Abstract
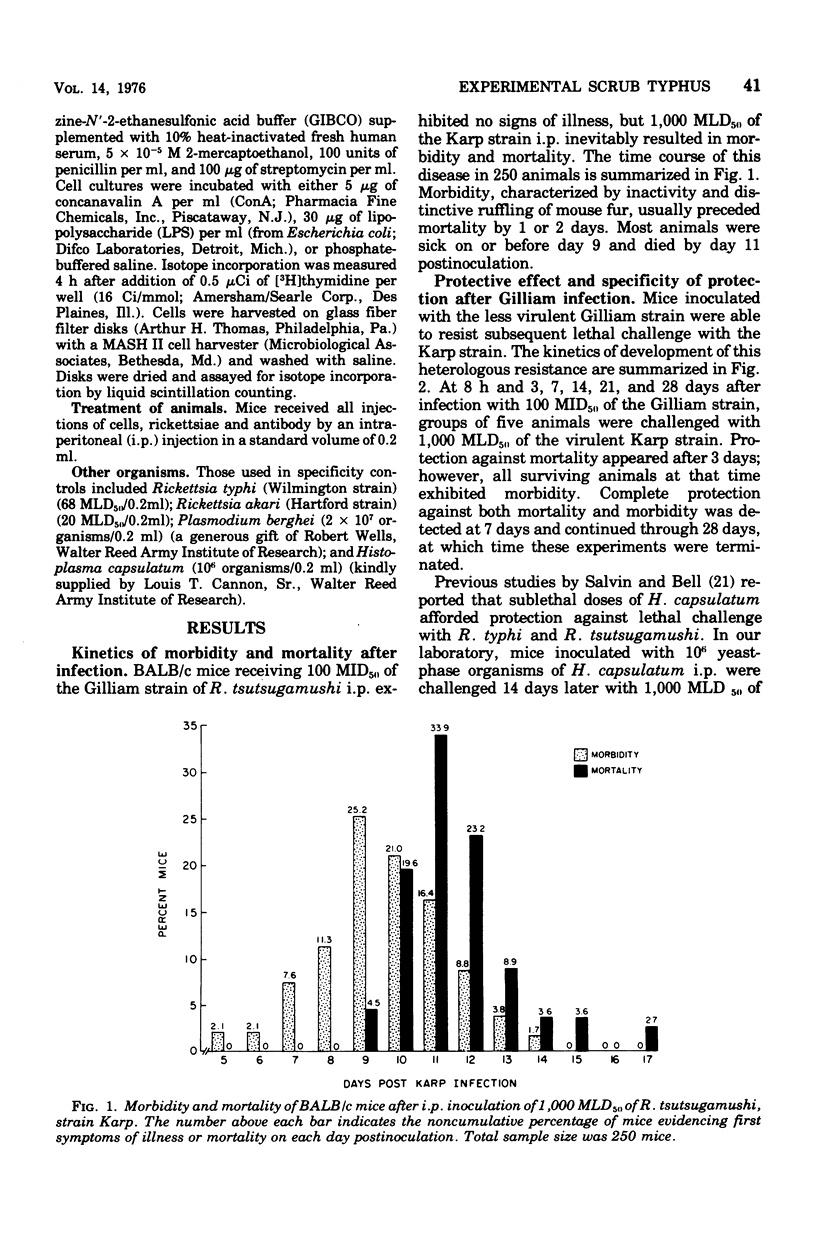
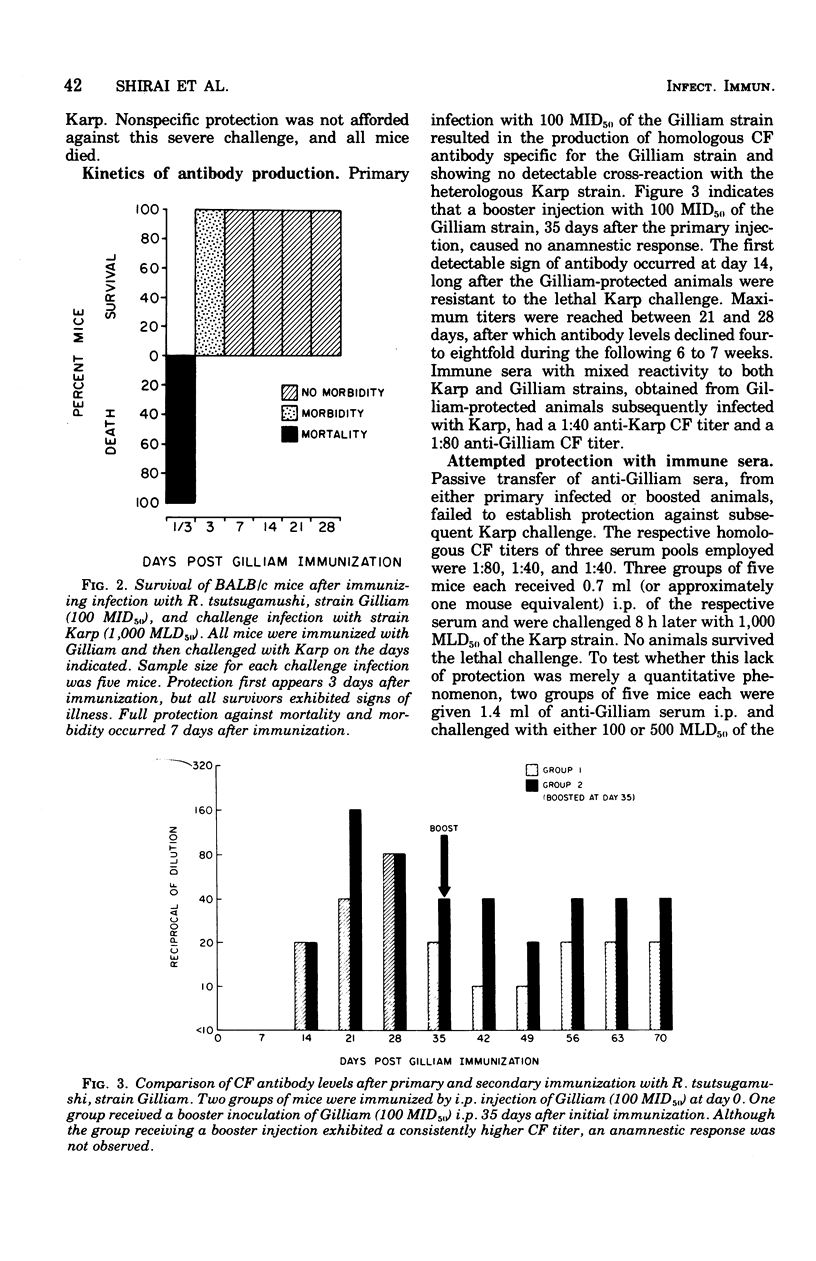
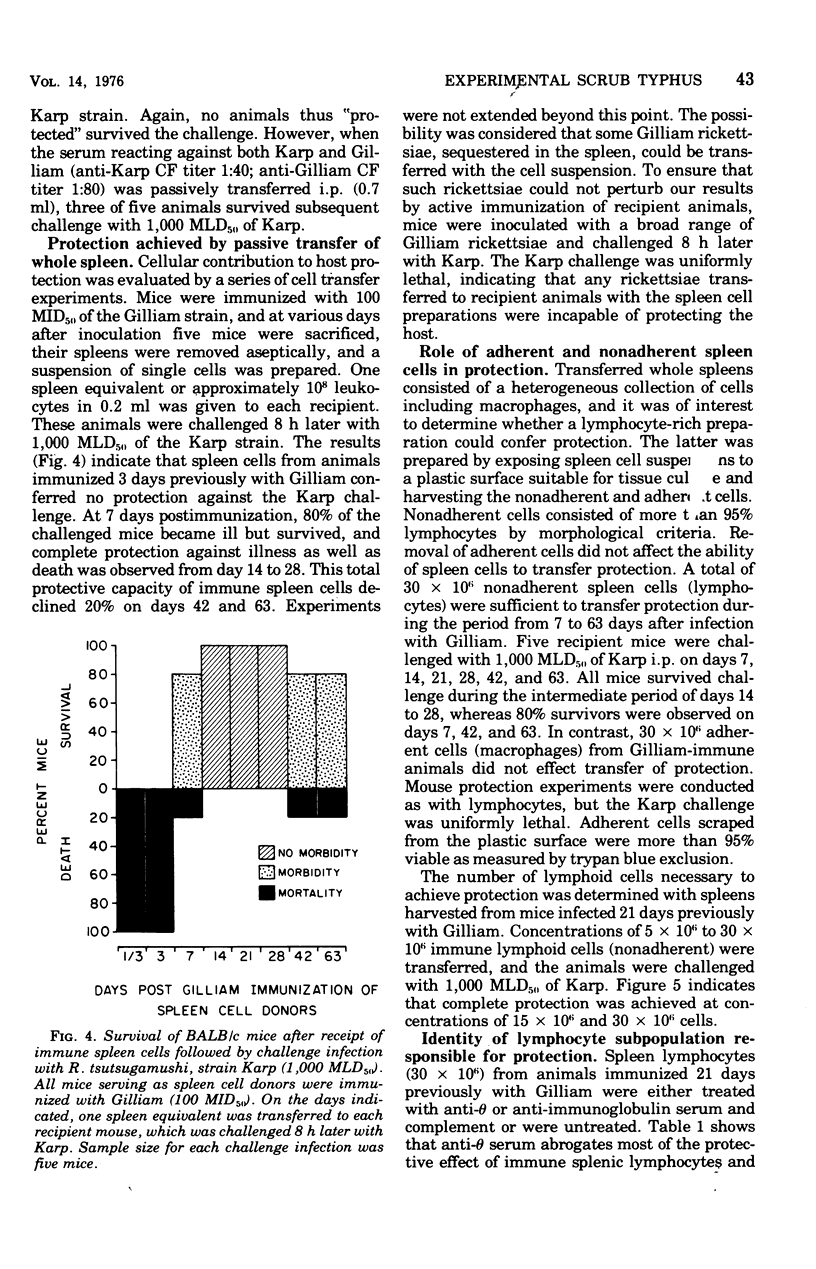
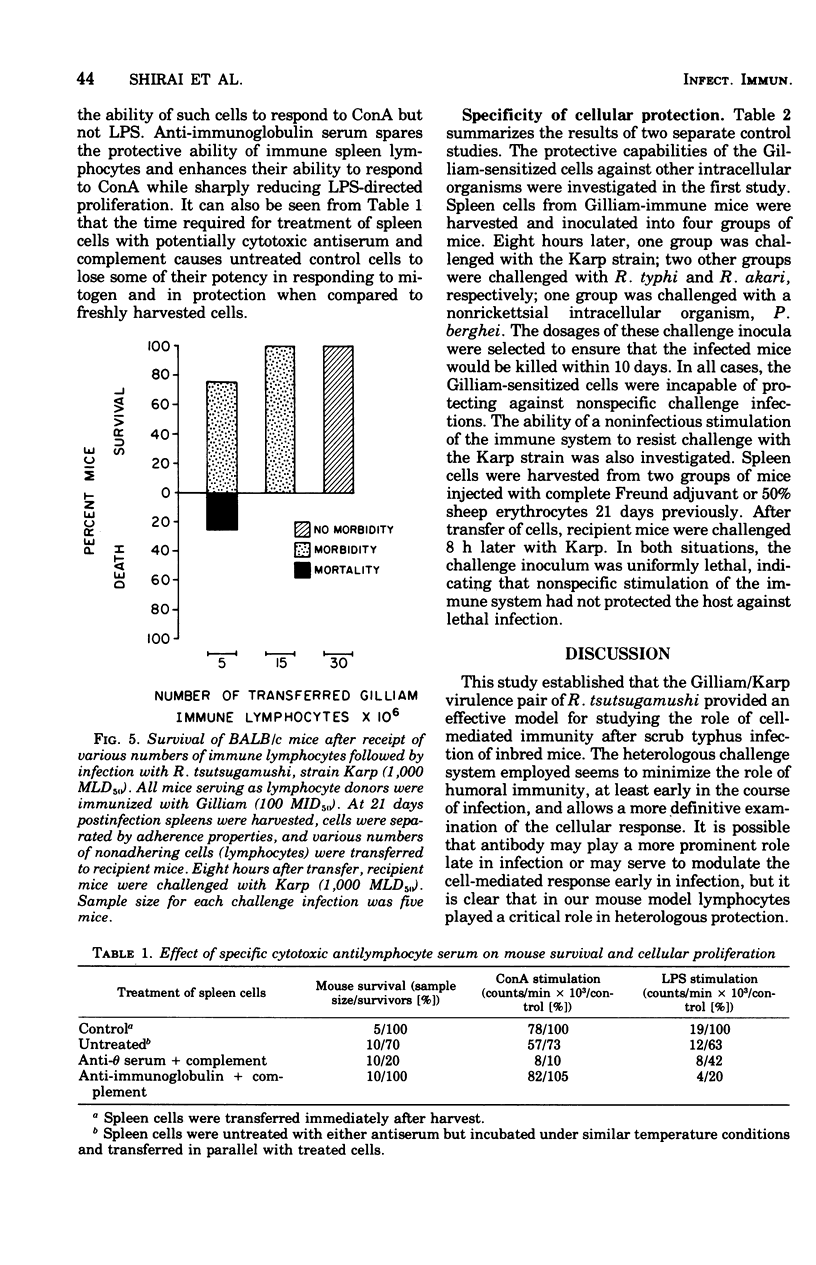
The relative contributions of cellular and humoral immunity in scrub typhus infections were studied in inbred mice employing paired strains of Rickettsia tsutsugamushi differing in virulence. An infectious dose (100 MID50) of the less virulent Gilliam strain resulted in heterologous immune protection against an otherwise lethal challenge (1,000 MLD50) of the virulent Karp strain. Partial heterologous protection against lethal Karp challenge was observed in animals preimmunized with the Gilliam strain as early as 3 days prior to challenge, whereas complete protection against illness and death existed in animals immunized at least 7 days prior to challenge. In the heterologous protection provided by prior Gilliam infection, the role of humoral immunity was not of primary importance for the following reasons: (i) significant levels of complement-fixing antibody against R. tsutsugamushi were not detectable until long after animals were solidly immune; (ii) antibody eventually appearing after Gilliam immunization exhibited a consistently low complement-fixing titer against the immunizing homologous (Gilliam) strain and contained no detectable activity against the heterologous challenge (Karp) strain; and (iii) passive transfer