Abstract
The B-cell receptor (BCR) is essential for normal B-cell development and maturation. In an increasing number of B-cell malignancies, BCR signaling is implicated as a pivotal pathway in tumorigenesis. Mechanisms of BCR activation are quite diverse and range from chronic antigenic drive by microbial or viral antigens to autostimulation of B-cells by self-antigens to activating mutations in intracellular components of the BCR pathway. Hepatitis C virus infection can lead to the development of splenic marginal zone lymphoma, while Helicobacter pylori infection is associated with the development of mucosa-associated lymphoid tissue lymphomas. In some of these cases, successful treatment of the infection removes the inciting antigen and results in resolution of the lymphoma. Chronic lymphocytic leukemia has been recognized for decades as a malignancy of auto-reactive B-cells and its clinical course is in part determined by the differential response of the malignant cells to BCR activation. In a number of B-cell malignancies, activating mutations in signal transduction components of the BCR pathway have been identified; prominent examples are activated B-cell-like (ABC) diffuse large B-cell lymphomas (DLBCL) that carry mutations in CD79B and CARD11 and displays chronic active BCR signaling resulting in constitutive activation of the NF-κB pathway. Despite considerable heterogeneity in biology and clinical course, many mature B-cell malignancies are highly sensitive to kinase inhibitors that disrupt BCR signaling. Thus, targeted therapy through inhibition of BCR signaling is emerging as a new treatment paradigm for many B-cell malignancies. Here, we review the role of the BCR in the pathogenesis of B-cell malignancies and summarize clinical results of the emerging class of kinase inhibitors that target this pathway.
Keywords: Ibrutinib, Idelalisib, Lymphoma, B-cell receptor, Antigen
Introduction
The B-cell receptor (BCR) signaling pathway, critical to the development and maturation of normal B cells, is emerging as a valuable target for the treatment of B-cell malignancies [1-5]. Several mechanisms activating this pathway have been identified in different B-cell malignancies; ranging from chronic antigenic drive due to hepatitis C virus (HCV) infection in splenic marginal zone lymphoma (SMZL) [6;7], over autostimulatory activation of the BCR in chronic lymphocytic leukemia (CLL) and follicular lymphoma (FL) [4;8-11], to antigen-independent signaling due to CARD11 mutations in a subset of activated B-cell like diffuse large B-cell lymphomas (ABC-DLBCL) [12]. Emerging data from clinical trials indicate that interruption of BCR signaling has substantial anti-tumor activity in a number of B-cell malignancies. In particular, kinase inhibitors directed against SYK [13], BTK [14-18] and PI3K [19], central “hubs” in the signal transduction network downstream of the BCR, are effective in CLL [19;20], mantle cell lymphoma (MCL) [15], follicular lymphoma (FL) [14], Waldenström’s macroglobulinemia (WM) [14], and in a subset of ABC-DLBCL [16]. In this review, we will concentrate on components of the BCR pathway that are targeted in clinical or preclinical trials for B-cell malignancies. We will give a brief overview of BCR signaling in normal B cells, discuss the evidence for its role in the pathogenesis of select B-cell malignancies, and summarize the available clinical experience (see table 1 for an overview).
Table 1.
Targeted therapies for the BCR pathway in clinical development for B-cell malignancies.
| Target | Function | Inhibitors | Malignancy, OR | Toxicity |
|---|---|---|---|---|
| LYN | Activate and terminate BCR signaling |
Dasatinib (also targets BTK) |
CLL 20% [33] | Neutropenia (40% grade 4) Thrombocytopenia (14% grade 4) Infection (14% grade 3) |
| SYK | Upstream amplification of BCR signaling, binds ITAM |
Fostamatinib (R406) |
CLL 55%, FL 10%, MCL 11%, DLBCL 22% [13], |
Diarrhea (54% grade 1/2) Neutropenia (25% ≥ grade 3) Thrombocytopenia (25% grade 1/2) Febrile neutropenia (8% ≥ grade 3) |
| PI3Kδ | Intermediary in BCR pathway, essential for antigen driven signaling, also in tonic signaling |
Idelalisib (GS- 1101, CAL- 101) |
CLL 26%, FL 62%, MCL 62%, DLBCL 0% [19;169;171] |
Pneumonia (24% ≥ grade 3) Neutropenia (24% ≥ grade 3) Trombocytopenia (7% ≥ grade 3) Febrile neutropenia (7% ≥ grade 3) |
| BTK | Downstream of LYN/SYK in BCR pathway, essential for NF-κB activation |
Ibrutinib (PCI- 32765) |
CLL 71%, MCL 75%, FL 27%, ABC DLBCL 40%, MZL 33%, WM 75% [14;154;162;163;172] |
Neutropenia (12.5% ≥ grade 3) Thrombocytopenia (7.2% ≥ grade 3) Respiratory (7.1% ≥ grade 3) Diarrhea (46.5%) |
| mTOR | Downstream of PI3K |
Everolimus (RAD001) |
CLL18%, MCL 20-32%, DLBCL 20% [68;71 ;72] |
Neutropenia (32% ≥ grade 3) Thrombocytopenia (50% ≥ grade 3) Infections (23% ≥ grade 3) ARDS (5% grade 4, class effect) |
| Temsirolimus (CCI-779) |
CLL 11%, FL 54%, MCL 38% DLBCL 28% [69;70] |
See text for further details and agents in preclinical development. Please note that the OR mentioned are mostly from preliminary clinical trial results. For CLL, higher proportions of OR are seen if PR with lymphocytosis is included.
The BCR signaling pathway
The BCR consists of two immunoglobulin heavy (IGH) and two immunoglobulin light (IGL) chains forming the extracellular, antigen binding part of the BCR (mIg). These are similar to the secreted antibodies of mature plasma cells and encoded by the same gene loci. During B-cell differentiation, genes encoding the IGH variable region (IGHV) and IGL variable region undergo recombination and somatic hypermutation, forming the multitude of distinct BCRs and antibodies that B cells are capable of expressing [21]. The extracellular antigen recognizing domain is complexed with CD79A and CD79B (Igα and Igβ, respectively) that form the cytoplasmic tail of the BCR.
While the intracellular signaling cascades downstream of the BCR are well characterized, the mechanism by which antigen binding triggers the cellular response is still a matter of investigation. In particular, the conformational state of the BCR in resting B cells awaits further elucidation. One model describes the BCRs of resting B cells as inhibited oligomers floating in the plasma membrane that, upon antigen binding, assume an open conformation conducive for signal propagation [22]. In contrast, another model proposes oligomerization upon antigen binding as the initial trigger [23].
Within the cytoplasmic part of the BCR, Immunoreceptor Tyrosine-based Activation Motifs (ITAMs) are critical for intracellular signal generation. ITAMs are highly conserved peptide motifs within CD79A and CD79B of the BCR complex [24]. ITAMs are also found in other immunoreceptors, for example in the CD3 component of the T-cell receptor and in Fc-receptors [25]. Binding of antigen to the extracellular part of the BCR activates upstream kinases that phosphorylate ITAMs, which then serve as docking sites for additional kinases and adaptor molecules. SRC family kinases, in particular LYN and SYK, are important for these initial steps of BCR activation [23].
LYN
Initiation of BCR signaling through phosphorylation of ITAMs on the cytoplasmic part of CD79A and CD79B involves LYN (see figure 1). LYN and two other SRC family kinases (FYN and BLK) are essential for BCR mediated pro-survival signaling during early B-cell development, with some redundancy between the three kinases [26]. LYN directly phosphorylates SYK that is essential for further signal propagation. At the same time, LYN activates phosphatases that in turn inhibit signal transduction through the BCR [2]. Through this dual mode of action, LYN both activates and terminates BCR signaling. LYN deficient mice have reduced numbers of B cells that are less responsive to antigenic BCR activation. However, these mice eventually develop a lupus-like autoimmune disease due to the accumulation of self-reactive IgM. Thus, LYN plays an essential role in downregulating BCR activation and limiting the expansion of autoreactive B cells [27;28]. LYN can be inhibited by dasatinib (approved for the treatment of chronic myeloid leukemia(CML)) that also targets a number of other kinases including BTK [29;30]. In a subgroup of CLL patients, LYN has been shown to phosphorylate and thereby activate hematopoietic cell-specific Lyn substrate-1 (HS1). HS1 is a cytoskeletal interactor in the BCR pathway and its phosphorylation has been correlated with poor outcome. In mice transplanted with splenocytes from the EμTCL1 transgenic model, dasatinib was effective in inhibiting the expansion of the tumor cells [31]. Due to the different kinases inhibited by dasatinib, its activity in clinical trials of CLL and against ABC-DLBCL cell lines in vitro may or may not be due to LYN inhibition [32;33]. Thus, validation of the clinical significance of targeting LYN will require further study.
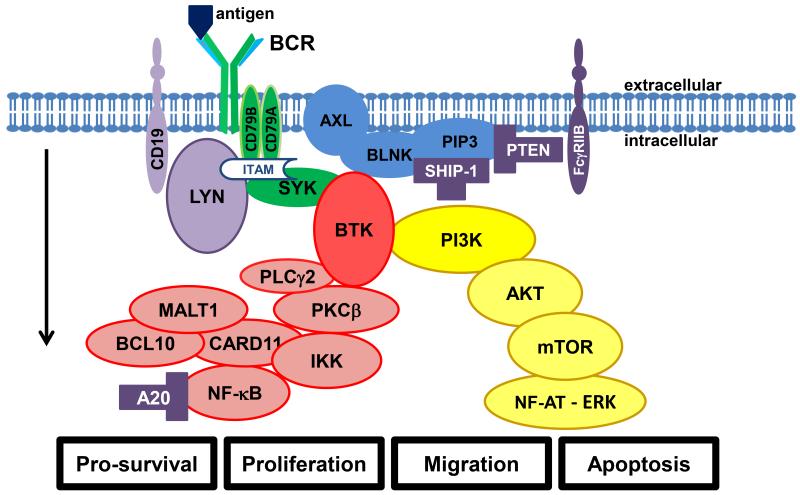
Fig. 1.
The B-cell receptor (BCR) and its downstream pathways. The arrow indicates direction of signaling from plasmamembrane towards effectors. Antigen binding or cell autologous interaction activates BCR, resulting in phosphorylation of ITAMs in the cytoplasmic domains of CD79A and CD79B. SYK amplifies the initial signal by autophosphorylation and further phosphorylation of ITAMs (the initial amplifying complex is marked in green). LYN has a double function in initiating and terminating BCR signaling depending on interaction with CD19 (inhibitory molecules marked purple, bifunctional molecules light purple). SYK also activates the PI3K arm of the pathway (marked in yellow). Phosphatidylinositol 4,5-bisphosphate (PIP2) is phosphorylated by PI3K to phosphatidylinositol 3,4,5-triphosphate (PIP3). PIP3, AXL, and BLNK form a signaling hub that recruits the upper part of the BCR pathway to the plasmamembrane. Inhibitory mechanisms include FcγRIIB that inhibits BCR signaling upon binding to immune complexes at the BCR. SHIP-1 and PTEN phosphatases inhibit the PI3K arm of the pathway by hydrolysis of PIP3. AKT and mTOR relay PI3K activation further to downstream targets and cell cycle regulation. The BTK arm of the pathway (marked in red) is initiated by recruitment of BTK to the plasmamembrane signaling hub. PLCγ2 is activated downstream of BTK, leading to subsequent activation of PKCβ. PKCβ phosphorylates IKK to activate NF-κB transcription factors that regulate gene expression of several survival factors. The complex of CARD11, MALT1, and BCL10 is an important part of the pathway activating NF-κB whereas A20 is a negative regulator of NF-κB. The downstream effectors can be modulated towards the pro-apoptotic NF-AT – ERK arm or the pro-survival NF-κB arm depending on balancing of the signaling cascades. Please see the sections on specific parts of the signaling pathways and activation in B-cell malignancies for further details.
SYK
SYK is a non-receptor tyrosine kinase essential for BCR signaling [34]. It is closely related to ZAP70, which is essential for T-cell receptor signaling, and whose expression in CLL cells is an adverse prognostic marker indicating more rapid disease progression [35]. Mice with a genetic deletion of SYK have a severe impairment of B-cell development at the pro-B cell to pre-B cell transition and lack mature B cells [36;37]. SYK binds directly to the phosphorylated ITAMs of CD79A/B via tandem SH2 domains. Binding of two phosphorylated ITAMs is necessary for maximal activation of SYK through autophosphorylation [38]. In addition, SYK can be phosphorylated by activated LYN. Thus, a SYK dependent amplification of the initial BCR signal promotes the activation of downstream signaling cascades (see figure 1, and recent reviews [34;39]).
