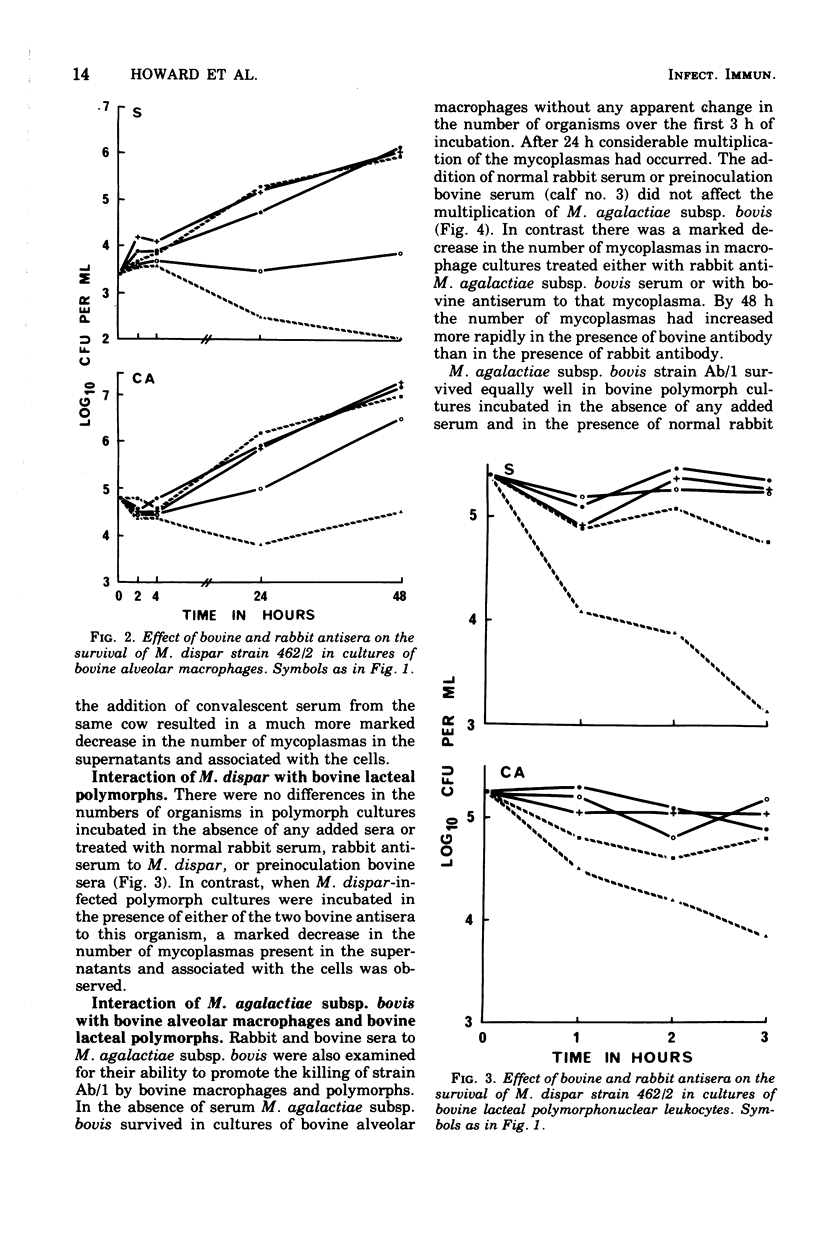
Abstract
Mycoplasma dispar and Mycoplasma agalactiae subsp. bovis survived or grew in cultures of bovine lacteal polymorphonuclear leukocytes or bovine alveolar macrophages. In the presence of specific bovine antibody, macrophages and polymorphonuclear leukocytes appeared to kill both species of mycoplasma. Specific rabbit antisera also promoted the killing of these mycoplasmas by bovine macrophages but had no demonstrable activity for bovine polymorphonuclear leukocytes. It is suggested that phagocytosis of these mycoplasmas by bovine cells occurs only in the presence of specific antibody. The experiments also indicate that differences exist between bovine polymorphonuclear leukocytes and macrophages with regard to their receptor sites for immunoglobulins.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. E., Leach R. H., Gourlay R. N., Howard C. J. Enhanced isolation of Mycoplasma dispar by substitution of ampicillin for benzylpenicillin in growth media. Vet Rec. 1973 Dec 8;93(23):603–603. doi: 10.1136/vr.93.23.603-a. [DOI] [PubMed] [Google Scholar]
- BLOBEL H., KATSUBE Y. EFFECTS OF EXPERIMENTALLY INDUCED LEUKOCYTOSIS IN BOVINE MAMMARY GLANDS UPON INFECTIONS WITH STAPHYLOCOCCUS AUREUS, STREPTOCOCCUS AGALACTIAE, AND AEROBACTER AEROGENES. Am J Vet Res. 1964 Jul;25:1085–1089. [PubMed] [Google Scholar]
- Brownlie J., Howard C. J., Gourlay R. N. Pathogenicity of certain Mycoplasma species in the bovine mammary gland. Res Vet Sci. 1976 May;20(3):261–266. [PubMed] [Google Scholar]
- Cole B. C., Ward J. R. Interaction of Mycoplasma arthritidis and other mycoplasmas with murine peritoneal macrophages. Infect Immun. 1973 May;7(5):691–699. doi: 10.1128/iai.7.5.691-699.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig C. P., Suter E. Extracellular factors influencing staphylocidal capacity of human polymorphonuclear leukocytes. J Immunol. 1966 Aug;97(2):287–296. [PubMed] [Google Scholar]
- Davis A. T., Estensen R., Quie P. G. Cytochalasin B. 3. Inhibition of human polymorphonuclear leukocyte phagocytosis. Proc Soc Exp Biol Med. 1971 May;137(1):161–164. doi: 10.3181/00379727-137-35535. [DOI] [PubMed] [Google Scholar]
- Gourlay R. N., Howard C. J., Brownlie J. Localized immunity in experimental bovine mastitis caused by Mycoplasma dispar. Infect Immun. 1975 Nov;12(5):947–950. doi: 10.1128/iai.12.5.947-950.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay R. N., Howard C. J., Thomas L. H., Stott E. J. Experimentally produced calf pneumonia. Res Vet Sci. 1976 Mar;20(2):167–173. [PubMed] [Google Scholar]