of large quantities of serum from Gilliam immune mice, themselves immune to Karp challenge, failed to protect recipients against a similar challenge. However, protection was afforded by the passive transfer of serum containing antibody against Karp, suggesting a major role for antibody in protection against homologous infection. This heterologous challenge system was particularly useful because it minimized the role of humoral immunity, at least early in the course of infection, and allowed a definitive examination of the cellular response. Cell-mediated immunity played a major role in the heterologous protection observed after Gilliam immunization. This was evidenced by the significant protection against Karp challenge afforded by the passive transfer of spleen cells from animals immunized with Gilliam 7 to 63 days previously. Of the immune spleen cells, only those which were nonadherent, presumably lymphocytes, were capable of transferring passive heterologous protection. This protective effect of nonadherent cells could be ablated by depleting the cell population of thymus-derived or T cells with anti-theta serum and complement prior to transfer but not by use of anti-immunoglobulin serum and complement, which selectively removes bone marrow-derived or B cells. These results suggested that the cell in immune spleens capable of conferring heterologous protection was a T lymphocyte.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro P. J., Shirai A., Hilderbrandt P. K., Osterman J. V. Host defenses in experimental scrub typhus: histopathological correlates. Infect Immun. 1976 Mar;13(3):861–875. doi: 10.1128/iai.13.3.861-875.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod J. D., Shepard C. C. Lymphocyte transformation in rickettsioses. J Immunol. 1971 Jan;106(1):209–216. [PubMed] [Google Scholar]
- Elisberg B. L., Campbell J. M., Bozeman F. M. Antigenic diversity of rickettsia tsutsugamushi: epidemiologic and ecologic significance. J Hyg Epidemiol Microbiol Immunol. 1968;12(1):18–25. [PubMed] [Google Scholar]
- Heggers J. P., Mallavia L. P., Hinrichs D. J. The cellular immune response to antigens of Coxiella burneti. Can J Microbiol. 1974 May;20(5):657–662. doi: 10.1139/m74-101. [DOI] [PubMed] [Google Scholar]
- JACKSON E. B., SMADEL J. E. Immunization against scrub typhus. II. Preparation of lyophilized living vaccine. Am J Hyg. 1951 May;53(3):326–331. doi: 10.1093/oxfordjournals.aje.a119457. [DOI] [PubMed] [Google Scholar]
- Jerrells T. R., Mallavia L. P., Hinrichs D. J. Detection of long-term cellular immunity to Coxiella burneti as assayed by lymphocyte transformation. Infect Immun. 1975 Feb;11(2):280–286. doi: 10.1128/iai.11.2.280-286.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENT J. F., FIFE E. H., Jr Precise standardization of reagents for complement fixation. Am J Trop Med Hyg. 1963 Jan;12:103–116. doi: 10.4269/ajtmh.1963.12.103. [DOI] [PubMed] [Google Scholar]