The therapeutic value of SYK inhibition has been evaluated in a phase I/II study with fostamatinib that included patients with different B-cell malignancies [13]. The response rate in CLL was the highest (55%), ahead of DLBCL (22%), MCL (11%), and FL (10%). Confirming the drug’s on target effects, inhibition of BCR signaling, and a decrease in cellular activation and proliferation was demonstrated in tumor cells of CLL patients treated with fostamatinib [40]. A study of fostamatinib in DLBCL and late stage clinical trials in rheumatoid arthritis are ongoing [41]. Fostamatinib also inhibits FLT3 and JAK1, which could result in clinical effects due to inhibition of multiple signaling pathways. Preclinical data on two other SYK inhibitors (PRT318 and P505-15) that seem to be more selective have recently been published [42].
PI3K (phosphatidylinositol 3 kinase)
PI3Ks are composed of a regulatory p85 subunit that binds via SH2 domains to phosphorylated tyrosine motifs, for example in the ITAMs, and a catalytic p110 subunit that phosphorylates phosphatidylinositol substrates, thus generating PIP3. The PI3Kδ isoform that is primarily expressed in leukocytes, and the ubiquitously expressed PI3Kα isoform are essential for B-cell development (both are class 1a PI3Ks that can generate PIP3, see So et al. for a recent review [43]). Mice lacking PI3Kα and δ show severe defects in B-cell development [44]. The importance of the PI3K pathway downstream of the BCR is further evidenced by the observation that resting B cells lacking BCR expression can be rescued from apoptosis by constitutively active PI3Kα [45;46]. Whether signaling through PI3Kδ vs. PI3Kα elicits qualitative or only quantitatively different responses remains to be elucidated [43].
PIP3 is a pivotal scaffold in the plasma membrane that recruits key components of the functional signaling complex downstream of the BCR including BTK, PLCγ2, and AKT (see figure 1). Hence, a hub for the initial phase of BCR signaling is formed at the plasmamembrane that in addition to PIP3 involves AXL and BLNK [47]. AXL is a receptor tyrosine kinase recently identified to be constitutively phosphorylated in CLL microvesicles [48]. AXL forms a complex with several molecules of the BCR pathway including LYN, SYK, PI3K, and PLCγ2 [49]. Similarly, BLNK (SLP65) functions as a multivalent adaptor molecule that also interacts with numerous components of the pathway including LYN, SYK, BTK, and PLCγ2 [50]. BLNK depends on interactions with CIN85 for plasma membrane translocation and the transmembrane protein CMTM7 for recruitment to the IgM class BCR [51]. Lack of any of these molecules due to mutations or inhibition seems to impair both B-cell development and BCR signaling. These molecules are thus potential candidates for the development of novel BCR inhibitors. While BTK (see below) can be tyrosine phosphorylated in the absence of PI3Kδ, downstream effects such as calcium mobilization and cell proliferation are severely impaired in the absence of PI3K [52]. Furthermore, the PI3K pathway regulates migration of B-cells, thus pointing towards an important role in the interaction of CLL cells with the microenvironment [43;44]. The significant preclinical and clinical results of PI3K inhibition in different B-cell malignancies are detailed in a later section.
BTK (Bruton’s Tyrosine Kinase)
BTK is a member of the TEC kinase family that also includes TEC (B-cells/T-cells/liver cells), ITK (IL2 inducible T-cell kinase), and BMX/ETK (bone marrow, endothelia, epithelia). Loss of BTK causes X-linked agammagloubulinaemia with absence of mature peripheral B cells and low serum immunoglobulin levels [53;54]. BTK is a non-receptor tyrosine kinase recruited early in the BCR signaling cascade in conjunction with SYK and PI3Kδ [55]. Upon activation of the BCR pathway, BTK attaches to the plasma membrane through its pleckstrin homology domain that binds to PIP3 [56]. BTK appears to be essential only in B cells and is required for BCR-induced calcium release, cell proliferation, and activation of the NF-κB pathway (see figure 1) [57;58]. BTK regulates actin dynamics and antigen processing during BCR activation [59]. An important downstream target of BTK is PKCβ, which in turn phosphorylates IκB kinase (IKK) resulting in release and translocation of NF-κB transcription factors to the nucleus [60]. BTK is also involved in B-cell trafficking mediated by the chemokine receptors CXCR4 and CXCR5 [61]. Results from clinical trials of BTK inhibitors in a range of B-cell malignancies are summarized in a later section.
mTOR (mammalian Target of Rapamycin)
mTOR is an ubiquitously expressed serine/threonine kinase. It is a downstream mediator of BCR signaling (through PI3K/AKT, see figure 1) as well as a cell cycle regulator at the transition from G1 to S phase [62;63]. Rapamycin (sirolimus, used as an immunosuppressant in organ transplants, isolated from Streptomyces hygroscopicus), was initially identified as a fungicide and later as an anti-tumor substance [64]. mTOR was identified as the primary target of rapamycin [65] and mTOR inhibition has effects on multiple cell cycle regulators [66]). The mTOR inhibitors everolimus and temsirolimus (indicated for treatment of some solid tumors), which have improved stability and oral availability compared to rapamycin, have been evaluated in FL, MCL, DLBCL, and CLL [67-72]. The overall response rate (OR) has been between 20 and 40%. While these agents have been tolerable, the immunosuppressive effect and especially the high frequency of respiratory infections have been a concern [70;72].
Inhibitory regulators of the BCR pathway and anergy
In addition to the dual role of LYN, which both promotes and inhibits BCR signaling (as outlined above) there are several additional mechanisms to limit and/or turn off BCR signaling. One inhibitory molecule is Fcγ receptor IIB (FcγRIIB). Upon antigen binding to the BCR, the immunoglobulin constant region is bound to FcγRIIB that inhibits further activation. The inhibitory effect of activated FcγRIIB is in part mediated by interaction with LYN and inhibition of BCR oligomer formation early in the signaling pathway [73].
Internalization of the BCR is another mechanism to dampen antigen-dependent activation of B cells. Repeat antigen stimulation results in decreased surface IgM expression and consequently reduced BCR signaling. BTK interacts with the actin cytoskeleton during BCR signaling causing both internalization of the BCR and migration of B cells [59]. Increased turnover of BCRs upon repeat antigen activation results in preferential expression of surface IgM molecules that are not fully N-glycosylated [2]. Recent findings demonstrate that BCR internalization is indeed involved in fine tuning the response to antigen. Inhibition of BCR internalization was shown to result in dysregulated gene expression due to hyperphosphorylation of kinases.[74]
Chronic BCR activation promotes anergy of B-cells characterized by the monophosphorylation of ITAMs and loss of SYK activation [75;76]. Phosphorylation and thereby activation of the phosphatase Src homology-2-containing inositol 5-phosphatase (SHIP-1) also contributes to anergy. SHIP-1 hydrolyses PIP3, thus inhibiting signaling through the PI3K arm and presumably the BTK arm of the BCR pathway (see figure 1). Furthermore, activation of PTEN can also contribute to turning off the PI3K arm of the BCR pathway [75]. For a recent review of molecular mechanisms of anergy, see Yarkoni [77].
Downstream from BTK towards NF-κB in the BCR pathway
PKCβ (Protein Kinase C β) functions downstream of BTK in the BCR pathway relaying signals further downstream through IKK to activation of NF-κB transcription factors. PKCβ has been targeted in preclinical and clinical trials for different B-cell malignancies by enzastaurin. However, the clinical results have been disappointing and no further clinical trials with this agent are expected [78]. Nevertheless, clinical and preclinical lessons from targeting PKCβ may prove helpful in further exploring the BCR pathway and guide combination therapy approaches [79]. Studies in mice demonstrate that CLL development in the murine TCL1 model is dependent on PKCβ expression [80]. However, whether PKCβ expression is required in B cells or stromal cells or both was not addressed. More, recently, an extension of the initial study demonstrated that disruption of PKCβ in stromal cells was sufficient to inhibit CLL cell survival in the murine TCL1 model [81].
As outlined in the next section and illustrated in figure 1, CARD11, BCL10, and MALT1 form a complex downstream of PKCβ that propagates signaling towards the NF-κB effectors [12;82;83] and plays a prominent role in B-cell malignancies. IKK is acting in conjunction with this complex just upstream of NF-κB. However, despite strong preclinical rationale for targeting IKK, only preclinical results with no successful translation into clinical studies have been reported [84].
NF-κB is constitutively activated in many B-cell malignancies. In part this may be due to NF-κB being a major downstream effector in the BCR pathway that conveys pro-survival and proliferation signaling [2;85]. NF-κB upregulates anti-apoptotic BCL2-family members [60], whose function is now also directly targeted with specific antagonists in clinical trials [86]. PBS-1086, a pan-Rel inhibitor that decreases DNA binding of subunits in both the canonical (p65, p50, Rel-C) and non-canonical (p52, Rel-B) NF-κB pathway was recently shown in vitro and in xenograft models of multiple myeloma to inhibit NF-κB activity. Apoptosis was induced in the models and synergism with bortezomib was demonstrated, in vitro effect was also demonstrated in CLL cells [85;87]. However, due to the ubiquitous role of NF-κB signaling, targeting this pathway directly could result in unwanted effects in other organ systems.
Antigen driven BCR activation in B-cell malignancies
Among the different types of B-cell malignancies, several different tactics are applied for activation of the BCR pathway. Even though they convey on the same pathway, understanding where the pathway is modulated helps unravel which B-cell malignancies would likely respond to which kind of targeted therapies.
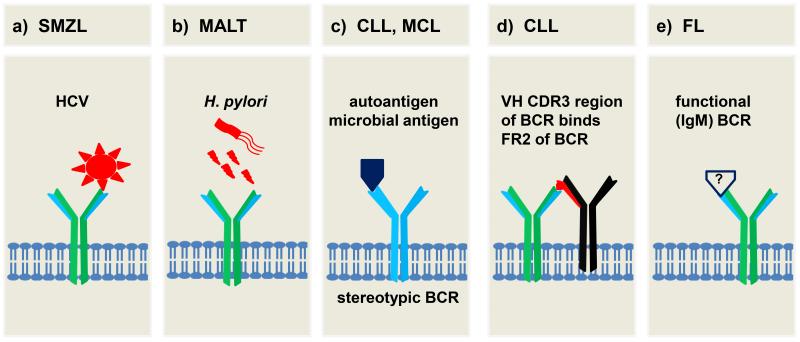
Evidence for a causal relationship between HCV infection, activation of the BCR by viral antigen, and lymphomagenesis is found in a subset of SMZL. Some of these lymphomas express a BCR that binds the E2 envelope protein of HCV (see figure 2a) [6;88], suggesting that a subgroup of these lymphomas arise as an expansion of HCV-reactive B cells. Consistently, antiviral treatment results in complete responses (CR) in about 75% of HCV positive non-Hodgkin lymphoma (NHL) patients, whereas no responses are seen in HCV negative NHL patients [89]. Thus, antigen-dependent BCR activation appears to be the driver of lymphomagenesis for some SMZL cases; and removal of the antigen can lead to clinical remissions in these patients.
Fig. 2.
Different mechanisms of BCR activation by antigen in B-cell malignancies. a) A HCV epitope may activate the BCR in SMZL. b) Helicobacter pylori infection causing antigenic and/or inflammatory drive in gastric MALT lymphoma. c) Stereotypic BCRs in CLL and MCL that recognize autoantigens or common microbial antigens provide an antigenic drive. d) BCR of CLL cells binds to epitope within the same or adjacent BCR, resulting in autostimulation. e) The conservation of a functional BCR in FL despite a translocation to the IGH locus is suggestive of a role for BCR activation. Indeed, a fourth of FL specimens have BCRs that recognize self-antigen. Please see text for further details.
Chronic exposure to microbial antigens has been implicated in the pathogenesis of other types of B-cell malignancies as well (figure 2b). For gastric mucosa associated lymphoid tissue (MALT) lymphomas, a clear association with Helicobacter pylori infection has been established. At least in early cases, remission of the lymphoma can be achieved by eradication of the helicobacter infection alone [90]. However, a direct link to the BCR pathway has not been demonstrated, and inflammation as well as direct antigenic drive may contribute to lymphomagenesis. Furthermore, CD4+ Th2 cells and follicular dendritic cells have been shown to be important for tumor formation in a murine model of Helicobacter pylori driven MALT lymphoma [91]. Thus, different pathways seem to contribute to lymphomagenesis in this case [90].
