Abstract
Glucocorticoids are steroid hormones which act through the glucocorticoid receptor. They regulate a wide variety of biological processes. Two glucocorticoids, the naturally occurring corticosterone and chemically produced dexamethasone, have been used to investigate the effect of glucocorticoids on Ca2+-signalling in cortical co-cultures of neurons and astrocytes. Dexamethasone and to a lesser degree corticosterone both induced a decrease in cytosolic Ca2+ concentration in neurons and astrocytes. The effect of both compounds can be blocked by inhibition of the plasmamembrane ATPase, calmodulin and by application of a glucocorticoid receptor antagonist, while inhibition of NMDA receptors or the endoplasmic reticulum calcium pump had no effect. Glucocorticoid treatment further protects against detrimental calcium signalling and cell death by modulating the delayed calcium deregulation in response to glutamate toxicity. At the concentrations used dexamethasone and corticosterone did not show cell toxicity of their own. Thus, these results indicate that dexamethasone and corticosterone might be used for protection of the cells from calcium overload.
Keywords: Calcium, Neuron, Astrocyte, Glucococorticoid, Corticosterone, Dexamethasone, Glutamate
1. Introduction
Glucocorticoids (GCs) are a class of steroid hormones that bind to the glucocorticoid receptor (GR), which is expressed on mammalian cells. Due to their anti-inflammatory activity they are widely used in medicine. A variety of synthetic glucocorticoids, some far more potent than cortisol, have been created for therapeutic use. Glucocorticoids have a wide range of physiological activities but for many of them the underlying mechanism is still unknown [1].
One such activity is the maintenance of calcium homeostasis within the cell. Calcium ions are universal regulators of cellular processes, from cell division to cell death. There is a vast amount of publications which suggest a role for glucocorticoids in calcium homeostasis. It has been shown that GC-reduced calcium absorption can result from reduced expression of calcium-processing genes [2]. Many studies have demonstrated that GC changes the intracellular calcium concentration under both physiological and pathological conditions [3]. Exogenous application of a high dose of GC-induced a decrease in [Ca2+]i in hypothalamic neurons [3]. This is opposite to what Reul et al. show, where increased corticosterone level enhance Ca2+ influx into CA1 pyramidal neurons [4]. High corticosterone modulated Ca2+ influx leads to altered physiological properties of the cell and network function [5,6]. Despite the apparent interest in this phenomenon the role of glucocorticoids in this process remains controversial. This might be due to the different cell types used by different groups as well as different glucocorticoids. To shed light on this disparity we used two different commonly used glucocorticoids, endogenous mammalian glucocorticoid corticosterone (CORT) produced in the rodent adrenal gland and a more potent synthetic preparation, dexamethasone (DEX) to investigate the effect of glucocorticoids on calcium homeostasis.
Dexamethasone (DEX) is a potent synthetic GC agonist, 25–30 times more potent than the natural steroid [7], known to cross the blood–brain barrier [8], whereas corticosterone (CORT) is a principal glucocorticoid synthesised in rodent adrenal cortex. DEX and CORT exerts their biochemical function mainly by binding to the glucocorticoid receptor (GR), which is expressed in almost all cell types but has varying effects in different cells [9]. Both are widely used therapeutically for many diseases such as neurological, neonatal respiratory distress syndrome, inflammatory, rheumatologic and autoimmune diseases. However, administration of GCs has many side effects, such as reduced growth and body weight [10], loss of memory and impaired logical thinking [11], disrupted hypothalamic-pituitary-adrenal (HAP) axis function [12] and reduction in calcium absorption [13]. Therefore it remains important to understand the exact mechanisms by which GCs exert their protective as well as detrimental effects.
Here we show the action of DEX and CORT on the cytosolic calcium concentration. They act through the GR, which modulates the cytosolic calcium concentrations through the plasmalemmal ATPase in a calmodulin dependent manner. We also demonstrate that glucocorticoids reduce the calcium signal activated by physiological (5 μM) and pathological (100 μM) concentrations of glutamate. Finally, this leads to a protective effect of DEX and CORT against glutamate induced excitotoxicity in primary neuronal cultures.
2. Methods
2.1. Cell culture
Mixed cultures of cortical neurones were prepared as described previously [14,15] with modifications, from Sprague–Dawley rat pups 2–4 days post-partum (UCL breeding colony). Hippocampi, cortex and midbrain were removed into ice-cold HBSS (Ca2+, Mg2+-free, Gibco-Invitrogen, Paisley, UK). The tissue was minced and trypsinised (0.1% for 15 min at 37 °C), triturated and plated on poly-d-lysine-coated coverslips and cultured in Neurobasal A medium (Gibco-Invitrogen, Paisley, UK) supplemented with B-27 (Gibco-Invitrogen, Paisley, UK) and 2 mM l-glutamine. Cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air, media changed twice a week. To avoid the age dependence of the delayed calcium deregulation, we used cells after 12–15 days in vivo in all experiments. Neurons were easily distinguishable from glia: they appeared phase bright, had small smooth rounded somata and distinct processes, and lay just above the focal plane of the glial layer.
2.2. Imaging [Ca2+]c
Hippocampal, cortical and midbrain neurons were loaded for 30 min at room temperature with 5 μM fura-ff AM, 5 μM fura-2 AM or 5 μM fluo-4 AM and 0.005% pluronic in a HEPES-buffered salt solution (HBSS) composed (mM): 156 NaCl, 3 KCl, 2MgSO4, 1.25 KH2PO4, 2 CaCl2, 10 glucose and 10 HEPES, pH adjusted to 7.35 with NaOH. For simultaneous measurement of [Ca2+]c and Δψm, Rh123 (10 μM, Molecular Probes) was added into the cultures during the last 15 min of the fura-2 loading period, and the cells were then washed.