- KUWATA T. Analysis of immunity in experimental Tsutsugamushi disease. J Immunol. 1952 Feb;68(2):115–120. [PubMed] [Google Scholar]
- LEY H. L., Jr, DIERCKS F. H., PATERSON P. Y., SMADEL J. E., WISSEMAN C. L., Jr, TRAUB R. Immunization against scrub typhus. IV. Living Karp vaccine and chemoprophylaxis in volunteers. Am J Hyg. 1952 Nov;56(3):303–312. [PubMed] [Google Scholar]
- Raff M. C., Sternberg M., Taylor R. B. Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature. 1970 Feb 7;225(5232):553–554. doi: 10.1038/225553a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Wortis H. H. Thymus dependence of theta-bearing cells in the peripheral lymphoid tissues of mice. Immunology. 1970 Jun;18(6):931–942. [PMC free article] [PubMed] [Google Scholar]
- Reif A. E., Allen J. M. Mouse thymic iso-antigens. Nature. 1966 Jan 29;209(5022):521–523. doi: 10.1038/209521b0. [DOI] [PubMed] [Google Scholar]
- SALVIN S. B., BELL E. J. Resistance of mice with experimental histoplasmosis to infection with Rickettsia typhi. J Immunol. 1955 Jul;75(1):57–62. [PubMed] [Google Scholar]
- SMADEL J. E., LEY H. L., Jr, DIERCKS F. H., TRAUB R., TIPTON V. J., FRICK L. P. Immunization against scrub typhus. I. Combined living vaccine and chemoprophylaxis in volunteers. Am J Hyg. 1951 May;53(3):317–325. [PubMed] [Google Scholar]