B-cell maturation involves somatic recombination and mutation of the IGHV genes that encode the antigen binding domains of the BCR. One of the first indications that antigen selection may play a role in the pathogenesis of B-cell malignancies was provided by observations that CLL cells use a restricted repertoire of IGHV genes, [92;93]. Furthermore, some cases express virtually identical BCRs, so called “stereotyped BCRs” that are predicted to recognize distinct antigens [94;95]. Furthermore, clinical disease progression is variable depending on whether the expressed IGHV gene has undergone somatic hypermutation (M-CLL) or not (U-CLL). [96] The U-CLL subtype is more rapidly progressive and often expresses ZAP-70, a paralogue of SYK that has been shown to enhance BCR signaling in vitro and that may contribute to an increased responsiveness of the U-CLL subtype to BCR activation [97-99].
An increasing number of antigens bound by the BCR on CLL cells have been identified (figure 2c) including autoantigens expressed on dying cells [100;101] as well as viral [102], bacterial [101], and fungal antigens [103]. Comparing purified CLL cells isolated concomitantly from the peripheral blood, bone marrow, and lymph node of patients; we recently showed that CLL cells in the lymph node contain increased levels of activated SYK and express genes upregulated in response to BCR activation. This indicates that antigenic signaling continues throughout the disease course and that the BCR is engaged primarily in the lymph node [99]. While both CLL subtypes showed evidence of antigen signaling in vivo, the more progressive U-CLL subtype had stronger BCR activation, higher expression of MYC and increased proliferation compared to M-CLL suggesting that BCR signaling may contribute to the clinical progression of CLL. Consistent with chronic antigen contact in vivo is the observation of a reversible down-modulation of surface IgM expression on CLL cells and the resemblance of these cells to anergic B-cells [104;105].
Recently, it has been shown that the BCR of many CLL cells recognizes an epitope that is part of the CLL BCR itself (figure 2d) leading to autostimulation on a single cell level [106]. In vivo, a number of additional elements may shape the cellular response, including the degree to which a BCR can react with multiple antigens (polyreactive BCRs are associated with more aggressive disease [107]), the strength of the intracellular response to antigenic activation (intracellular responses are stronger in disease subsets with inferior outcome [97;99;108]), the availability of co-stimulatory signals in the tissue microenvironment (BCR signaling is stronger in CLL cells in the lymph node than in the peripheral blood [99]) and the extent to which the cells have been anergized by chronic stimulation (resulting in a dampened response to BCR activation [104;105]. In summary, several lines of evidence indicate that BCR signaling plays a pivotal role in the pathogenesis of CLL, and it recently has become the target of some of the most promising therapeutic advances in this disease [4;109-113].
Initial antigen selection during transformation or continued antigenic drive appears to play a role in additional B-cell malignancies, including MCL [114;115], FL [8], WM [116], and HCL [117;118]. In MCL, similar to what has been described in CLL, there is a bias towards usage of certain IGHV genes and expression of stereotyped receptors [114], albeit often with usage of IGHV genes distinct from those used in CLL [119]. While for about 25% of FL self-reactivity has been shown (figure 2e) [8], a more common mechanism of BCR activation may be crosslinking of the BCR by mannose-binding lectins in the tissue microenvironment due to increased N-glycosylation [120].
Combining extrinsic and intrinsic mechanisms of BCR activation in B-cell malignancies
While in most cases of B-cell malignancies specific disease promoting antigens have remained elusive, it is remarkable that most mature B-cell malignancies express an IgM type BCR, irrespective of pre- or post-germinal center origin. Given that IgM signaling promotes primarily NF-κB activation and cell proliferation, while IgG signaling promotes plasmacytic differentiation, a plausible explanation for this observation is that these malignancies derive a selection bias from antigen-dependent BCR signaling [121]. In ABC-DLBCL, acquired mutations in the IGH switch regions prevent isotype switching and thereby maintain the expression of an IgM class BCR on the tumor cells [122]. Further evidence for a selective pressure towards retaining a functional IgM type BCR is exemplified by FL, which is characterized by the chromosomal translocations t(14;18). In addition to the translocated allele that leads to overexpression of BCL-2, these cells have a productively rearranged IgH locus that encodes the IgM BCR expressed on the cell surface [123-125]. Thus, there appears to be a strong selective pressure for these malignant B-cells to maintain a functional IgM-type BCR in addition to intrinsic genetic abnormalities.
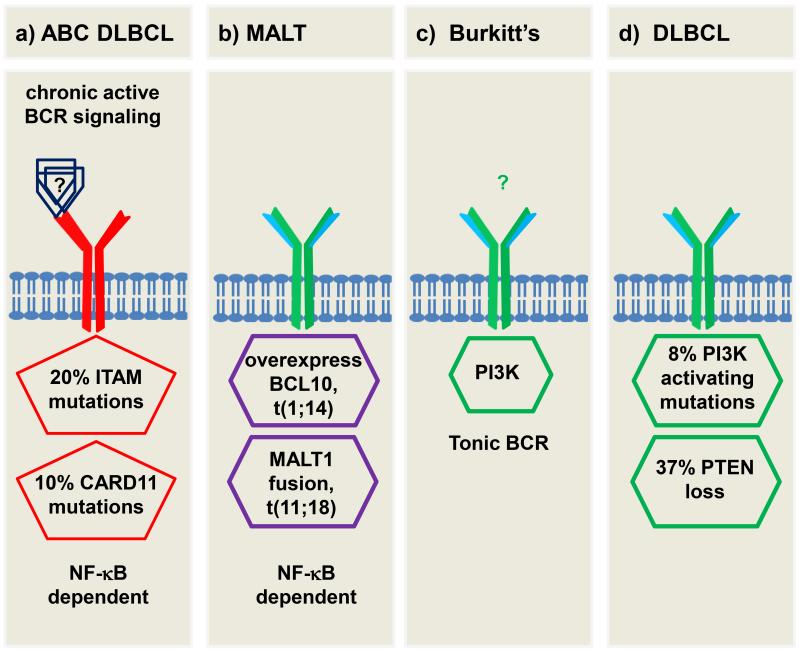
Activation of BCR signaling through a combination of extrinsic signaling, by antigenic drive, and intrinsic signaling, due to acquired mutations, is observed in a subset of ABC DLBCLs. This lymphoma is characterized by constitutive activation of the NF-κB pathway. Using an RNAi screen it was found that most ABC-DLBCL cell lines depend on expression of a functional BCR and genetic knockdown of BCR components (IGH, CD79A, CD79B) or BCR signal transduction components (SYK, PI3Kδ, BTK) effectively killed these cells [32]. This dependency on activation of the BCR pathway with or without extrinsic antigenic involvement in ABC DLBCL has been called “chronic active BCR signaling” (figure 3a). About a fifth of primary ABC DLBCL cases and several cell lines derived from this lymphoma carry a mutation of a critical tyrosine residue in the ITAM of CD79B. Interestingly, these mutations are not sufficient to initiate BCR activation but rather increase the signaling response by preventing BCR internalization and by interfering with activation of LYN. In this subset of ABC DLBCL cells, PI3K and BTK signaling remain essential for NF-κB activation [126]. In addition to CD79B mutations, activating mutations of CARD11, a key scaffolding protein that connects BCR activation to NF-κB signaling, have been identified in about a tenth of ABC DLBCL cases. This mutation is sufficient to intrinsically activate survival signaling in the malignant B cells and obviates the need for upstream BCR signaling in a subset of ABC DLBCL [12]. Also, loss of function mutations in a negative regulator of NF-κB, the tumor suppressor A20, contributes to NF-κB pro-survival signaling in 24% of ABC DLBCL cases [127].
Fig. 3.
Extrinsic and intrinsic perturbations of BCR signaling in B-cell malignancies a) Chronic active signaling in ABC subtype DLBCL, CARD11 mutations confer BCR independency. b) In MALT lymphomas, overexpression of BCL10 or MALT1 fusion protein results in activation of NF-κB driven proliferation. c) Burkitt’s lymphoma depending on PI3K activity that resembles tonic signaling in resting normal B cells. d) In subsets of DLBCLs, activating mutations of PI3K or loss of inhibitory PTEN induce PI3K driven proliferation. Please see text for further details.
Another example of a transition from a dependence on extrinsic BCR activation to intrinsic activation has been well described in MALT lymphomas, which, as described above, likely arise in the setting of chronic antigen stimulation and, may be cured by elimination of the infectious agent. However, in more advanced cases recurrent chromosomal translocations are observed; t(11;18) results in a fusion transcript of API2-MALT1 and t(1;14) leads to overexpression of BCL10 under the control of the IGH locus [128-130]. MALT1, a paracaspase, BCL10, and CARD11 form a complex that recruits IKKβ to activate the classical NF-κB pathway. The MALT1 fusion protein and BCL10 overexpression can activate NF-κB independent of upstream BCR signaling (figure 3b). [82;173] Consequently, the presence of these translocations, or overexpression of nuclear BCL10 by immunohistochemistry identifies a subset of MALT lymphomas that often fail to regress after eradication of the underlying infection [174; 175].
While most cases of B-cell malignancies discussed so far depend on antigenic activation of the BCR resulting in activation of the NF-κB pathway, tonic survival signaling through PI3K may play a role in Burkitt’s lymphoma (BL). The hallmark of BL is a translocation of MYC to the IGH locus. However, MYC has strong pro-apoptotic effects and requires activation of pro-survival signaling through the PI3K pathway. In BL activation of PI3K resembles the tonic signaling in normal resting B cells (figure 3c). [131;132] Consistently, BL cells are sensitive to genetic knockdown of CD79A or SYK and pharmacologic inhibition of PI3K but are not affected by knockdown of BTK [131].
Disinhibition or direct activation of the PI3K arm of the BCR pathway is also implicated in some B-cell malignancies. In a murine model, it is shown that concomitant deletion of SHIP-1 and PTEN (both inhibitors of the PI3K arm of the BCR pathway) induces lethal B-cell malignancies resembling marginal zone lymphoma or follicular lymphoma [133]. In a study of DLBCL specimens, 37% of the samples showed reduction or lack of PTEN whereas 8% of the samples showed activating mutations in the PIK3CA domain of PI3K [134]. In another study, loss of PTEN was solely seen in the GCB subtype of DLBCL (11% of the GCB samples) [135]. In a Hodgkin lymphoma cell line, deletion of one of the SH2 domains of PI3K that results in PI3K activation has also been identified [136]. Thereby, GCB DLBCL that is independent of BCR signaling still seems to depend upon intrinsic activation of the PI3K pathway (figure 3d). It is important to mention that the PIK3CA mutations are not inhibited by idelalisib, which is specific for PI3Kδ (see below). Thus, PI3Kα or pan PI3K inhibitors may be of importance for some B-cell malignancies [43].
BCR pathway inhibitors: PI3K targeting agents
The PI3Kδ isoform specific kinase inhibitor Idelalisib (GS-1101, CAL-101) has shown clinical efficacy in B-cell malignancies, particularly for CLL patients. In vitro, it decreases AKT and ERK phosphorylation downstream in the BCR pathway and induces apoptosis in ALL, MCL, and CLL primary cells as well as in MCL, FL, and DLBCL cell lines [137;138]. In addition to direct effects in the BCR pathway, idelalisib inhibits microenvironmental signals through CD40L, BAFF, TNFα, and fibronectin [139;140]. Moreover, idelalisib inhibits secretion of cytokines and chemokines in a dose dependent manner. In vivo, CCL3 and CCL4 that are upregulated in CLL cells in a BCR dependent manner, show a rapid decrease in CLL patients treated with idelalisib [140-142]. T cell viability is not affected by idelalisib, although T cell secretion of some inflammatory and anti-apoptotic cytokines seems to be inhibited [139]. Thus, T cell modulation may also contribute to the effect of idelalisib against B-cell malignancies.