- Gourlay R. N., Mackenzie A., Cooper J. E. Studies of the microbiology and pathology of pneumonic lungs of calves. J Comp Pathol. 1970 Oct;80(4):575–584. doi: 10.1016/0021-9975(70)90055-1. [DOI] [PubMed] [Google Scholar]
- HALE H. H., HELMBOLDT C. F., PLASTRIDGE W. N., STULA E. F. Bovine mastitis caused by a Mycoplasma species. Cornell Vet. 1962 Oct;52:582–591. [PubMed] [Google Scholar]
- Howard C. J., Gourlay R. N., Collins J. Serological comparison and haemagglutinating activity of Mycoplasma dispar. J Hyg (Lond) 1974 Dec;73(3):457–466. doi: 10.1017/s0022172400042820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction in vitro of Mycoplasma pulmonis with mouse peritoneal macrophages and L-cells. J Exp Med. 1971 Feb 1;133(2):231–259. doi: 10.1084/jem.133.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus G. G. Cytochalasin B. Dissociation of pinocytosis and phagocytosis by peritoneal macrophages. Exp Eye Res. 1973 Apr;79(1):73–78. [PubMed] [Google Scholar]
- Patterson R., Suszko I. M. Passive immune elimination and in vitro phagocytosis of antigen-antibody complexes in relation to species origin of antibody. J Immunol. 1966 Jul;97(1):138–149. [PubMed] [Google Scholar]
- Powell D. A., Clyde W. A., Jr Opsonin-reversible resistance of Mycoplasma pneumoniae to in vitro phagocytosis by alveolar macrophages. Infect Immun. 1975 Mar;11(3):540–550. doi: 10.1128/iai.11.3.540-550.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberkoff M. S., Elsbach P. The interaction in vitro between polymorphonuclear leukocytes and mycoplasma. J Exp Med. 1971 Dec 1;134(6):1417–1430. doi: 10.1084/jem.134.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Purcell R. H., Wong D. C., Chanock R. M. A colour test for the measurement of antibody to certain mycoplasma species based upon the inhibition of acid production. J Hyg (Lond) 1966 Mar;64(1):91–104. doi: 10.1017/s0022172400040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple A., Loewi G., Davies P., Howard A. Cytotoxicity of immune guinea-pig cells. II. The mechanism of macrophage cytotoxicity. Immunology. 1973 Apr;24(4):655–669. [PMC free article] [PubMed] [Google Scholar]
- Thomas L. H., Howard C. J., Gourlay R. N. Isolation of Mycoplasma agalactiae var bovis from a calf pneumonia outbreak in the south of England. Vet Rec. 1975 Jul 19;97(3):55–56. doi: 10.1136/vr.97.3.55. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D., Davidson M., Thomas L. The interaction of mycoplasmas with mammalian cells. I. HeLa cells, neutrophils, and eosinophils. J Exp Med. 1966 Sep 1;124(3):521–532. doi: 10.1084/jem.124.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]