Fluorescence measurements were obtained on an epifluorescence inverted microscope equipped with a 20× fluorite objective. [Ca2+]i and Δψm were monitored in single cells using excitation light provided by a Xenon arc lamp, the beam passing sequentially through 10 nm band pass filters centred at 340, 380 and 490 nm housed in computer-controlled filter wheel (Cairn Research, Kent, UK). Emitted fluorescence light was reflected through a 515 nm long-pass filter to a cooled CCD camera (Retiga, QImaging, Canada). All imaging data were collected and analysed using software from Andor (Belfast, UK). The fura-2 or fura-ff data have not been calibrated in terms of [Ca2+]i because of the uncertainty arising from the use of different calibration techniques. Fluo-4 signal was excited by 490 nm and measured above 515 nm. Accumulation of Rh123 in polarised mitochondria quenches the fluorescent signal; in response to depolarisation the fluorescence signal is dequenched; an increase in Rh123 signal therefore indicates mitochondrial depolarisation. We have normalised the signals between resting level (set to 0) and a maximal signal generated in response to the uncoupler FCCP (1 μM; set to 100%).
2.3. Toxicity experiments
For toxicity assays cells were exposed to 5 μM propidium iodide (PI) and 5 μM Hoechst 33342 (Molecular Probes, Eugene, OR) for 30 min prior to imaging. The PI is excluded from viable cells and exhibits a red fluorescence following a loss of membrane integrity, while the Hoechst 33342 labels all nuclei blue. This allows expression of the number of dead (red stained) cells as a fraction of the total number of nuclei counted. Using phase contrast optics, a bright field image allowed identification of neurones, which look quite different to the flatter glial cells and also lie in a different focal plane, above the glial layer. A total number of 100–300 neurons were counted in 4-5 fields of each coverslip. Each experiment was repeated four or more times using separate cultures.
2.4. Statistical analysis
Statistical analysis was performed with the aid of Origin 8 (Microcal Software Inc., Northampton, MA, USA) software. Means expressed ±the standard error of the mean (S.E.M.).
3. Results
3.1. Effect of glucocorticoids on [Ca2+]c in neurons and astrocytes
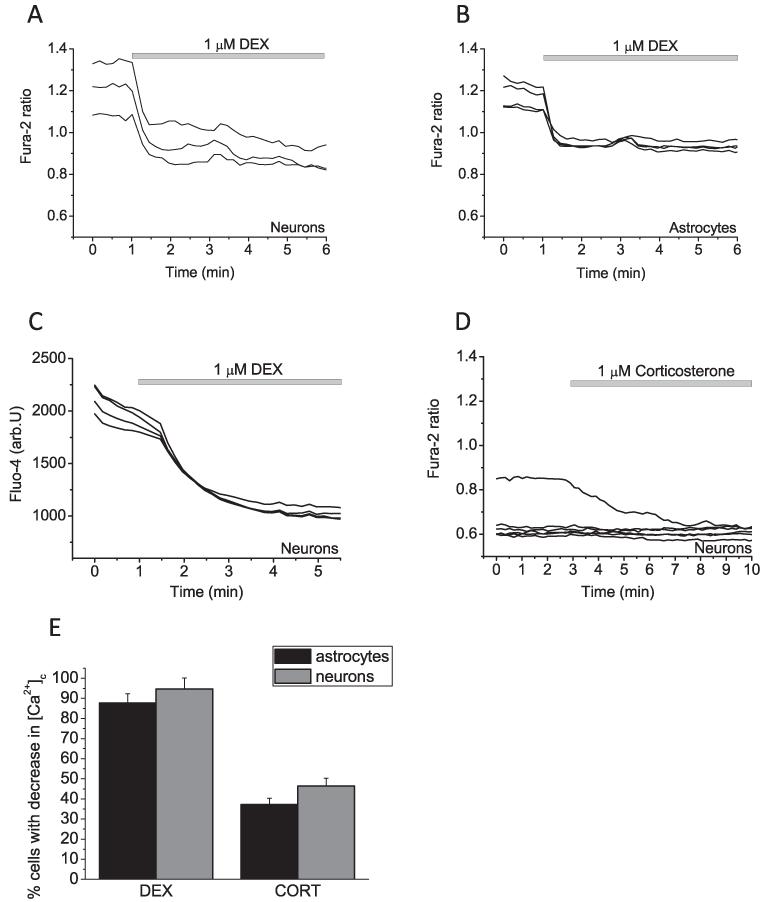
The application of DEX (1 μM) to co-cultured neurons and astrocytes loaded with the fluorescent calcium indicator fura-2, induced a significant decrease of cytosolic calcium concentration [Ca2+]c in both cell types (0.93 ± 0.06 vs 1.21 ± 0.07; P < 0.01, n = 104 neurons and 93 astrocytes 0.94 ± 0.01 vs 1.15 ± 0.03; P < 0.05; Fig. 1A and B). To avoid any artefacts of quenching the fura-2 signal by DEX, we also used another calcium indicator fluo-4. Adding of DEX (1 μM) to fluo-4 loaded neurons and astrocytes had the same Ca2+-reducing effect (n = 20 neurons and 22 astrocytes; Fig. 1C). Corticosterone (1 μM) was slightly less potent in reducing the basal [Ca2+]c in cells, but effectively decrease calcium in stimulated cells (n = 99 neurons and 96 astrocytes; Fig. 1D). Thus, CORT decreased [Ca2+]c in 46.4 ± 3.9% of neurons and 37.2 ± 3.1% of astrocytes compare 94.7 ± 5.4% and 87.7 ± 4.6% in neurons and astrocytes in response to DEX; Fig. 1E.