Safety and activity of idelalisib in hematologic malignancies were evaluated in a phase I study enrolling fifty four patients with CLL. The OR by IWCLL criteria [143] was 26% [19;144]. However, 80% of patients had a reduction in lymphadenopathy by ≥50%. Many of these patients did not meet criteria for response by IWCLL criteria due to a transient increase in the absolute lymphocyte count. Initial lymphocytosis has been seen with several targeted therapies for CLL in otherwise responding patients without any sign of progressive disease [13;17;18]. As discussed recently by Cheson et al., the peripheral lymphocytosis seen with most targeted drugs in CLL may warrant amendment of the response criteria for CLL [145]. Progression free survival (PFS) was not reached at >11 months and responses were independent of classic risk factors, including responses in patients with 17p deletion. Grade ≥3 adverse events included pneumonia (24%), neutropenia (24%), thrombocytopenia (7%), neutropenic fever (7%), anemia (6%), and increased liver enzymes (6%). Studies in which idelalisib is combined with bendamustine and/or rituximab, fludarabine, ofatumumab, chlorambucil, and chlorambucil + rituximab maintenance are currently ongoing. Preliminary results from some of these studies include OR up to 87% and 1 year PFS rates up to 88% [146; 147]. No limiting safety concerns have hitherto been identified for any of the combinations.
Several PI3K inhibitors with different isotype specificities are in pre-clinical and early clinical studies in hematologic malignancies. Isotype specific PI3K inhibitors seem to confer different effects, i.e. the PI3Kα inhibitors PIK-90 and PI-103 are more effective than PI3Kδ or PI3Kβ/δ specific inhibitors at inhibiting CLL cell migration to CXCL12 and in antagonizing stromal cell mediated survival signals [148]. Rigosertib, a PI3Kα/β inhibitor in phase III studies for myelodysplastic syndrome, induces apoptosis in CLL cells cultured in contact with stromal cells [149]. A pan-PI3K inhibitor (NVP-BKM120) is cytotoxic for Burkitt’s lymphoma cell lines that depend on pro-survival PI3K activation [131]. SAR245408 is another pan PI3K inhibitor that is well tolerated in patients with solid tumors [5;150]. In addition to idelalisib, other PI3Kδ specific inhibitors are in preclinical and early clinical trials in CLL, including IPI145 (ongoing phase I), PWT143, and TGR1202 [151;152]. As mentioned previously, a mutant constitutively active PIK3CA subunit that could confer resistance to idelalisib was identified in a subset of ABC DLBCL [134]. Thus, results from clinical trials of PI3Kα-specific or pan-PI3K inhibitors are awaited for these conditions. Taken together, isotype specific PI3K inhibitors may be attractive for treatment of B-cell malignancies. A caveat with highly selective inhibitors may by that activation or upregulation of other PI3K isoforms that are not targeted may give rise to drug resistance [153].
BCR pathway inhibitors: BTK targeting agents
The BTK inhibitor Ibrutinib (PCI-32765) is a BCR inhibitor with very promising results, especially in MCL [154], WM, the ABC subset of DLBCL [163], and CLL [18]. It binds covalently to the cysteine Cys-481 of BTK and thereby irreversibly inactivates the kinase [57;155]. In addition to blocking BCR signaling and integrin-mediated adhesion, migration of primary CLL cells to CXCL12, CXCL13, and CCL19 is inhibited by ibrutinib in vitro [156]. The on-target effect is reflected in the downregulation of BCR regulated genes and by decreased NF-κB activity in tumor cells from both peripheral blood and lymph nodes of CLL patients treated with ibrutinib [157]. Herman et al. show that ibrutinib not only inhibits BCR signaling but also disrupts the protective effect of stromal cells, and inhibits CD40, BAFF, TLR, and cytokine signaling [158]. Furthermore, ibrutinib inhibits adhesion to stromal elements such as fibronectin and VCAM1 [159]. ABC DLBCL cell lines that depend on constitutive active BCR signaling can be killed by genetic knock down of BTK as well as by ibrutinib treatment [32]. Primary ALL and HCL cells/cell lines also show decreased proliferation and increased apoptosis upon ibrutinib treatment [160;161]. Studies in a mouse model of autoimmune disease demonstrates reduction of circulating autoantibodies and objective clinical responses have been described in dogs with spontaneous non-Hodgkin lymphoma [58]. Inhibition of BCR signaling with no effect on T-cell receptor signaling has been demonstrated in these models.
In the first clinical trial reported with ibrutinib, an OR of 54% across different B-cell malignancies was reported, 7/9 for MCL, 11/16 for CLL, 6/16 for FL (later updated with response in 11/16 patients), 2/7 for DLBCL (no data on subtype), and 3/4 for WM [14;162]. Another study for DLBCL patients showed responses for ABC but not GCB subtypes in agreement with the preclinical data on the importance of BTK signaling preferentially in ABC DLBCL (two partial responses (PR) and one CR out of 10 patients) [163]. More recently, OR of 71% for treatment naïve CLL patients, 67% for relapsed or refractory patients, and 50% for high risk patients has been reported [18]. If PR with lymphocytosis is included according to the proposed amendment to CLL response criteria [145], the response rates increase to 81%, 87%, and 79% respectively. The estimated PFS at 26 months was 75% for the relapsed/refractory cohort and 96% for treatment naïve patients, demonstrating a remarkable duration of response with single agent therapy. In addition, patients with high risk CLL, including those with del17p, benefit from ibrutinib treatment with rapid and apparently persistent disease control [164]. Preliminary results from combination therapy with ibrutinib and rituximab, ofatumumab, or bendamustine in high risk CLL patients show OR rates approximating 100%. Adverse events are reported to be manageable [165-167]. A recent report from a phase II study in relapsed MCL (n=111, 48 previously bortezomib treated) showed OR of 68% and CR of 21%; after a median of 15.3 months follow up the estimated PFS was 13.9 months [154].
Another selective, orally available BTK inhibitor, AVL-292, has been tested in early clinical trials. Preliminary data showed stable disease in 8 of 8 CLL patients with median decrease in lymph node size of 28% and initial augmented peripheral blood lymphocytosis in most patients [168]. Less effect of AVL-292 compared to ibrutinib may in part be due to differences in kinase specificity, in part due to differences in pharmacokinetics. Preliminary data indicates higher OR in patient groups treated with higher doses or twice daily dosing [169]. Several other BTK inhibitors are in preclinical testing (GDC-0834, LFM-A13, AVL-101) [170] with no clinical studies registered at clinicaltrials.gov.
Conclusion
The BCR pathway has emerged as the driving pathway of lymphoma development and evolution in many mature B-cell malignancies. In contrast to the homogenous, monogenetic disease entity of CML, where the development of tyrosine kinase inhibitors for the BCR-ABL fusion protein of the Philadelphia chromosome t(9;22) has been a turning point in the management of the disease, the genetic background of these B-cell malignancies is heterogeneous and may pose multiple challenges for the successful translation of targeted therapy. This heterogeneity non-withstanding, BCR signaling appears to be a “hub” of such importance that successful translation of targeted approaches to many of these diseases appears within reach.
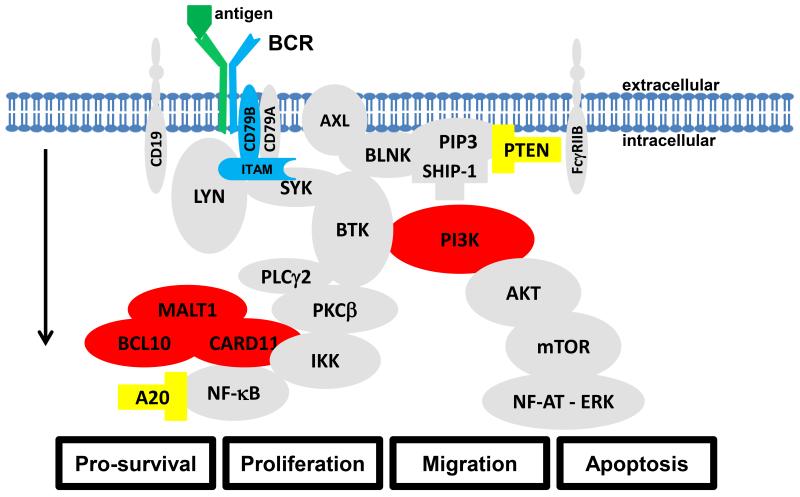
The different mechanisms of BCR activation in different B-cell malignancies testify to the proto-oncogenic nature of this pathway, which regulates proliferation, survival, and differentiation of B-cells. Different aspects of BCR activation may be viewed as stages in a multistep process that increasingly corrupt the normal function of the pathway. Chronic antigenic drive as seen in SMZL, CLL, and other lymphomas may be part of a first stage in the development of an antigen-dependent malignancy (Figure 4, in green), whereas the acquisition of mutations in CD79B that enhance chronic active BCR signaling in ABC DLBCL may be viewed as a second event (Figure 4, in blue). Gain of function mutations in downstream BCR components (Figure 4, in red) and/or loss-of-function mutations in pathway inhibitors (Figure 4, in yellow) then represent further hits that may promote disease entities with antigen/BCR independent proliferation and pro-survival signaling. While such a multistep process in the development of the different entities is hypothetical, it may serve well to stratify treatment approaches. Entities strongly dependent on extrinsic antigenic signaling appear to be sensitive to pathway inhibitors targeting PI3K and BTK, while an entity such as BL, that depends on tonic signaling, is not sensitive to BTK inhibitors, and lymphomas that have activating mutations in downstream signaling molecules, such as ABC-DLBCL with CARD11 mutations are resistant to both BTK and PI3K inhibitors.
Fig. 4.
Multiple event model for B-cell lymphomagenesis. The BCR pathway as depicted in figure 1. Antigenic drive is considered as an initiating event (green). Mutations within the CD79B part of the BCR that increase the response to antigenic drive (blue). Activating mutations (red) and loss of function mutations (yellow) in downstream signal transduction components may confer antigen independent pro-survival signaling and are considered tertiary events. Please see text and figure 2-3 for details on which events are implicated in which types of B-cell malignancies.
For many patients, BCR pathway inhibitors are rapidly becoming the preferred option for treatment. In recognition of this, the FDA has granted breakthrough designation to ibrutinib for patients with WM, MCL, and CLL with 17p deletion. Challenges will be to individualize treatment goals and approaches, to define optimal combination therapies, and to integrate molecular characterization for response prediction.
