TAE68413 (Fig. 5). We are currently investigating the reasons for this unexpected result.
Table 1. Pharmacokinetic parameters of GSK2578215A.
| Route | Matrix | AUClast (hr ng/mL) | T1/2 (h) | CL (mL/min/kg) | Vss (L/kg) | Brain/plasma (AUClast) ratio | %F |
|---|---|---|---|---|---|---|---|
| IV | Plasma | 519.6 | 1.14 | 30.0 | 2.3 | 1.4 | 12.2 |
| Brain | 708.7 | 1.11 | 22.1 | 1.6 | – | ||
| PO | Plasma | 635.3 | – | – | – | 2.4 | – |
| Brain | 1539.4 | – | – | – | – |
Experiments were done in male Swiss Albino Mice following single intravenous (IV, 1 mg/kg) and oral (PO, 10 mg/kg) administration. AUC = area under the curve (measure of exposure), T1/2 = half life, CL = plasma clearance, Vss = volume of distribution, F = oral bioavailability.
Figure 5. Pharmacodynamic analysis for GSK2578215A.
Pharmacodynamic study of GSK2578215A from brain, spleen and kidney following intraperitoneal injection at dose of 100 mg/kg. Tissues were collected and endogenous LRRK2 was resolved by SDS–PAGE and blotted with a phospho-specific antibody directed against Ser910, Ser935 and total LRRK2.
In summary, we have discovered that GSK2578215A is a potent biochemical and cellular inhibitor of LRRK2 kinase activity which represents a novel chemotype with respect to all previously reported inhibitors of LRRK2, or indeed of any protein kinase Importantly, GSK2578215A exhibits exquisite selectivity across the kinome that is superior to previously reported LRRK2 inhibitor tool compounds. As such, GSK2578215A provides a significant addition to the battery of available tools to be deployed for elucidation of the functions of LRRK2. Detailed characterization of GSK2578215A using LRRK2-IN-1 as a bench mark revealed that these two compounds had quite similar potency against wild-type LRRK2 and LRRK2[G2019S] mutant both in vitro and in vivo. The A2016T and G2019S + A2016T LRRK2 mutations induce significant resistance to LRRK2-IN-1 but not to GSK2578215A. The ability of GSK2578215A to reduce phosphorylation levels of Ser910 and Ser935 in peripheral tissues on dosing to mice supports the notion that these phosphoepitopes can serve as markers of LRRK2 inhibitor activity in animal studies. Interestingly, whilst GSK2578215A achieves good exposure to mouse brain following oral administration, it failed to induce significant inhibition of Ser910 or Ser935 phosphorylation of LRRK2 in brain. It remains to be determined whether this reflects some pharmacokinetic limitation of this tool or phosphorylation of these sites in brain by non-LRRK2 kinases. Further development of 2-arylmethyloxy-5-substitutent-N-arylbenzamides may result in the identification of pharmacological agents to investigate the impact of LRRK2 inhibition in brain in preclinical animal models and eventually in humans.
Supplementary Material
Acknowledgments
Mouse pharmacokinetic studies were performed at SAI Advantium (http://www.saiadvantium.com/) following their standard operating procedures which are subject to SAI’s policies on care, welfare and treatment of laboratory animals which were also reviewed and approved by the animal welfare committee of the Dana Farber Cancer Institute. All animal studies undertaken in the laboratory of Prof. Dario Alessi were ethically reviewed and carried out in accordance with Animals (Scientific Procedures) Act 1986 and Institute Policy on the Care, Welfare and Treatment of Animals.
We wish to thank Angela Bridges, Rob Tanner and Ryan Bingham (GSK) for reagent provision & screening assay development, staff at the National Centre for Protein Kinase Profiling (http://www.kinase-screen.mrc.ac.uk) for undertaking Dundee kinase specificity screening as well as Nicholas Dzamko for providing the LRRK2 rabbit monoclonal antibodies. We also thank Faycal Hentati Institut National de Neurologie, Tunis, Tunisia for help in generating the human lymphoblastoid cells. SAI Advantium for performing pharmacokinetic studies, and the antibody purification teams [Division of Signal Transduction Therapy (DSTT), University of Dundee] coordinated by Hilary McLauchlan and James Hastie for generation of antibodies. This work was supported in part by a Therapeutics Development Initiative Award from the Michael J. Fox Foundation for Parkinson’s Research (A. Reith), NIH Grant P41 GM079575-03 (N. Gray), the Medical Research Council (D. Alessi), the Michael J Fox Foundation for Parkinson’s Research (N. Gray & D. Alessi) and the pharmaceutical companies supporting the DSTT (AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck KgaA and Pfizer) (D. Alessi). Requests for provision of GSK2578215A should be directed to Alastair Reith (alastair.d.reith@gsk.com).
References and notes
- 1.Gandhi PN, Chen SG, Wilson-Delfosse AL. J. Neurosci. Res. 2009;87:1283. doi: 10.1002/jnr.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM. Neurology. 2007;68:384. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 3.Daniels V, Baekelandt V, Taymans JM. Neuro-Signals. 2011;19:1. doi: 10.1159/000324488. [DOI] [PubMed] [Google Scholar]
- 4.Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW. Lancet Neurol. 2008;7:583. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dächsel JC, Farrer M. J. Arch. Neurol. 2010;67:542. doi: 10.1001/archneurol.2010.79. [DOI] [PubMed] [Google Scholar]
- 6.Greggio E, Cookson MR. ASN Neuro. 2009;1 doi: 10.1042/AN20090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Cookson MR. Expert Rev. Mol. Med. 2011;13:e20. doi: 10.1017/S146239941100192X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng X, Dzamko N, Prescott A, Davies P, Liu Q, Yang Q, Lee JD, Patricelli MP, Nomanbhoy TK, Alessi DR, Gray NS. Nat. Chem. Biol. 2011;7:203. doi: 10.1038/nchembio.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsden N, Perrin J, Ren Z, Lee BD, Zinn N, Dawson VL, Tam D, Bova M, Lang M, Drewes G, Bantscheff M, Bard F, Dawson TM, Hopf C. ACS Chem. Biol. 2011;1021:6. doi: 10.1021/cb2002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patent Application WO2011/038572.
- 11.Dai et al., in preparation.
- 12.Nichols RJ, Dzamko N, Hutti JE, Cantley LC, Deak M, Moran J, Bamborough P, Reith AD, Alessi DR. Biochem. J. 2009;424:47. doi: 10.1042/BJ20091035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Deng X, Choi HG, Alessi DR, Gray NS. Bioorg. Med. Chem. Lett. 1864;2012:22. doi: 10.1016/j.bmcl.2012.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. Biochem. J. 2007;408:297. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabian MA, Biggs WH, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, Galvin M, Gerlach JL, Grotzfeld RM, Herrgard S, Insko DE, Insko MA, Lai AG, Lelias JM, Mehta SA, Milanov ZV, Velasco AM, Wodicka LM, Patel HK, Zarrinkar PP, Lockhart DJ. Nat. Biotechnol. 2005;23:329. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 16.Dzamko N, Deak M, Hentati F, Reith AD, Prescott AR, Alessi DR, Nichols RJ. Biochem. J. 2010;430:405. doi: 10.1042/BJ20100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.