Fig. 1.
The effect of glucocorticoids on [Ca2+]c in co-cultured neurons and astrocytes. (A and B) Representative recording of fura-2 ratio from both cell types following exposure to DEX (1 μM; A and B) and corticosterone (1 μM; D). (C) DEX (1 μM) also reducing effect of [Ca2+]c in fluo-4 loaded neurons. (E) Percentage of the cells in the experiment with calcium response to glucocorticoids.
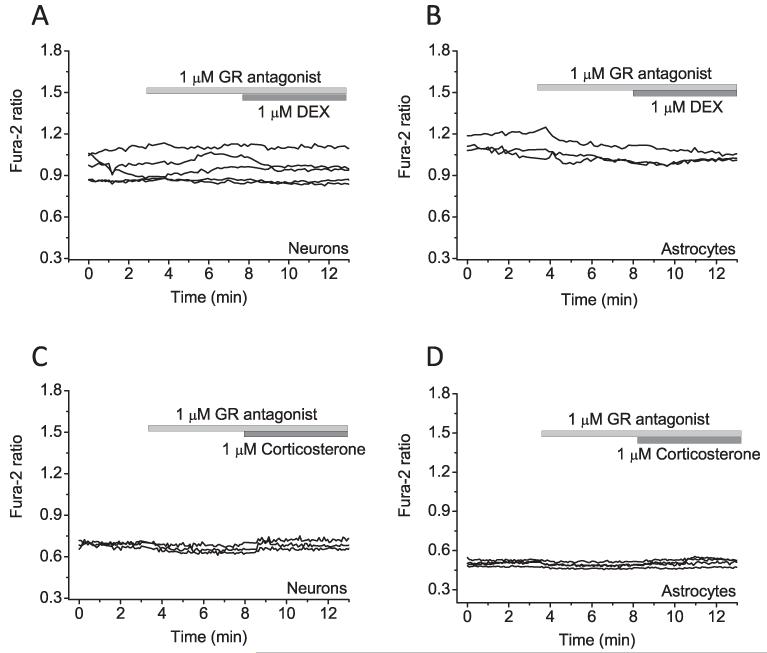
DEX and corticosterone are both glucocorticoids and can bind glucocorticoid receptors [16]. We used a specific antagonist (GR antagonist) to check whether the [Ca2+]c-reducing effect of these compounds is indeed through binding of glucocorticoid receptors. Pre-incubation of cells with the GR antagonist completely prevented the effect of DEX and corticosterone on [Ca2+]c (n = 54 neurons and 58 astrocytes for DEX and n = 57 neurons and 61 astrocytes for CORT) (Fig. 2A-D; see also summary histogram Fig. 4G).
Fig. 2.
The effect of glucocorticoid receptor antagonist on glucocorticoid-induced Ca2+ decrease. Application of glucocorticoid receptor antagonist, inhibited the effect of DEX (A and B) and corticosterone-reduced (C and D) Ca2+ signal in primary co-culture neurons and astrocytes.
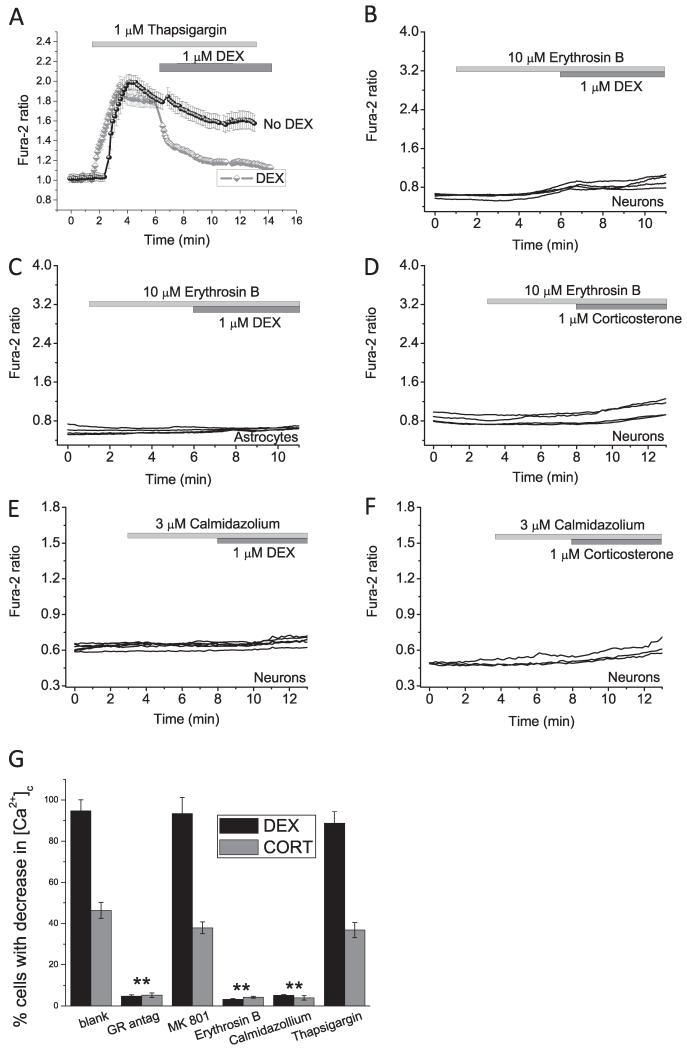
Fig. 4.