Acknowledgments
CUN is supported by the Danish Cancer Society and AW is supported by the intramural research program of the Heart, Lung, Blood Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat.Rev.Drug Discov. 2013 Mar;12(3):229–43. doi: 10.1038/nrd3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevenson FK, Krysov S, Davies AJ, Steele AJ, Packham G. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2011 Oct 20;118(16):4313–20. doi: 10.1182/blood-2011-06-338855. [DOI] [PubMed] [Google Scholar]
- 3.Wiestner A. Targeting B-Cell receptor signaling for anticancer therapy: the Bruton’s tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J.Clin.Oncol. 2013 Jan 1;31(1):128–30. doi: 10.1200/JCO.2012.44.4281. [DOI] [PubMed] [Google Scholar]
- 4.Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012 Aug 9;120(6):1175–84. doi: 10.1182/blood-2012-02-362624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davids MS, Brown JR. Targeting the B cell receptor pathway in chronic lymphocytic leukemia. Leuk.Lymphoma. 2012 Dec;53(12):2362–70. doi: 10.3109/10428194.2012.695781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinn ER, Chan CH, Hadlock KG, Foung SK, Flint M, Levy S. The B-cell receptor of a hepatitis C virus (HCV)-associated non-Hodgkin lymphoma binds the viral E2 envelope protein, implicating HCV in lymphomagenesis. Blood. 2001 Dec 15;98(13):3745–9. doi: 10.1182/blood.v98.13.3745. [DOI] [PubMed] [Google Scholar]
- 7.Hermine O, Lefrere F, Bronowicki JP, Mariette X, Jondeau K, Eclache-Saudreau V, Delmas B, Valensi F, Cacoub P, Brechot C, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N.Engl.J.Med. 2002 Jul 11;347(2):89–94. doi: 10.1056/NEJMoa013376. [DOI] [PubMed] [Google Scholar]
- 8.Sachen KL, Strohman MJ, Singletary J, Alizadeh AA, Kattah NH, Lossos C, Mellins ED, Levy S, Levy R. Self-antigen recognition by follicular lymphoma B-cell receptors. Blood. 2012 Nov 15;120(20):4182–90. doi: 10.1182/blood-2012-05-427534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duhren-von MM, Ubelhart R, Schneider D, Wossning T, Bach MP, Buchner M, Hofmann D, Surova E, Follo M, Kohler F, et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 2012 Sep 13;489(7415):309–12. doi: 10.1038/nature11309. [DOI] [PubMed] [Google Scholar]
- 10.Zwick C, Fadle N, Regitz E, Kemele M, Stilgenbauer S, Buhler A, Pfreundschuh M, Preuss KD. Autoantigenic targets of B-cell receptors derived from chronic lymphocytic leukemias bind to and induce proliferation of leukemic cells. Blood. 2013 Jun 6;121(23):4708–17. doi: 10.1182/blood-2012-08-447904. [DOI] [PubMed] [Google Scholar]
- 11.Agathangelidis A, Darzentas N, Hadzidimitriou A, Brochet X, Murray F, Yan XJ, Davis Z, van Gastel-Mol EJ, Tresoldi C, Chu CC, et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood. 2012 May 10;119(19):4467–75. doi: 10.1182/blood-2011-11-393694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW, Dave SS, Zhao H, Xu W, Rosenwald A, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008 Mar 21;319(5870):1676–9. doi: 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- 13.Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, Schaefer-Cutillo J, De VS, Sinha R, Leonard JP, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010 Apr 1;115(13):2578–85. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, Kolibaba KS, Furman RR, Rodriguez S, Chang BY, et al. Bruton Tyrosine Kinase Inhibitor Ibrutinib (PCI-32765) Has Significant Activity in Patients With Relapsed/Refractory B-Cell Malignancies. J.Clin.Oncol. 2012 Oct 22; doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M, Rule SA, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, McGreivy J, et al. Interim Results of an International, Multicenter, Phase 2 Study of Bruton’s Tyrosine Kinase (BTK) Inhibitor, Ibrutinib (PCI-32765), in Relapsed or Refractory Mantle Cell Lymphoma (MCL): Durable Efficacy and Tolerability with Longer Follow-up. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):904. [Google Scholar]
- 16.Staudt LM, Dunleavy K, Buggy JJ, Hedrick E, Lucas N, Pittaluga S, Jhavar S, Schmitz R, Williams M, Lih J, et al. The Bruton’s Tyrosine Kinase (Btk) Inhibitor PCI-32765 Modulates Chronic Active BCR Signaling and Induces Tumor Regression in Relapsed/Refractory ABC DLBCL. ASH Annual Meeting Abstracts. 2011 Nov 18;118(21):2716. [Google Scholar]
- 17.Farooqui M, Aue G, Valdez J, Saba N, Herman SEM, Lipsky A, Bojanowski L, Wells A, Soto S, Liu D, et al. Rapid Decrease in Overall Tumor Burden On Ibrutinib (PCI-32765) in CLL Despite Transient Increase in ALC Indicates a Significant Degree of Treatment Induced Cell Death. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):2899. [Google Scholar]
- 18.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N.Engl.J.Med. 2013 Jul 4;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coutre SE, Byrd JC, Furman RR, Brown JR, Benson DM, Wagner-Johnston ND, Flinn IW, Kahl BS, Spurgeon SEF, Lannutti BJ, et al. Phase I study of CAL-101, an isoform-selective inhibitor of phosphatidylinositol 3-kinase P110d, in patients with previously treated chronic lymphocytic leukemia. ASCO Meeting Abstracts. 2011 Jun 9;29(15_suppl):6631. [Google Scholar]
- 20.Herman SEM, Farooqui M, Bezabhie R, Aue G, Wiestner A. In Vivo Effects of Ibrutinib On BCR Signaling, Tumor Cell Activation and Proliferation in Blood and Tissue-Resident Cells of Chronic Lymphocytic Leukemia Patients. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):185. [Google Scholar]
- 21.Borghesi L, Milcarek C. From B cell to plasma cell: regulation of V(D)J recombination and antibody secretion. Immunol.Res. 2006;36(1-3):27–32. doi: 10.1385/IR:36:1:27. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Reth M. Oligomeric organization of the B-cell antigen receptor on resting cells. Nature. 2010 Sep 23;467(7314):465–9. doi: 10.1038/nature09357. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Meckel T, Tolar P, Sohn HW, Pierce SK. Antigen affinity discrimination is an intrinsic function of the B cell receptor. J.Exp.Med. 2010 May 10;207(5):1095–111. doi: 10.1084/jem.20092123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reth M. Antigen receptor tail clue. Nature. 1989 Mar 30;338(6214):383–4. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- 25.Bezbradica JS, Medzhitov R. Role of ITAM signaling module in signal integration. Curr.Opin.Immunol. 2012 Feb;24(1):58–66. doi: 10.1016/j.coi.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Saijo K, Schmedt C, Su IH, Karasuyama H, Lowell CA, Reth M, Adachi T, Patke A, Santana A, Tarakhovsky A. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat.Immunol. 2003 Mar;4(3):274–9. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]
- 27.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995 Nov;3(5):549–60. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 28.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995 Oct 20;83(2):301–11. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 29.Hantschel O, Rix U, Schmidt U, Burckstummer T, Kneidinger M, Schutze G, Colinge J, Bennett KL, Ellmeier W, Valent P, et al. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc.Natl.Acad.Sci.U.S.A. 2007 Aug 14;104(33):13283–8. doi: 10.1073/pnas.0702654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi H, Zhang CJ, Chen GY, Yao SQ. Cell-based proteome profiling of potential dasatinib targets by use of affinity-based probes. J.Am.Chem.Soc. 2012 Feb 15;134(6):3001–14. doi: 10.1021/ja208518u. [DOI] [PubMed] [Google Scholar]
- 31.Ten HE, Scielzo C, Bertilaccio MT, Scarfo L, Apollonio B, Barbaglio F, Stamatopoulos K, Ponzoni M, Ghia P, Caligaris-Cappio F. Targeting the LYN/HS1 signaling axis in chronic lymphocytic leukemia. Blood. 2013 Mar 21;121(12):2264–73. doi: 10.1182/blood-2012-09-457119. [DOI] [PubMed] [Google Scholar]
- 32.Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010 Jan 7;463(7277):88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amrein PC, Attar EC, Takvorian T, Hochberg EP, Ballen KK, Leahy KM, Fisher DC, Lacasce AS, Jacobsen ED, Armand P, et al. Phase II study of dasatinib in relapsed or refractory chronic lymphocytic leukemia. Clin.Cancer Res. 2011 May 1;17(9):2977–86. doi: 10.1158/1078-0432.CCR-10-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat.Rev.Immunol. 2010 Jun;10(6):387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orchard JA, Ibbotson RE, Davis Z, Wiestner A, Rosenwald A, Thomas PW, Hamblin TJ, Staudt LM, Oscier DG. ZAP-70 expression and prognosis in chronic lymphocytic leukaemia. Lancet. 2004 Jan 10;363(9403):105–11. doi: 10.1016/S0140-6736(03)15260-9. [DOI] [PubMed] [Google Scholar]
- 36.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995 Nov 16;378(6554):303–6. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 37.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VL. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995 Nov 16;378(6554):298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 38.Rowley RB, Burkhardt AL, Chao HG, Matsueda GR, Bolen JB. Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Ig alpha/Ig beta immunoreceptor tyrosine activation motif binding and autophosphorylation. J.Biol.Chem. 1995 May 12;270(19):11590–4. doi: 10.1074/jbc.270.19.11590. [DOI] [PubMed] [Google Scholar]
- 39.Efremov DG, Laurenti L. The Syk kinase as a therapeutic target in leukemia and lymphoma. Expert.Opin.Investig.Drugs. 2011 May;20(5):623–36. doi: 10.1517/13543784.2011.570329. [DOI] [PubMed] [Google Scholar]
- 40.Herman SE, Barr PM, McAuley EM, Liu D, Wiestner A, Friedberg JW. Fostamatinib inhibits B-cell receptor signaling, cellular activation and tumor proliferation in patients with relapsed and refractory chronic lymphocytic leukemia. Leukemia. 2013 Feb 6; doi: 10.1038/leu.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Genovese MC, Kavanaugh A, Weinblatt ME, Peterfy C, DiCarlo J, White ML, O’Brien M, Grossbard EB, Magilavy DB. An oral Syk kinase inhibitor in the treatment of rheumatoid arthritis: a three-month randomized, placebo-controlled, phase II study in patients with active rheumatoid arthritis that did not respond to biologic agents. Arthritis Rheum. 2011 Feb;63(2):337–45. doi: 10.1002/art.30114. [DOI] [PubMed] [Google Scholar]
- 42.Hoellenriegel J, Coffey GP, Sinha U, Pandey A, Sivina M, Ferrajoli A, Ravandi F, Wierda WG, O’Brien S, Keating MJ, et al. Selective, novel spleen tyrosine kinase (Syk) inhibitors suppress chronic lymphocytic leukemia B-cell activation and migration. Leukemia. 2012 Jul;26(7):1576–83. doi: 10.1038/leu.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.So L, Fruman DA. PI3K signalling in B- and T-lymphocytes: new developments and therapeutic advances. Biochem.J. 2012 Mar 15;442(3):465–81. doi: 10.1042/BJ20112092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baracho GV, Miletic AV, Omori SA, Cato MH, Rickert RC. Emergence of the PI3-kinase pathway as a central modulator of normal and aberrant B cell differentiation. Curr.Opin.Immunol. 2011 Apr;23(2):178–83. doi: 10.1016/j.coi.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, Kutok JL, Kearney JF, Otipoby KL, Rajewsky K. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009 Oct 30;139(3):573–86. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramadani F, Bolland DJ, Garcon F, Emery JL, Vanhaesebroeck B, Corcoran AE, Okkenhaug K. The PI3K isoforms p110alpha and p110delta are essential for pre-B cell receptor signaling and B cell development. Sci.Signal. 2010;3(134):ra60. doi: 10.1126/scisignal.2001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat.Rev.Mol.Cell Biol. 2008 Feb;9(2):99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 48.Ghosh AK, Secreto CR, Knox TR, Ding W, Mukhopadhyay D, Kay NE. Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: implications for disease progression. Blood. 2010 Mar 4;115(9):1755–64. doi: 10.1182/blood-2009-09-242719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghosh AK, Secreto C, Boysen J, Sassoon T, Shanafelt TD, Mukhopadhyay D, Kay NE. The novel receptor tyrosine kinase Axl is constitutively active in B-cell chronic lymphocytic leukemia and acts as a docking site of nonreceptor kinases: implications for therapy. Blood. 2011 Feb 10;117(6):1928–37. doi: 10.1182/blood-2010-09-305649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oellerich T, Bremes V, Neumann K, Bohnenberger H, Dittmann K, Hsiao HH, Engelke M, Schnyder T, Batista FD, Urlaub H, et al. The B-cell antigen receptor signals through a preformed transducer module of SLP65 and CIN85. EMBO J. 2011 Aug 31;30(17):3620–34. doi: 10.1038/emboj.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyazaki A, Yogosawa S, Murakami A, Kitamura D. Identification of CMTM7 as a transmembrane linker of BLNK and the B-cell receptor. PLoS.One. 2012;7(2):e31829. doi: 10.1371/journal.pone.0031829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta N, Scharenberg AM, Fruman DA, Cantley LC, Kinet JP, Long EO. The SH2 domain-containing inositol 5′-phosphatase (SHIP) recruits the p85 subunit of phosphoinositide 3-kinase during FcgammaRIIb1-mediated inhibition of B cell receptor signaling. J.Biol.Chem. 1999 Mar 12;274(11):7489–94. doi: 10.1074/jbc.274.11.7489. [DOI] [PubMed] [Google Scholar]
