Abstract
Objective
To determine the relationship of perioperative hyperglycemia and insulin administration on outcomes in elective colon/rectal and bariatric operations.
Background
There is limited evidence to characterize the impact of perioperative hyperglycemia and insulin on adverse outcomes in patients, with and without diabetes, undergoing general surgical procedures.
Methods
The Surgical Care and Outcomes Assessment Program is a Washington State quality improvement benchmarking-based initiative. We evaluated the relationship of perioperative hyperglycemia (>180 mg/dL) and insulin administration on mortality, reoperative interventions, and infections for patients undergoing elective colorectal and bariatric surgery at 47 participating hospitals between fourth quarter of 2005 and fourth quarter of 2010.
Results
Of the 11,633 patients (55.4 ± 15.3 years; 65.7% women) with a serum glucose determination on the day of surgery, postoperative day 1, or postoperative day 2, 29.1% of patients were hyperglycemic. After controlling for clinical factors, those with hyperglycemia had a significantly increased risk of infection [odds ratio (OR) 2.0; 95% confidence interval (CI), 1.63–2.44], reoperative interventions (OR, 1.8; 95% CI, 1.41–2.3), and death (OR, 2.71; 95% CI, 1.72–4.28). Increased risk of poor outcomes was observed both for patients with and without diabetes. Those with hyperglycemia on the day of surgery who received insulin had no significant increase in infections (OR, 1.01; 95% CI, 0.72–1.42), reoperative interventions (OR, 1.29; 95% CI, 0.89–1.89), or deaths (OR, 1.21; 95% CI, 0.61–2.42). A dose-effect relationship was found between the effectiveness of insulin-related glucose control (worst 180–250 mg/dL, best <130 mg/dL) and adverse outcomes.
Conclusions
Perioperative hyperglycemia was associated with adverse outcomes in general surgery patients with and without diabetes. However, patients with hyperglycemia who received insulin were at no greater risk than those with normal blood glucoses. Perioperative glucose evaluation and insulin administration in patients with hyperglycemia are important quality targets.
Keywords: diabetes, insulin, perioperative hyperglycemia, quality improvement
Hyperglycemia is a common occurrence in patients undergoing surgery, and undiagnosed insulin resistance identified on the day of surgery (DOS) is increasingly common.1,2 Postoperative blood glucose greater than 140 mg/dL is present in as many as 40% of noncardiac surgery patients, with 25% of those patients having a blood glucose level greater than 180 mg/dL.2 Perioperative hyperglycemia has been associated with postoperative complications in vascular surgery,3 mastectomies,4 neurosurgery,5,6 spine surgery,7,8 transplant surgery,9 colorectal surgery,10 hepatobiliary-pancreatic surgery,11 and cholecystectomy.12 The oldest and most extensive evidence of this association is in cardiac surgery13–15 and surgical patients in intensive care units.16–19 Despite these observations, glycemic monitoring and control are often overlooked among general surgery patients.20–22 One study reported that glucose monitoring occurred in only 59% of hospitalized patients, and only 54% of those patients with elevated glucose received insulin therapy.1
There is evidence that suggests that hyperglycemia is a modifiable, independent predictor and possibly a causal factor of adverse outcomes in diabetic patients. In cardiac surgery, using insulin to improve glucose control was associated with reduced in-hospital mortality and infection rates among diabetic patients decreasing their rates to the rate of nondiabetic patients.15,23,24 Other studies among critically ill patients have shown similar findings.16,19,25 There are limited data on the impact of perioperative hyperglycemia on general surgery patients. Two retrospective cohort studies at single, academic institution noted an association of hyperglycemia with outcomes but did not explore the impact of insulin on reducing adverse outcomes.26,27 One recent multi-institutional randomized trial (N = 211) found that improved glycemic control using a basal-bolus regimen of insulin in general surgery patients with type 2 diabetes was associated with a reduction in average and maximum glucose levels and a significant decrease in a composite of outcomes21 when compared with less effective sliding scale insulin. The impact of insulin for hyperglycemia in general surgery patients without diabetes has yet to be evaluated.
We performed an observational evaluation of the association of perioperative hyperglycemia and outcomes among a broad network of hospitals in Washington State participating in Surgical Care and Outcomes Assessment Program (SCOAP). SCOAP is a prospectively gathered clinical care benchmarking and quality improvement activity now implemented at nearly all statewide hospitals where surgery is performed (n = 55).28 The purpose of this study was to evaluate the relationship of perioperative hyperglycemia, degree and timing of hyperglycemia, and the impact of perioperative insulin administration on mortality and complications in patients undergoing elective colon/rectal and bariatric operations.