- SMADEL J. E., LEY H. L., Jr, DIERCKS R. H., CAMERON J. A. P. Persistence of Rickettsia tsutsugamushi in tissues of patients recovered from scrub typhus. Am J Hyg. 1952 Nov;56(3):294–302. doi: 10.1093/oxfordjournals.aje.a119553. [DOI] [PubMed] [Google Scholar]
- Schramek S. Deoxyribonucleic acid base composition of members of the typhus group of rickettsiae. Acta Virol. 1972 Sep;16(5):447–447. [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. I. Responsiveness to and surface binding of concanavalin A and phytohemagglutinin. J Immunol. 1972 Jan;108(1):1–17. [PubMed] [Google Scholar]
- TRAUB R., JOHNSON P. T., MIESSE M. L., ELBEL R. E. Isolation of Rickettsia tsutsugamushi from rodents from Thailand. Am J Trop Med Hyg. 1954 Mar;3(2):356–359. doi: 10.4269/ajtmh.1954.3.356. [DOI] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Weiss E., Millar D. B., Bozeman F. M., Ormsbee R. A. DNA base composition of rickettsiae. Science. 1973 Apr 27;180(4084):415–417. doi: 10.1126/science.180.4084.415. [DOI] [PubMed] [Google Scholar]
- Wisseman C. L., Jr DNA composition in Rickettsia mooseri by base analysis. Acta Virol. 1973 Sep;17(5):443–443. [PubMed] [Google Scholar]
- Wisseman C. L., Jr, el Batawi Y., Wood W. H., Jr, Noriega A. R. Gross and microscopic skin reactions to killed typhus Rickettsiae in human beings. J Immunol. 1967 Jan;98(1):194–209. [PubMed] [Google Scholar]