- 53.Hendriks RW, Bredius RG, Pike-Overzet K, Staal FJ. Biology and novel treatment options for XLA, the most common monogenetic immunodeficiency in man. Expert.Opin.Ther.Targets. 2011 Aug;15(8):1003–21. doi: 10.1517/14728222.2011.585971. [DOI] [PubMed] [Google Scholar]
- 54.BRUTON OC. Agammaglobulinemia. Pediatrics. 1952 Jun;9(6):722–8. [PubMed] [Google Scholar]
- 55.Humphries LA, Dangelmaier C, Sommer K, Kipp K, Kato RM, Griffith N, Bakman I, Turk CW, Daniel JL, Rawlings DJ. Tec kinases mediate sustained calcium influx via site-specific tyrosine phosphorylation of the phospholipase Cgamma Src homology 2-Src homology 3 linker. J.Biol.Chem. 2004 Sep 3;279(36):37651–61. doi: 10.1074/jbc.M311985200. [DOI] [PubMed] [Google Scholar]
- 56.Murayama K, Kato-Murayama M, Mishima C, Akasaka R, Shirouzu M, Fukui Y, Yokoyama S. Crystal structure of the Bruton’s tyrosine kinase PH domain with phosphatidylinositol. Biochem.Biophys.Res.Commun. 2008 Dec 5;377(1):23–8. doi: 10.1016/j.bbrc.2008.09.055. [DOI] [PubMed] [Google Scholar]
- 57.Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int.Rev.Immunol. 2012 Apr;31(2):119–32. doi: 10.3109/08830185.2012.664797. [DOI] [PubMed] [Google Scholar]
- 58.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc.Natl.Acad.Sci.U.S.A. 2010 Jul 20;107(29):13075–80. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma S, Orlowski G, Song W. Btk regulates B cell receptor-mediated antigen processing and presentation by controlling actin cytoskeleton dynamics in B cells. J.Immunol. 2009 Jan 1;182(1):329–39. doi: 10.4049/jimmunol.182.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saijo K, Mecklenbrauker I, Santana A, Leitger M, Schmedt C, Tarakhovsky A. Protein kinase C beta controls nuclear factor kappaB activation in B cells through selective regulation of the IkappaB kinase alpha. J.Exp.Med. 2002 Jun 17;195(12):1647–52. doi: 10.1084/jem.20020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Gorter DJ, Beuling EA, Kersseboom R, Middendorp S, van Gils JM, Hendriks RW, Pals ST, Spaargaren M. Bruton’s tyrosine kinase and phospholipase Cgamma2 mediate chemokine-controlled B cell migration and homing. Immunity. 2007 Jan;26(1):93–104. doi: 10.1016/j.immuni.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 62.Giles FJ, Albitar M. Mammalian target of rapamycin as a therapeutic target in leukemia. Curr.Mol.Med. 2005 Nov;5(7):653–61. doi: 10.2174/156652405774641034. [DOI] [PubMed] [Google Scholar]
- 63.Decker T, Hipp S, Ringshausen I, Bogner C, Oelsner M, Schneller F, Peschel C. Rapamycin-induced G1 arrest in cycling B-CLL cells is associated with reduced expression of cyclin D3, cyclin E, cyclin A, and survivin. Blood. 2003 Jan 1;101(1):278–85. doi: 10.1182/blood-2002-01-0189. [DOI] [PubMed] [Google Scholar]
- 64.Douros J, Suffness M. New antitumor substances of natural origin. Cancer Treat.Rev. 1981 Mar;8(1):63–87. doi: 10.1016/s0305-7372(81)80006-0. [DOI] [PubMed] [Google Scholar]
- 65.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991 Aug 23;253(5022):905–9. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 66.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012 Apr 13;149(2):274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Decker T, Sandherr M, Goetze K, Oelsner M, Ringshausen I, Peschel C. A pilot trial of the mTOR (mammalian target of rapamycin) inhibitor RAD001 in patients with advanced B-CLL. Ann.Hematol. 2009 Mar;88(3):221–7. doi: 10.1007/s00277-008-0582-9. [DOI] [PubMed] [Google Scholar]
- 68.Zent CS, LaPlant BR, Johnston PB, Call TG, Habermann TM, Micallef IN, Witzig TE. The treatment of recurrent/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) with everolimus results in clinical responses and mobilization of CLL cells into the circulation. Cancer. 2010 May 1;116(9):2201–7. doi: 10.1002/cncr.25005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Witzig TE, Geyer SM, Ghobrial I, Inwards DJ, Fonseca R, Kurtin P, Ansell SM, Luyun R, Flynn PJ, Morton RF, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J.Clin.Oncol. 2005 Aug 10;23(23):5347–56. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 70.Smith SM, van BK, Karrison T, Dancey J, McLaughlin P, Younes A, Smith S, Stiff P, Lester E, Modi S, et al. Temsirolimus has activity in non-mantle cell non-Hodgkin’s lymphoma subtypes: The University of Chicago phase II consortium. J.Clin.Oncol. 2010 Nov 1;28(31):4740–6. doi: 10.1200/JCO.2010.29.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Renner C, Zinzani PL, Gressin R, Klingbiel D, Dietrich PY, Hitz F, Bargetzi M, Mingrone W, Martinelli G, Trojan A, et al. A multicenter phase II trial (SAKK 36/06) of single-agent everolimus (RAD001) in patients with relapsed or refractory mantle cell lymphoma. Haematologica. 2012 Jul;97(7):1085–91. doi: 10.3324/haematol.2011.053173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Witzig TE, Reeder CB, LaPlant BR, Gupta M, Johnston PB, Micallef IN, Porrata LF, Ansell SM, Colgan JP, Jacobsen ED, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia. 2011 Feb;25(2):341–7. doi: 10.1038/leu.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu W, Won SH, Tolar P, Meckel T, Pierce SK. Antigen-induced oligomerization of the B cell receptor is an early target of Fc gamma RIIB inhibition. J.Immunol. 2010 Feb 15;184(4):1977–89. doi: 10.4049/jimmunol.0902334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chaturvedi A, Martz R, Dorward D, Waisberg M, Pierce SK. Endocytosed BCRs sequentially regulate MAPK and Akt signaling pathways from intracellular compartments. Nat.Immunol. 2011 Nov;12(11):1119–26. doi: 10.1038/ni.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Neill SK, Getahun A, Gauld SB, Merrell KT, Tamir I, Smith MJ, Dal Porto JM, Li QZ, Cambier JC. Monophosphorylation of CD79a and CD79b ITAM motifs initiates a SHIP-1 phosphatase-mediated inhibitory signaling cascade required for B cell anergy. Immunity. 2011 Nov 23;35(5):746–56. doi: 10.1016/j.immuni.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adachi T, Wienands J, Tsubata T, Kurosaki T. Interdomain A is crucial for ITAM-dependent and - independent regulation of Syk. Biochem.Biophys.Res.Commun. 2007 Dec 7;364(1):111–7. doi: 10.1016/j.bbrc.2007.09.100. [DOI] [PubMed] [Google Scholar]
- 77.Yarkoni Y, Getahun A, Cambier JC. Molecular underpinning of B-cell anergy. Immunol.Rev. 2010 Sep;237(1):249–63. doi: 10.1111/j.1600-065X.2010.00936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ysebaert L, Morschhauser F. Enzastaurin hydrochloride for lymphoma: reassessing the results of clinical trials in light of recent advances in the biology of B-cell malignancies. Expert.Opin.Investig.Drugs. 2011 Aug;20(8):1167–74. doi: 10.1517/13543784.2011.590130. [DOI] [PubMed] [Google Scholar]
- 79.Liffraud C, Quillet-Mary A, Fournie JJ, Laurent G, Ysebaert L. Protein phosphatase-2A activation is a critical step for enzastaurin activity in chronic lymphoid leukemia cells. Leuk.Lymphoma. 2012 May;53(5):966–72. doi: 10.3109/10428194.2011.634041. [DOI] [PubMed] [Google Scholar]
- 80.Holler C, Pinon JD, Denk U, Heyder C, Hofbauer S, Greil R, Egle A. PKCbeta is essential for the development of chronic lymphocytic leukemia in the TCL1 transgenic mouse model: validation of PKCbeta as a therapeutic target in chronic lymphocytic leukemia. Blood. 2009 Mar 19;113(12):2791–4. doi: 10.1182/blood-2008-06-160713. [DOI] [PubMed] [Google Scholar]
- 81.Lutzny G, Kocher T, Schmidt-Supprian M, Rudelius M, Klein-Hitpass L, Finch AJ, Durig J, Wagner M, Haferlach C, Kohlmann A, et al. Protein kinase c-beta-dependent activation of NF-kappaB in stromal cells is indispensable for the survival of chronic lymphocytic leukemia B cells in vivo. Cancer Cell. 2013 Jan 14;23(1):77–92. doi: 10.1016/j.ccr.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan W, Schaffer TB, Pomerantz JL. A quantitative signaling screen identifies CARD11 mutations in the CARD and LATCH domains that induce Bcl10 ubiquitination and human lymphoma cell survival. Mol.Cell.Biol. 2013 Jan;33(2):429–43. doi: 10.1128/MCB.00850-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou H, Wertz I, O’Rourke K, Ultsch M, Seshagiri S, Eby M, Xiao W, Dixit VM. Bcl10 activates the NF-kappaB pathway through ubiquitination of NEMO. Nature. 2004 Jan 8;427(6970):167–71. doi: 10.1038/nature02273. [DOI] [PubMed] [Google Scholar]
- 84.Everett PC, Meyers JA, Makkinje A, Rabbi M, Lerner A. Preclinical assessment of curcumin as a potential therapy for B-CLL. Am.J.Hematol. 2007 Jan;82(1):23–30. doi: 10.1002/ajh.20757. [DOI] [PubMed] [Google Scholar]
- 85.Fabre C, Mimura N, Bobb K, Kong SY, Gorgun G, Cirstea D, Hu Y, Minami J, Ohguchi H, Zhang J, et al. Dual inhibition of canonical and noncanonical NF-kappaB pathways demonstrates significant antitumor activities in multiple myeloma. Clin.Cancer Res. 2012 Sep 1;18(17):4669–81. doi: 10.1158/1078-0432.CCR-12-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, Carney DA, He SZ, Huang DC, Xiong H, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J.Clin.Oncol. 2012 Feb 10;30(5):488–96. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tudhope SJ, Mulligan EA, Hunter JE, Summerfield GP, Marshall S, Wallis J, Marr H, Evans P, Lowe C, Durkacz BW, et al. P B S-1086, a “Pan-Rel” Inhibitor, Decreases Viability of Chronic Lymphocytic Leukemia Cells. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):867. [Google Scholar]
- 88.Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011 Feb 10;117(6):1792–8. doi: 10.1182/blood-2010-06-275818. [DOI] [PubMed] [Google Scholar]
- 89.Gisbert JP, Garcia-Buey L, Pajares JM, Moreno-Otero R. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment.Pharmacol.Ther. 2005 Mar 15;21(6):653–62. doi: 10.1111/j.1365-2036.2005.02395.x. [DOI] [PubMed] [Google Scholar]
- 90.Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N.Engl.J.Med. 1994 May 5;330(18):1267–71. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 91.Mueller A, O’rourke J, Chu P, Chu A, Dixon MF, Bouley DM, Lee A, Falkow S. The role of antigenic drive and tumor-infiltrating accessory cells in the pathogenesis of helicobacter-induced mucosa-associated lymphoid tissue lymphoma. Am.J.Pathol. 2005 Sep;167(3):797–812. doi: 10.1016/S0002-9440(10)62052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fais F, Ghiotto F, Hashimoto S, Sellars B, Valetto A, Allen SL, Schulman P, Vinciguerra VP, Rai K, Rassenti LZ, et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J.Clin.Invest. 1998 Oct 15;102(8):1515–25. doi: 10.1172/JCI3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tobin G, Thunberg U, Karlsson K, Murray F, Laurell A, Willander K, Enblad G, Merup M, Vilpo J, Juliusson G, et al. Subsets with restricted immunoglobulin gene rearrangement features indicate a role for antigen selection in the development of chronic lymphocytic leukemia. Blood. 2004 Nov 1;104(9):2879–85. doi: 10.1182/blood-2004-01-0132. [DOI] [PubMed] [Google Scholar]