Effect of Ca2+ pumps and calmodulin on the action of DEX and CORT. Depletion of the intracellular calcium pool with an inhibitor of the ER Ca2+ pump, thapsigargin (1 μM) (A) did not block the effect of DEX-reduced Ca2+ response in the cell (grey trace). Black trace represents the effect of 1 μM of thapsigargin without DEX. (B–D) Inhibitor of calcium ATPase – plasmalemmal Ca2+-ATPase – erythrosine B (10 μM) completely blocked the effect of DEX or corticosterone on the level of cytosolic calcium. Application of the inhibitor of calmodulin – calmidazolium (3 μM) blocked the effect of DEX (E) and CORT (F) on cytosolic calcium concentration of neurons and astrocytes. (G) Percentage of the neurons with response to DEX and CORT in the presence of inhibitors. **P < 0.001.
3.2. Mechanism of glucocorticoid-induced reduction of [Ca2+]c
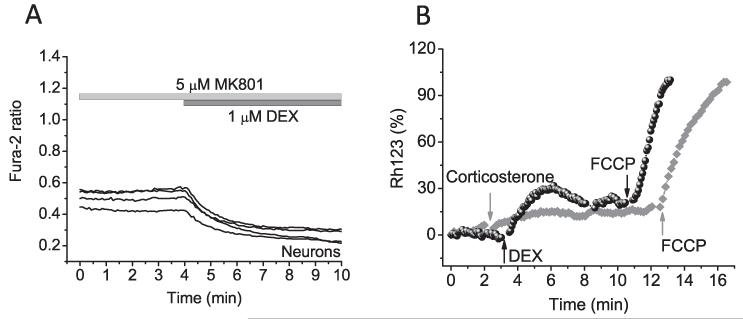
Glucocorticoids are known to suppress NMDA receptors [17]. In order to investigate whether the decreased [Ca2+]c seen after DEX treatment was NMDA receptor mediated, we used a selective inhibitor of this receptor, MK801 (5 μM). Application of MK801 did not change the level of cytosolic calcium in neurons and astrocytes and also had no effect on the response to DEX (n = 34 neurons and 42 astrocytes; Figs. 3A and 4G).
Fig. 3.
Glutamate receptor or mitochondrial calcium uptake are not involved in the effect of DEX or CORT on [Ca2+]c in neurons and astrocytes. Incubation cells with MK801 did not change the effect of DEX on [Ca2+]c (A). (B) Changes in Δψm were measured using Rh123 in dequench mode (the loss of potential is seen as an increase in fluorescence). Corticosterone (1 μM) or DEX (1 μM) induced mild depolarisation of Δψm in both cell types. The Rh123 fluorescence signal in these traces is normalised between zero, representing the resting Rh123 fluorescence and 100, representing the maximal increase Rh123 fluorescence in response to complete mitochondrial depolarisation by 1 μM FCCP.
The effect of glucocorticoids on [Ca2+]c in neurons and astrocytes can be induced by activation of active ion transport or buffering of Ca2+ by mitochondria. Mitochondrial calcium uptake is directly dependent on the mitochondrial membrane potential (Δψm). Application of corticosterone or DEX to neurons and astrocytes induced a mild depolarisation of Δψm in both cell types (21.42 ± 2.18% for corticosterone and 25.65 ± 7.43% for DEX; Fig. 3B). These data strongly suggests that both glucocorticoids do not modulate calcium handling through mitochondrial uptake, as we see a slight decrease in Δψm, usually a mitochondrial depolarisation leads to the opposite effect, a rise in cytosolic calcium [18].
The effects of corticosterone and DEX on cellular calcium may be induced by activation of the calcium pump of the reticulum (SERCA). Application of DEX to the cells pre-treated with SERCA inhibitor (thapsigargin, 1 μM) induced a significant decrease in [Ca2+]c in neurons and astrocytes (n = 26 neurons and 27 astrocytes) (Fig. 4A and G) which strongly suggests that the effect of DEX in calcium is not induced by activation of SERCA in the cells.
Finally, pre-treatment of the cells with an inhibitor of another calcium ATPase – the plasmalemmal Ca2+-ATPase (erythrosine B (10 μM)) did not change [Ca2+]c neurons and astrocytes by itself but completely prevents the decrease of cytosolic calcium after DEX or corticosterone treatment (n = 43 neurons and 42 astrocytes for DEX and n = 41 neurons and 39 astrocytes for CORT) (Fig. 4B-D and G). Thus, corticosteroid and DEX activate the plasmalemmal Ca2+-ATPase and reduce the [Ca2+]c. Furthermore, the plasmalemmal Ca2+-ATPase is known to be regulated by calmodulin [19]. Inhibition of calmodulin by the specific inhibitor calmidazolium (3 μM) also blocks the effect of DEX and CORT on cytosolic calcium concentration in neurons and astrocytes (n = 32 neurons and 40 astrocytes for DEX and n = 36 neurons and 39 astrocytes for CORT) (Fig. 4E-G).
3.3. Physiological and pathological implications for glucocorticoid actions on [Ca2+]c
One of the major processes in which calcium homeostasis plays a role in the brain, is the cells response to glutamate induced calcium signalling. Glutamate is the most commonly used neurotransmitter in the brain, however, it is widely accepted that accumulation of extracellular glutamate mediates cell death. This is particularly prominent following periods of cerebral ischaemia or anoxia [20]. Glutamate induced neuronal cell death through excitotoxicity is stimulated by calcium overload. In order to investigate whether glucocorticoids affect physiological and pathological glutamate induced calcium signalling we study the effects of glucocorticoids on low (5 μM) and high toxic (100 μM) concentrations of glutamate.