- 94.Agathangelidis A, Darzentas N, Hadzidimitriou A, Brochet X, Murray F, Yan XJ, Davis Z, van Gastel-Mol EJ, Tresoldi C, Chu CC, et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood. 2012 May 10;119(19):4467–75. doi: 10.1182/blood-2011-11-393694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Messmer BT, Albesiano E, Efremov DG, Ghiotto F, Allen SL, Kolitz J, Foa R, Damle RN, Fais F, Messmer D, et al. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J.Exp.Med. 2004 Aug 16;200(4):519–25. doi: 10.1084/jem.20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N.Engl.J.Med. 2005 Feb 24;352(8):804–15. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 97.Chen L, Apgar J, Huynh L, Dicker F, Giago-McGahan T, Rassenti L, Weiss A, Kipps TJ. ZAP-70 directly enhances IgM signaling in chronic lymphocytic leukemia. Blood. 2005 Mar 1;105(5):2036–41. doi: 10.1182/blood-2004-05-1715. [DOI] [PubMed] [Google Scholar]
- 98.Gobessi S, Laurenti L, Longo PG, Sica S, Leone G, Efremov DG. ZAP-70 enhances B-cell-receptor signaling despite absent or inefficient tyrosine kinase activation in chronic lymphocytic leukemia and lymphoma B cells. Blood. 2007 Mar 1;109(5):2032–9. doi: 10.1182/blood-2006-03-011759. [DOI] [PubMed] [Google Scholar]
- 99.Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B, Gibellini F, Njuguna N, Lee E, Stennett L, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011 Jan 13;117(2):563–74. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chu CC, Catera R, Hatzi K, Yan XJ, Zhang L, Wang XB, Fales HM, Allen SL, Kolitz JE, Rai KR, et al. Chronic lymphocytic leukemia antibodies with a common stereotypic rearrangement recognize nonmuscle myosin heavy chain IIA. Blood. 2008 Dec 15;112(13):5122–9. doi: 10.1182/blood-2008-06-162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lanemo MA, Hellqvist E, Sidorova E, Soderberg A, Baxendale H, Dahle C, Willander K, Tobin G, Backman E, Soderberg O, et al. A new perspective: molecular motifs on oxidized LDL, apoptotic cells, and bacteria are targets for chronic lymphocytic leukemia antibodies. Blood. 2008 Apr 1;111(7):3838–48. doi: 10.1182/blood-2007-11-125450. [DOI] [PubMed] [Google Scholar]
- 102.Steininger C, Widhopf GF, Ghia EM, Morello CS, Vanura K, Sanders R, Spector D, Guiney D, Jager U, Kipps TJ. Recombinant antibodies encoded by IGHV1-69 react with pUL32, a phosphoprotein of cytomegalovirus and B-cell superantigen. Blood. 2012 Mar 8;119(10):2293–301. doi: 10.1182/blood-2011-08-374058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hoogeboom R, van Kessel KP, Hochstenbach F, Wormhoudt TA, Reinten RJ, Wagner K, Kater AP, Guikema JE, Bende RJ, van Noesel CJ. A mutated B cell chronic lymphocytic leukemia subset that recognizes and responds to fungi. J.Exp.Med. 2013 Jan 14;210(1):59–70. doi: 10.1084/jem.20121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mockridge CI, Potter KN, Wheatley I, Neville LA, Packham G, Stevenson FK. Reversible anergy of sIgM-mediated signaling in the two subsets of CLL defined by VH-gene mutational status. Blood. 2007 May 15;109(10):4424–31. doi: 10.1182/blood-2006-11-056648. [DOI] [PubMed] [Google Scholar]
- 105.Muzio M, Apollonio B, Scielzo C, Frenquelli M, Vandoni I, Boussiotis V, Caligaris-Cappio F, Ghia P. Constitutive activation of distinct BCR-signaling pathways in a subset of CLL patients: a molecular signature of anergy. Blood. 2008 Jul 1;112(1):188–95. doi: 10.1182/blood-2007-09-111344. [DOI] [PubMed] [Google Scholar]
- 106.Duhren-von MM, Ubelhart R, Schneider D, Wossning T, Bach MP, Buchner M, Hofmann D, Surova E, Follo M, Kohler F, et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 2012 Sep 13;489(7415):309–12. doi: 10.1038/nature11309. [DOI] [PubMed] [Google Scholar]
- 107.Binder M, Muller F, Jackst A, Lechenne B, Pantic M, Bacher U, Zu EC, Veelken H, Mertelsmann R, Pasqualini R, et al. B-cell receptor epitope recognition correlates with the clinical course of chronic lymphocytic leukemia. Cancer. 2011 May 1;117(9):1891–900. doi: 10.1002/cncr.25755. [DOI] [PubMed] [Google Scholar]
- 108.Guarini A, Chiaretti S, Tavolaro S, Maggio R, Peragine N, Citarella F, Ricciardi MR, Santangelo S, Marinelli M, De Propris MS, et al. BCR ligation induced by IgM stimulation results in gene expression and functional changes only in IgV H unmutated chronic lymphocytic leukemia (CLL) cells. Blood. 2008 Aug 1;112(3):782–92. doi: 10.1182/blood-2007-12-127688. [DOI] [PubMed] [Google Scholar]
- 109.Burger JA. Inhibiting B-cell receptor signaling pathways in chronic lymphocytic leukemia. Curr.Hematol.Malig.Rep. 2012 Mar;7(1):26–33. doi: 10.1007/s11899-011-0104-z. [DOI] [PubMed] [Google Scholar]
- 110.Efremov DG, Wiestner A, Laurenti L. Novel Agents and Emerging Strategies for Targeting the B-Cell Receptor Pathway in CLL. Mediterr.J.Hematol.Infect.Dis. 2012;4(1):e2012067. doi: 10.4084/MJHID.2012.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stevenson FK, Krysov S, Davies AJ, Steele AJ, Packham G. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2011 Oct 20;118(16):4313–20. doi: 10.1182/blood-2011-06-338855. [DOI] [PubMed] [Google Scholar]
- 112.Davids MS, Brown JR. Targeting the B cell receptor pathway in chronic lymphocytic leukemia. Leuk.Lymphoma. 2012 Dec;53(12):2362–70. doi: 10.3109/10428194.2012.695781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wiestner A. Emerging role of kinase-targeted strategies in chronic lymphocytic leukemia. Blood. 2012 Dec 6;120(24):4684–91. doi: 10.1182/blood-2012-05-423194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hadzidimitriou A, Agathangelidis A, Darzentas N, Murray F, Delfau-Larue MH, Pedersen LB, Lopez AN, Dagklis A, Rombout P, Beldjord K, et al. Is there a role for antigen selection in mantle cell lymphoma? Immunogenetic support from a series of 807 cases. Blood. 2011 Sep 15;118(11):3088–95. doi: 10.1182/blood-2011-03-343434. [DOI] [PubMed] [Google Scholar]
- 115.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011 Jan 6;117(1):26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Varettoni M, Zibellini S, Capello D, Arcaini L, Rossi D, Pascutto C, Rattotti S, Mangiacavalli S, Pochintesta L, Gotti M, et al. Clues to pathogenesis of Waldenstrom macroglobulinemia and immunoglobulin M monoclonal gammopathy of undetermined significance provided by analysis of immunoglobulin heavy chain gene rearrangement and clustering of B-cell receptors. Leuk.Lymphoma. 2013 Apr 17; doi: 10.3109/10428194.2013.779689. [DOI] [PubMed] [Google Scholar]
- 117.Arons E, Roth L, Sapolsky J, Suntum T, Stetler-Stevenson M, Kreitman RJ. Evidence of canonical somatic hypermutation in hairy cell leukemia. Blood. 2011 May 5;117(18):4844–51. doi: 10.1182/blood-2010-11-316737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Forconi F, Cencini E, Sicuranza A, Sozzi E, Lauria F. Molecular insight into the biology and clinical course of hairy cell leukemia utilizing immunoglobulin gene analysis. Leuk.Lymphoma. 2011 Jan;52(1):15–23. doi: 10.3109/10428194.2010.530362. [DOI] [PubMed] [Google Scholar]
- 119.Thelander EF, Rosenquist R. Molecular genetic characterization reveals new subsets of mantle cell lymphoma. Leuk.Lymphoma. 2008 Jun;49(6):1042–9. doi: 10.1080/10428190801947559. [DOI] [PubMed] [Google Scholar]
- 120.Coelho V, Krysov S, Ghaemmaghami AM, Emara M, Potter KN, Johnson P, Packham G, Martinez-Pomares L, Stevenson FK. Glycosylation of surface Ig creates a functional bridge between human follicular lymphoma and microenvironmental lectins. Proc.Natl.Acad.Sci.U.S.A. 2010 Oct 26;107(43):18587–92. doi: 10.1073/pnas.1009388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat.Rev.Drug Discov. 2013 Mar;12(3):229–43. doi: 10.1038/nrd3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lenz G, Nagel I, Siebert R, Roschke AV, Sanger W, Wright GW, Dave SS, Tan B, Zhao H, Rosenwald A, et al. Aberrant immunoglobulin class switch recombination and switch translocations in activated B cell-like diffuse large B cell lymphoma. J.Exp.Med. 2007 Mar 19;204(3):633–43. doi: 10.1084/jem.20062041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Staudt LM. A closer look at follicular lymphoma. N.Engl.J.Med. 2007 Feb 15;356(7):741–2. doi: 10.1056/NEJMcibr067155. [DOI] [PubMed] [Google Scholar]
- 124.Vaandrager JW, Schuuring E, Kluin-Nelemans HC, Dyer MJ, Raap AK, Kluin PM. DNA fiber fluorescence in situ hybridization analysis of immunoglobulin class switching in B-cell neoplasia: aberrant CH gene rearrangements in follicle center-cell lymphoma. Blood. 1998 Oct 15;92(8):2871–8. [PubMed] [Google Scholar]
- 125.Zuckerman NS, McCann KJ, Ottensmeier CH, Barak M, Shahaf G, Edelman H, Dunn-Walters D, Abraham RS, Stevenson FK, Mehr R. Ig gene diversification and selection in follicular lymphoma, diffuse large B cell lymphoma and primary central nervous system lymphoma revealed by lineage tree and mutation analyses. Int.Immunol. 2010 Nov;22(11):875–87. doi: 10.1093/intimm/dxq441. [DOI] [PubMed] [Google Scholar]
- 126.Kloo B, Nagel D, Pfeifer M, Grau M, Duwel M, Vincendeau M, Dorken B, Lenz P, Lenz G, Krappmann D. Critical role of PI3K signaling for NF-kappaB-dependent survival in a subset of activated B-cell-like diffuse large B-cell lymphoma cells. Proc.Natl.Acad.Sci.U.S.A. 2011 Jan 4;108(1):272–7. doi: 10.1073/pnas.1008969108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Compagno M, Lim WK, Grunn A, Nandula SV, Brahmachary M, Shen Q, Bertoni F, Ponzoni M, Scandurra M, Califano A, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009 Jun 4;459(7247):717–21. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maes B, Demunter A, Peeters B, De Wolf-Peeters C. BCL10 mutation does not represent an important pathogenic mechanism in gastric MALT-type lymphoma, and the presence of the API2-MLT fusion is associated with aberrant nuclear BCL10 expression. Blood. 2002 Feb 15;99(4):1398–404. doi: 10.1182/blood.v99.4.1398. [DOI] [PubMed] [Google Scholar]
- 129.Willis TG, Jadayel DM, Du MQ, Peng H, Perry AR, Abdul-Rauf M, Price H, Karran L, Majekodunmi O, Wlodarska I, et al. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell. 1999 Jan 8;96(1):35–45. doi: 10.1016/s0092-8674(00)80957-5. [DOI] [PubMed] [Google Scholar]
- 130.Dierlamm J, Baens M, Wlodarska I, Stefanova-Ouzounova M, Hernandez JM, Hossfeld DK, De Wolf-Peeters C, Hagemeijer A, Van den Berghe H, Marynen P. The apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11;18)(q21;q21) associated with mucosa-associated lymphoid tissue lymphomas. Blood. 1999 Jun 1;93(11):3601–9. [PubMed] [Google Scholar]
- 131.Schmitz R, Young RM, Ceribelli M, Jhavar S, Xiao W, Zhang M, Wright G, Shaffer AL, Hodson DJ, Buras E, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012 Oct 4;490(7418):116–20. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sander S, Calado DP, Srinivasan L, Kochert K, Zhang B, Rosolowski M, Rodig SJ, Holzmann K, Stilgenbauer S, Siebert R, et al. Synergy between PI3K signaling and MYC in Burkitt lymphomagenesis. Cancer Cell. 2012 Aug 14;22(2):167–79. doi: 10.1016/j.ccr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miletic AV, Anzelon-Mills AN, Mills DM, Omori SA, Pedersen IM, Shin DM, Ravetch JV, Bolland S, Morse HC, III, Rickert RC. Coordinate suppression of B cell lymphoma by PTEN and SHIP phosphatases. J.Exp.Med. 2010 Oct 25;207(11):2407–20. doi: 10.1084/jem.20091962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Abubaker J, Bavi PP, Al-Harbi S, Siraj AK, Al-Dayel F, Uddin S, Al-Kuraya K. PIK3CA mutations are mutually exclusive with PTEN loss in diffuse large B-cell lymphoma. Leukemia. 2007 Nov;21(11):2368–70. doi: 10.1038/sj.leu.2404873. [DOI] [PubMed] [Google Scholar]