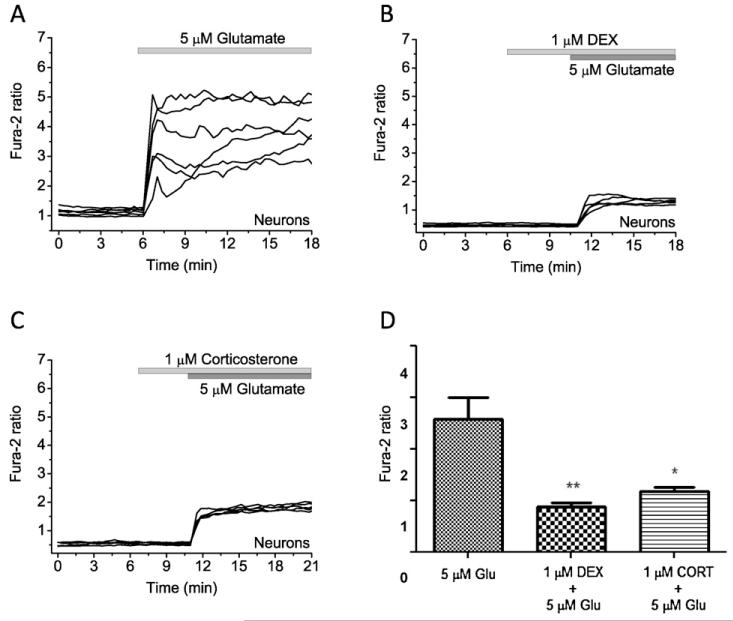
Application of 5 μM glutamate to cortical neurons induced a transient increase in [Ca2+]c (n = 140 neurons; Fig. 5A). Pre-incubation of cells with 1 μM DEX or 1 μM corticosterone significantly reduced the effect of 5 μM glutamate on [Ca2+]c cortical neurons (n = 98 neurons and 80 astrocytes for DEX and n = 73 neurons and 92 astrocytes for CORT) (Fig. 5B-D).
Fig. 5.
The effect of glucocorticoids on glutamate-induced calcium signal in co-cultured neurons and astrocytes. (A) 5 μM glutamate induced typical [Ca2+]c rise cortical neurons. Application of DEX (1 μM, B) or corticosterone (1 μM, C) significantly decreased the amplitude of calcium rise in response to 5 μM glutamate. (D) Differences in the amplitude (fura-2 ratio) of glutamate (5 μM)-induced calcium signal in the presence of DEX (1 μM) and corticosterone (1 μM) (*P < 0.05 and **P < 0.01).
It is well established that glutamate toxicity is induced by delayed calcium deregulation (DCD), defined as an initial transient increase in cytosolic calcium concentration [Ca2+]c followed by a delayed, irreversible rise in [Ca2+]c, simultaneously with mitochondrial depolarization [14,15]. DCD can be detected using low affinity calcium indicators such as fura-ff. We measured the appearance of the glutamate induced DCD in cortical, neurons using fura-ff and Rh123.
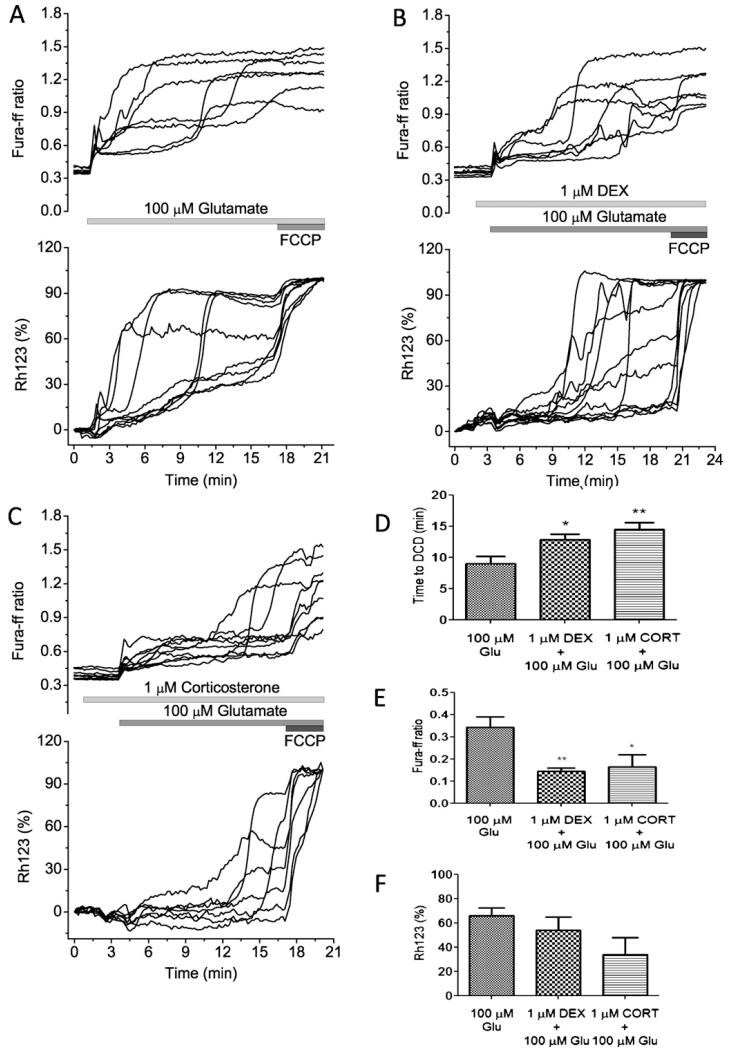
We and others have previously shown that application of 100 μM glutamate to neurons causes a stereotypical response that consists of an initial transient increase in [Ca2+]c, followed by a secondary delayed increase to a plateau (Fig. 6A) [21,22]. Simultaneous measurements of mitochondrial membrane potential showed that the initial phase may be accompanied by a modest and slow mitochondrial depolarisation, while the delayed secondary increase in [Ca2+]c is accompanied by a progressive and occasionally complete loss of mitochondrial potential (n = 70 neurons; Fig. 5A) [21,22]. Pre-incubation cells for (3 min) with 1 μM DEX or 1 μM corticosterone significantly delayed the appearance of DCD (from 9.04 ± 4.14 min to 12.82 ± 3.94 min for DEX (P < 0.05) (n = 50 neurons and 81 astrocytes for DEX) and to 14.56 ± 4.77 min for corticosterone (P < 0.01) (n = 102 neurons and 89 astrocytes for CORT); Fig. 6B-D, upper panel). This results in a decrease in the glutamate induced [Ca2+]c changes (from 0.34 ± 0.12 to 0.143 ± 0.04 for DEX (P < 0.01) to 0.16 ± 0.13 (P < 0.05) for corticosterone; Fig. 6E). Although DEX and corticosterone induced a small mitochondrial depolarisation by themselves (Figs. 3B and 5B-C), both compounds did not enhance the glutamate-induced depolarisation at the time of DCD (Fig. 6B and C lower panel, F).
Fig. 6.
The effect of glucocorticoids on glutamate-induced calcium signal and mitochondrial membrane potential in co-cultured neurons and astrocytes. Simultaneous measurements of changes in [Ca2+]c (fura-ff ratio) and Δψm (relative Rh123 fluorescence) were made from single neurons. Glutamate (100 μM) and glycine (10 μM) were applied in a Mg2+-free solution. An increase in Rh123 fluorescence reflects mitochondrial depolarisation. (A) The calcium response to glutamate shows initial phase followed by calcium deregulation and loss of mitochondrial membrane potential. (B and C) The differences in the dynamics of glutamate-induced changes in [Ca2+]c and Δψm in the presence of 1 μM DEX or 1 μM corticosterone. The mitochondrial uncoupler FCCP (1 μM) was applied at the end of this and subsequent experiments to indicate the level of Rh123 fluorescence that reflected complete dissipation of Δψm. (D) Time (min) from application of glutamate and appearance of DCD; (E) differences in the amplitude (fura-ff ratio) of initial peak of glutamate (100 μM) induced calcium signal; (F) both glucocorticoids decrease the average of glutamate-induced Rh123 signal in neurons (*P < 0.05 and **P < 0.001).
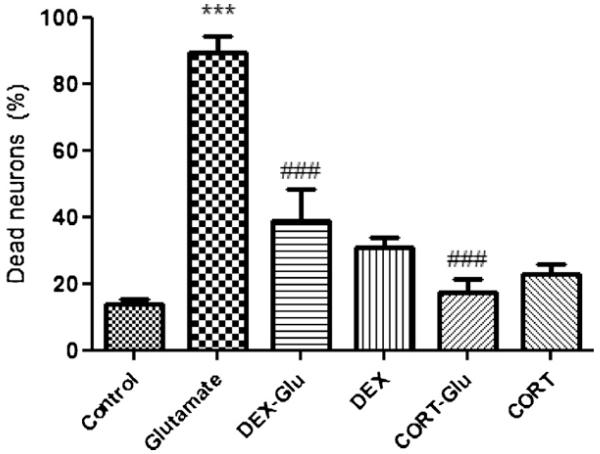
Finally, considering the protective role of DEX and CORT against glutamate-induced DCD, we examine the effect of the two compounds on cell viability after 15 min of 100 μM glutamate exposure (estimated after 24 h). We found that most (89.37 ± 5.04%) of the neurons died within a 24 h period after glutamate exposure (Fig. 7, n = 4 experiments). Pre-incubation with DEX reduced cell death of cortical neurons by ~50% (38.85 ± 9.24% dead neurons, P < 0.001; n = 4 experiments; Fig. 7). Interestingly, corticosterone was even more protective (17.29 ± 3.93% dead neurons; P < 0.001; n = 4).
Fig. 7.
Glucocorticoids protects neurons against glutamate-induced cell death. The viability of neurons from rat cortex was measured 24 after 10 min exposure to 100 μM glutamate using PI fluorescence. Dead cells were counted with respect to the total number of cells present, identified by staining nuclei with Hoechst 33342. 1 μM DEX and 1 μM corticosterone were significantly protective, but caused significant toxicity when applied without glutamate. ***P < 0.0001 compared with control,###P < 0.001 compared to glutamate. All data are expressed as mean ± S.E.M., n = 4.
4. Discussion
Maintenance of [Ca2+]c in cells is tightly controlled. A calcium signal is usually activated by opening of channels which elevates the [Ca2+]c, while active transport brings the levels of back to basal (~100 nM). Here we demonstrate that activation of the plasmalemmal Ca2+ ATPase by glucocorticoids can not only stimulate recovery of [Ca2+]c after stimulus but also significantly reduce the basal level of cytosolic Ca2+ in neurons and astrocytes. This effect has previously been shown for high doses of dexamethasone [3] in hypothalamic neurons. Activation of the Ca2+ pump by another endogenous compound, vitamin B12, also induced a reduction of the basal [Ca2+]c [23], suggesting that calcium reducing effect by natural compounds can have physiological relevance.