- 135.Lenz G, Wright GW, Emre NC, Kohlhammer H, Dave SS, Davis RE, Carty S, Lam LT, Shaffer AL, Xiao W, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc.Natl.Acad.Sci.U.S.A. 2008 Sep 9;105(36):13520–5. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hofmann BT, Jucker M. Activation of PI3K/Akt signaling by n-terminal SH2 domain mutants of the p85alpha regulatory subunit of PI3K is enhanced by deletion of its c-terminal SH2 domain. Cell Signal. 2012 Oct;24(10):1950–4. doi: 10.1016/j.cellsig.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 137.Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, Byrd JC, Tyner JW, Loriaux MM, Deininger M, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011 Jan 13;117(2):591–4. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rudelius M, Pittaluga S, Nishizuka S, Pham TH, Fend F, Jaffe ES, Quintanilla-Martinez L, Raffeld M. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood. 2006 Sep 1;108(5):1668–76. doi: 10.1182/blood-2006-04-015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, Jones J, Andritsos L, Puri KD, Lannutti BJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010 Sep 23;116(12):2078–88. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, Giese N, O’Brien S, Yu A, Miller LL, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011 Sep 29;118(13):3603–12. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Burger JA, Quiroga MP, Hartmann E, Burkle A, Wierda WG, Keating MJ, Rosenwald A. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood. 2009 Mar 26;113(13):3050–8. doi: 10.1182/blood-2008-07-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B, Gibellini F, Njuguna N, Lee E, Stennett L, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011 Jan 13;117(2):563–74. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008 Jun 15;111(12):5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Furman RR, Byrd JC, Brown JR, Coutre SE, Benson DM, Jr., Wagner-Johnston ND, Flinn IW, Kahl BS, Spurgeon SE, Lannutti B, et al. CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110{delta}, Demonstrates Clinical Activity and Pharmacodynamic Effects In Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia. ASH Annual Meeting Abstracts. 2010 Nov 19;116(21):55. [Google Scholar]
- 145.Cheson BD, Byrd JC, Rai KR, Kay NE, O’Brien SM, Flinn IW, Wiestner A, Kipps TJ. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J.Clin.Oncol. 2012 Aug 10;30(23):2820–2. doi: 10.1200/JCO.2012.43.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Coutre SE, Leonard JP, Furman RR, Barrientos JC, De Vos S, Flinn IW, Schreeder MT, Wagner-Johnston ND, Sharman J, Boyd TE, et al. Combinations of the Selective Phosphatidylinositol 3-Kinase-Delta (PI3Kdelta) Inhibitor GS-1101 (CAL-101) with Rituximab and/or Bendamustine Are Tolerable and Highly Active in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL): Results From a Phase I Study. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):191. [Google Scholar]
- 147.Furman RR, Barrientos JC, Sharman JP, De Vos S, Leonard J, Coutre SE, Schreeder MT, Wagner-Johnston ND, Boyd TE, Fowler NH, et al. A phase I/II study of the selective phosphatidylinositol 3-kinase-delta (PI3K{delta}) inhibitor, GS-1101 (CAL-101), with ofatumumab in patients with previously treated chronic lymphocytic leukemia (CLL) ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):6518. [Google Scholar]
- 148.Niedermeier M, Hennessy BT, Knight ZA, Henneberg M, Hu J, Kurtova AV, Wierda WG, Keating MJ, Shokat KM, Burger JA. Isoform-selective phosphoinositide 3′-kinase inhibitors inhibit CXCR4 signaling and overcome stromal cell-mediated drug resistance in chronic lymphocytic leukemia: a novel therapeutic approach. Blood. 2009 May 28;113(22):5549–57. doi: 10.1182/blood-2008-06-165068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chapman CM, Sun X, Roschewski M, Aue G, Farooqui M, Stennett L, Gibellini F, Arthur D, Perez-Galan P, Wiestner A. ON 01910.Na is selectively cytotoxic for chronic lymphocytic leukemia cells through a dual mechanism of action involving PI3K/AKT inhibition and induction of oxidative stress. Clin.Cancer Res. 2012 Apr 1;18(7):1979–91. doi: 10.1158/1078-0432.CCR-11-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brown JR, Davids MS, Rodon J, Abrisqueta P, DeCillis AP, Rockich K, Egile C, Kelly A, Xu Y, Lager J, et al. Phase I Trial of SAR245408 (S08), a Pan-Phosphatidylinositol 3 Kinase (PI3K) Inhibitor, in Patients with Chronic Lymphocytic Leukemia (CLL) and Lymphoma. ASH Annual Meeting Abstracts. 2011 Nov 18;118(21):2683. [Google Scholar]
- 151.Friedman DR, Lanasa MC, Brander DM, Allgood SD, Davis ED, Miskin H, Viswanadha S, Vakkalanka S, Weinberg JB. Comparison of the PI3K-{delta} Inhibitors TGR1202 and GS-1101 in Inducing Cytotoxicity and Inhibiting Phosphorylation of Akt in CLL Cells in Vitro. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):3914. [Google Scholar]
- 152.O’Farrell M, Ventura R, Tai A, Tyner JW, Loriaux MM, Mahadevan D, Morales C, Brown SD, Matthews DJ. Preclinical Characterization of PWT143, a Novel Selective and Potent Phosphatidylinositol 3-kinase Delta (PI3K delta) Inhibitor with Ex-Vivo Activity in Hematologic Malignancies. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):2907. [Google Scholar]
- 153.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat.Rev.Mol.Cell Biol. 2010 May;11(5):329–41. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 154.Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, et al. Targeting BTK with Ibrutinib in Relapsed or Refractory Mantle-Cell Lymphoma. N.Engl.J.Med. 2013 Jun 19; doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pan Z, Scheerens H, Li SJ, Schultz BE, Sprengeler PA, Burrill LC, Mendonca RV, Sweeney MD, Scott KC, Grothaus PG, et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem. 2007 Jan;2(1):58–61. doi: 10.1002/cmdc.200600221. [DOI] [PubMed] [Google Scholar]
- 156.de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, Pals ST, Spaargaren M. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012 Mar 15;119(11):2590–4. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 157.Herman SE, Farooqui M, Bezabhie R, Aue G, Wiestner A. In Vivo Effects of Ibrutinib On BCR Signaling, Tumor Cell Activation and Proliferation in Blood and Tissue-Resident Cells of Chronic Lymphocytic Leukemia Patients. 2012. [Google Scholar]
- 158.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, Flynn J, Jones J, Blum KA, Buggy JJ, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011 Jun 9;117(23):6287–96. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, Keating MJ, O’Brien S, Chiorazzi N, Burger JA. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012 Feb 2;119(5):1182–9. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim E, Koehrer S, Rosin NY, Thomas DA, Ravandi F, Kornblau SM, Kantarjian HM, O’Brien S, Estrov Z, Buggy JJ, et al. Activity of Bruton’s Tyrosine Kinase (BTK) Inhibitor Ibrutinib (PCI-32765) in B-Cell Acute Lymphoblastic Leukemia (B-ALL) ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):2569. [Google Scholar]
- 161.Sivina M, Kreitman RJ, Arons E, Buggy JJ, Ravandi F, Burger JA. Bruton’s Tyrosine Kinase (BTK) Inhibitor Ibrutinib (PCI-32765) Blocks Hairy Cell Leukemia (HCL) Survival, Proliferation, and BCR Signaling: A New Therapeutic Approach for HCL. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):1802. [Google Scholar]
- 162.Fowler NH, Advani RH, Sharman J, Smith SM, McGreivy J, Kunkel L, Troung V, Zhou C, Boyd TE. The Bruton’s Tyrosine Kinase Inhibitor Ibrutinib (PCI-32765) Is Active and Tolerated in Relapsed Follicular Lymphoma. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):156. [Google Scholar]
- 163.Wilson WH, Gerecitano JF, Goy A, De Vos S, Kenkre VP, Barr PM, Blum KA, Shustov AR, Advani RH, Lih J, et al. The Bruton’s Tyrosine Kinase (BTK) Inhibitor, Ibrutinib (PCI-32765), Has Preferential Activity in the ABC Subtype of Relapsed/Refractory De Novo Diffuse Large B-Cell Lymphoma (DLBCL): Interim Results of a Multicenter, Open-Label, Phase 2 Study. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):686. [Google Scholar]
- 164.Wiestner A, Farooqui M, Valdez J, Jones J, Marti GE, Arthur DC, Lipsky A, Thomas F, ian X, aric I, et al. Single agent ibrutinib (PCI-32765) is highly effective in chronic lymphocytic leukemia patients with 17p deletion. Hematol Oncol. 2013 Jun 1;31(S1):96–150. [Google Scholar]
- 165.Jaglowski SM, Jones JA, Flynn JM, Andritsos LA, Maddocks KJ, Blum KA, Grever MR, Geyer SM, Woyach JA, Johnson AJ, et al. A phase Ib/II study evaluating activity and tolerability of BTK inhibitor PCI-32765 and ofatumumab in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and related diseases. ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):6508. [Google Scholar]
- 166.O’Brien SM, Barrientos JC, Flinn IW, Barr PM, Burger JA, Navarro T, James DF, Hedrick E, Friedberg JW, Brown JR. Combination of the Bruton’s tyrosine kinase (BTK) inhibitor PCI-32765 with bendamustine (B)/rituximab (R) (BR) in patients (pts) with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): Interim results of a phase Ib/II study. ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):6515. [Google Scholar]
- 167.Burger JA, Keating MJ, Wierda WG, Hoellenriegel J, Ferrajoli A, Faderl S, Lerner S, Zacharian G, Huang X, James DF, et al. The Btk Inhibitor Ibrutinib (PCI-32765) in Combination with Rituximab Is Well Tolerated and Displays Profound Activity in High-Risk Chronic Lymphocytic Leukemia (CLL) Patients. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):187. [Google Scholar]
- 168.Brown JR, Sharman JP, Harb WA, Kelly KR, Schreeder MT, Sweetenham JW, Barr PM, Foran JM, Gabrilove JL, Kipps TJ, et al. Phase Ib trial of AVL-292, a covalent inhibitor of Bruton’s tyrosine kinase (Btk), in chronic lymphocytic leukemia (CLL) and B-non-Hodgkin lymphoma (B-NHL) ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):8032. [Google Scholar]
- 169.Brown JR, Harb WA, Sharman J, Hill B, Ma S, Miller TP, Schreeder MT, Barr PM, Foran JM, Gabrilove JL, et al. Phase 1 study of single agent CC-292, a highly selective Bruton’s tyrosine kinase (BTK) inhibitor, in relapsed/refractory chronic lymphocytic leukemia (CLL) and B-cell Non-Hodgkin lymphoma (B-NHL) Haematologica. 2013 Jun;98(s1):217. [Google Scholar]
- 170.Robak T, Robak E. Tyrosine kinase inhibitors as potential drugs for B-cell lymphoid malignancies and autoimmune disorders. Expert.Opin.Investig.Drugs. 2012 Jul;21(7):921–47. doi: 10.1517/13543784.2012.685650. [DOI] [PubMed] [Google Scholar]
- 171.Kahl B, Byrd JC, Flinn IW, Wagner-Johnston N, Spurgeon S, Benson DM, Jr., Furman RR, Brown JR, Coutre S, Lannutti B, et al. Clinical Safety and Activity In a Phase 1 Study of CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110{delta}, In Patients with Relapsed or Refractory Non-Hodgkin Lymphoma. ASH Annual Meeting Abstracts. 2010 Nov 19;116(21):1777. [Google Scholar]
- 172.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Sharman J, Grant B, Jones J, Wierda WG, et al. The Bruton’s Tyrosine Kinase (BTK) Inhibitor Ibrutinib (PCI-32765) Promotes High Response Rate, Durable Remissions, and Is Tolerable in Treatment Naïve (TN) and Relapsed or Refractory (RR) Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL) Patients Including Patients with High-Risk (HR) Disease: New and Updated Results of 116 Patients in a Phase Ib/II Study. 2012. [Google Scholar]
- 173.McAllister-Lucas LM, Baens M, Lucas PC. MALT1 protease: a new therapeutic target in B lymphoma and beyond? Clin Cancer Res. 2011 Nov 1;17(21):6623–31. doi: 10.1158/1078-0432.CCR-11-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, Ye H, Molina T, Bouhnik Y, Hamoudi RA, Diss TC, Dogan A, Megraud F, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet. 2001 Jan 6;357(9249):39–40. doi: 10.1016/S0140-6736(00)03571-6. [DOI] [PubMed] [Google Scholar]
- 175.Ye H, Gong L, Liu H, Ruskone-Fourmestraux A, de Jong D, Pileri S, Thiede C, Lavergne A, Boot H, Caletti G, et al. Strong BCL10 nuclear expression identifies gastric MALT lymphomas that do not respond to H pylori eradication. Gut. 2006 Jan;55(1):137–8. doi: 10.1136/gut.2005.081117. [DOI] [PMC free article] [PubMed] [Google Scholar]