Importantly, in agreement with previously published reports [24], in our experiments both glucocorticoids had no effects on another calcium pump – SERCA. The plasmalemmal Ca2+ ATPase in contrast to SERCA is a calmodulin-dependent calcium ATPase [25], and in our experiments the [Ca2+]c-reducing effect of glucocorticoids could be blocked by an inhibitor of calmodulin, calmidasolium, which suggest the involvement of this protein in glucocorticoid signalling. Considering the fact that the effects of CORT and DEX could be blocked by antagonists of glucocorticoid receptors, we can suggest a mechanism in which then binding of the glucocorticoids to receptors activates calmodulin following the activation of the plasmalemmal Ca2+ ATPase. It is not very clear how activation of the glucocorticoid receptors can stimulate a calcium-dependent protein. One could imagine that binding to the glucocorticoid receptors may stimulate a local Ca2+ increase which leads to binding of calmodulin and activation of the plasmalemmal Ca2+ ATPase.
Both corticosterone and DEX induced a mild mitochondrial depolarisation in neurons and astrocytes. This effect is likely to be explained by inhibition of mitochondrial respiration, which has been previously demonstrated for synthetic glucocorticoids[26,27].
In the present study we investigate the effects of glucocorticoids on neurons under different physiological and pathological conditions. Incubation with 5 μM glutamate represents physiological conditions whereas 100 μM glutamate is induces excitotoxicity [21]. Under physiological condition, pre-treated with DEX or corticosterone decreased the initial amplitude in the [Ca2+]c rise after addition of glutamate (5 μM). It possibly can lead to the described side effects of GC, such as loss of memory and impaired logical thinking and mood (for review see [28]). The potential action of glucocorticoids on glutamate receptors is very controversial. We show that in our experiments, the effect is induced by activation of calcium removal from the cells rather than suppression of glutamate receptors. Under pathological conditions (high concentration of glutamate (100 μM)), pre-treated with DEX or corticosterone produced significantly delays the appearance of DCD, which suggests that glucocorticoids are able to protect cells from excitotoxicity. Interestingly, the DEX or CORT-induced mild mitochondrial depolarisation did not enhance the toxic effects of high doses of glutamate unlike other mitochondrial inhibitors [15,29].
In conclusion, this study demonstrated that DEX and corticosterone can protect against glutamate-induced cell death by decreasing [Ca2+]c signalling in cortical neurons. This discovery points to the contribution of glucocorticoids as potential protective agents, through decreasing of the calcium signals in toxic elevation of [Ca2+]c.
Acknowledgments
This work was supported by The Thailand Research Fund (TRF)-Royal Golden Jubilee Ph.D. Program and Mahidol University to WS and BC. AYA is a Parkinson’s UK Senior Research Fellow.
Footnotes
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
References
- [1].Barnes P. Glucocorticosteroids: current and future directions. British Journal of Pharmacology. 2011;163:29–43. doi: 10.1111/j.1476-5381.2010.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kim M, Lee G, Jung E, Choi K, Oh G, Jeung E. Dexamethasone differentially regulates renal and duodenal calcium-processing genes in calbindin-D9k and D28k knockout mice. Experimental Physiology. 2009;94:138–151. doi: 10.1113/expphysiol.2008.044339. [DOI] [PubMed] [Google Scholar]
- [3].Chen S, Wang X, Zhang X, Wang W, Liu D, Long Z, Dai W, Chen Q, Xu M, Zhou J. High-dose glucocorticoids induce decreases calcium in hypothalamus neurons via plasma membrane Ca2+ pumps. NeuroReport. 2011;22:660–663. doi: 10.1097/WNR.0b013e32834a282a. [DOI] [PubMed] [Google Scholar]
- [4].Reul JMHM, Bosch J.R.V.d., de ERK. Relative occupation of type 1 and type 2 corticosteroid receptors in rat brain following stress and dexamethasone treatment: functional implications. Journal of Endocrinology. 1987;115:459–467. doi: 10.1677/joe.0.1150459. [DOI] [PubMed] [Google Scholar]
- [5].Beato M, Klug J. Steroid hormone receptors: an update. Human Reproduction Update. 2000;6:225–236. doi: 10.1093/humupd/6.3.225. [DOI] [PubMed] [Google Scholar]
- [6].Karst H, Nair S, Velzing E, Essen L, Slagter E, Shinnick-Gallagher P, Joels M. Glucocorticoids alter calcium conductance and calcium channel subunit expression in basolateral amygdala neurons. European Journal of Neuroscience. 2002;16:1083–1089. doi: 10.1046/j.1460-9568.2002.02172.x. [DOI] [PubMed] [Google Scholar]
- [7].Kawata M. Roles of steroid hormones and their receptors in structural organization in the nervous system. Neuroscience Research. 1995;24:1–46. doi: 10.1016/0168-0102(96)81278-8. [DOI] [PubMed] [Google Scholar]
- [8].Joels KERM, Holsboer F. Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- [9].Cole T. Glucocorticoid action and the development of selective glucocorticoid receptor ligands. Biotechnology Annual Review. 2006;12:269–300. doi: 10.1016/S1387-2656(06)12008-6. [DOI] [PubMed] [Google Scholar]
- [10].Seckl J. Glucocorticoid programming of the fetus: adult phenotypes and molecular mechanisms. Molecular and Cellular Endocrinology. 2001;185:61–71. doi: 10.1016/s0303-7207(01)00633-5. [DOI] [PubMed] [Google Scholar]
- [11].Young A, Sahakian B, Robbins T, Cowe P. The effects of chronic administration of hydrocortisone on cognitive function in normal volunteers. Psychopharmacology. 1999;145:260–266. doi: 10.1007/s002130051057. The Third Generation of Progress, Raven Press, New York. [DOI] [PubMed] [Google Scholar]
- [12].Lajic S, Nordenstrom A, Hirvikoski T. Long-term outcome of prenatal dexamethasone treatment of 21-hydroxylase deficiency. Endocrine Development. 2011;20:96–105. doi: 10.1159/000321228. [DOI] [PubMed] [Google Scholar]
- [13].Jia D, O’Brien C, Stewart S, Manolagas S, Weinstein R. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592–5599. doi: 10.1210/en.2006-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stout A, Reynolds I. High-affinity calcium indicators underestimate increases in intracellular calcium concentrations associated with excitotoxic glutamate stimulations. Neuroscience. 1999;89:91–100. doi: 10.1016/s0306-4522(98)00441-2. [DOI] [PubMed] [Google Scholar]
- [15].Abramov A, Duchen M. Impaired mitochondrial bioenergetics determines glutamate-induced delayed calcium deregulation in neurons. Biochimica et Biophysica Acta. 2010;1800:297–304. doi: 10.1016/j.bbagen.2009.08.002. [DOI] [PubMed] [Google Scholar]
- [16].Menconi M, Gonnella P, Petkova V, Lecker S, Hasselgren P. Dexamethasone and corticosterone induced similar, but not identical, muscle wasting responses in cultured L6 and C2C12 myotubes. Journal of Cellular Biochemistry. 2008;105:353–364. doi: 10.1002/jcb.21833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang Y, Sheng H, Qi J, Ma B, Sun J, Li S, Ni X. Glucocorticoid acts on a putative G protein-coupled receptor to rapidly regulate the activity of NMDA receptors in hippocampal neurons. American Journal of Physiology: Endocrinology and Metabolism. 2012;302:E747–E758. doi: 10.1152/ajpendo.00302.2011. [DOI] [PubMed] [Google Scholar]
- [18].Domijan A, Kovac S, Abramov A. Impact of fumonisin B1 on glutamate toxicity and low magnesium-induced seizure activity in neuronal primary culture. Neuroscience. 2012;202:10–16. doi: 10.1016/j.neuroscience.2011.12.005. [DOI] [PubMed] [Google Scholar]
- [19].Moreau B, Straube S, Fisher R, Putney J, Parekh A. Ca2+-calmodulin-dependent facilitation and Ca2+ inactivation of Ca2+ release-activated Ca2+ channels. Journal of Biological Chemistry. 2005;280:8776–8783. doi: 10.1074/jbc.M409619200. [DOI] [PubMed] [Google Scholar]
- [20].Sasaki A, Matsubara A, Tabuchi K, Hara A, Namba A, Yamamoto Y, Shinkawa H. Immunoelectron microscopic analysis of neurotoxic effect of glutamate in the vestibular end organs during ischemia. Acta Otolaryngologica. 2012;132:686–692. doi: 10.3109/00016489.2012.656322. [DOI] [PubMed] [Google Scholar]
- [21].Abramov A, Duchen M. Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochimica et Biophysica Acta. 2008;1777:953–964. doi: 10.1016/j.bbabio.2008.04.017. [DOI] [PubMed] [Google Scholar]
- [22].Vergun O, Keelan J, Khodorov B, Duchen M. Glutamate-induced mitochondrial depolarization and perturbation of calcium homeostasis in cultured rat hippocampal neurons. The Journal of Physiology. 1999;519:451–466. doi: 10.1111/j.1469-7793.1999.0451m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sukocheva O, Abramov A, Levitskaya J, Gagelgans A, Carpenter D. Modulation of intracellular Ca(2+) concentration by vitamin B12 in rat thymocytes. Blood Cells, Molecules, and Diseases. 2001;27:812–824. doi: 10.1006/bcmd.2001.0450. [DOI] [PubMed] [Google Scholar]
- [24].Waring P, Beaver J. Cyclosporin A rescues thymocytes from apoptosis induced by very low concentration of thapsigargin: effect on mitochondrial function. Experimental Cell Research. 1996;227:264–276. doi: 10.1006/excr.1996.0276. [DOI] [PubMed] [Google Scholar]
- [25].Carafoli E, Garcia-Martin E, Guerini D. The plasma membrane calcium pump: recent developments and future perspectives. Experientia. 1996;52:1091–1100. doi: 10.1007/BF01952107. [DOI] [PubMed] [Google Scholar]
- [26].Martens M, Peterson P, Lee C. In vitro effects of glucocorticoid on mitochondrial energy metabolism. Biochimica et Biophysica Acta. 1991;1058:152–160. doi: 10.1016/s0005-2728(05)80232-4. [DOI] [PubMed] [Google Scholar]
- [27].Morin C, Zini R, Simon N, Charbonnier P, Tillement J, Louet HL. Low glucocorticoid concentrations decrease oxidative phosphorylation of isolated rat brain mitochondria: an additional effect of dexamethasone. Fundamental and Clinical Pharmacology. 2000;14:493–500. doi: 10.1111/j.1472-8206.2000.tb00432.x. [DOI] [PubMed] [Google Scholar]
- [28].Brown ES. Effects of glucocorticoids on mood, memory, and the hippocampus. Treatment and preventive therapy. Annals of the New York Academy of Sciences. 2009;1179:41–55. doi: 10.1111/j.1749-6632.2009.04981.x. [DOI] [PubMed] [Google Scholar]
- [29].Domijan A, Abramov A. Fumonisin B1 inhibits mitochondrial respiration and deregulates calcium homeostasis-implication to mechanism of cell toxicity. International Journal of Biochemistry and Cell Biology. 2011;43:897–904. doi: 10.1016/j.biocel.2011.03.003. [DOI] [PubMed] [Google Scholar]