METHODS
Study Design
This study was approved by the University of Washington Human Subject Review Committee and the Washington State Department of Health. A retrospective cohort study was conducted using SCOAP’s prospectively gathered data drawn from in-hospital medical records by trained, audited abstractors using standardized definitions (http://www.scoap.org/documents/index.html). Data from 47 of 55 Washington State hospitals currently participating in SCOAP were available by the time of this analysis. Nonparticipating hospitals are smaller hospitals that do not perform many bariatric and/or colorectal operations. Records of inpatient hospitalization between fourth quarter of 2005 and fourth quarter of 2010 were used to assess outcomes for patients undergoing elective colon/rectal resections and bariatric operations.
Data Specification
Clinical Risk Factors
SCOAP records were used to obtain patient’s sociodemographic characteristics, clinical comorbidities, and operative details. Health status was classified using the Deyo modification29 of the Charlson comorbidity to calculate a weighted index of comorbid conditions for each patient (categorized 0–3, with 3 or more indicating greatest comorbidity).29 Body mass index, smoking status, diabetes (including insulin dependency), history of coronary artery disease, history of cancer, and current immunosuppression medication use were available. Normothermia was defined as first recovery room temperature of 36.0°C or greater. Prophylactic antibiotic was defined as antibiotics given within 60 minutes before incision.
Type/Method of Operation
Bariatric operations included laparoscopic and open Rouxen-Y gastric bypass, laparoscopic gastric band placement, sleeve gastrectomy, biliopancreatic bypass with and without duodenal switch, vertical banded gastroplasty, and revision of gastric bypass. Colon operations included right/transverse and left hemicolectomy, low anterior resection, abdominal perineal resection, total abdominal colectomy, stoma takedown, perineal proctectomy, and abdominal proctectomy. Method of operation was specified as laparoscopic, open, laparoscopic converted to open, and laparoscopic/hand-assisted.
Hyperglycemia and Insulin Use
SCOAP collects information on the highest blood glucose level at 3 different time periods: DOS, postoperative day (POD) 1, and POD 2. As a statewide quality improvement program designed to feedback information to hospitals and surgeons regarding process and outcome at a minimal expense, SCOAP does not record all blood glucose values available and thus does not have information on variability or hypoglycemia. DOS consists of fasting blood glucose before incision on DOS, blood glucose intraoperatively, or within 60 minutes of operative close time. POD 1 is defined as the 24-hour period beginning at midnight after surgery. POD 2 is defined as the 24-hour period that began at midnight after POD 1. The American Diabetes Association recommends random blood glucose levels to be kept below 180 mg/dL in noncritically ill patients.30 Accordingly, hyperglycemia was defined as glucose levels above 180 mg/dL. SCOAP contained records of administration of insulin only during the DOS period.
Outcomes
The prospectively defined primary outcome was the rate of infection. Infection in SCOAP is clinically defined as antibiotics being started for presumed or confirmed infections, wound being reopened secondary to presumed infection, and record of abscess drainage procedure during the hospitalization and within 30 days. These 3 clinical interventions were used to define composite infections. Secondary outcomes explored included in-hospital death, reoperative interventions, length of stay (in days), and myocardial infarctions.
Analysis
Patient characteristics and outcomes were summarized using frequency distributions for categorical variables and means and standard deviations for continuous variables stratified by hyperglycemia. Descriptive statistics were produced for primary and secondary outcomes for the whole cohort, for colorectal and bariatric patient populations separately, and for diabetic and nondiabetic patients separately. P values for the differences between groups were obtained using the independent 2-sample Student t test for unequal variances on continuous variables and Pearson χ2 statistics for categorical variables. Logistic regression models were created a priori to evaluate the association between hyperglycemia and outcomes adjusting for patient, clinical, and operative characteristics identified as statistically significant (P < 0.05) on bivariate evaluation or found to be important in previous studies. We also looked at the effect of degree of glucose elevation on outcomes. Adjusted logistic regression model was used for patients categorized by glucose values in incrementals of 10 mg/dL. Next, we looked at those with glucose checks in all 3 days and used the adjusted logistic regression model on infection by the timing of when patients had hyperglycemia (DOS or postoperative).
Finally, we analyzed data from the “hyperglycemia on DOS-only” group given that postoperative hyperglycemia may be a marker of early postoperative complications (ie, infections may have caused postoperative hyperglycemia), although this is less likely in the first 2 days postoperatively. Considering all patients with DOS glucose checks, we used the same logistic regression model to look at the relationship of DOS hyperglycemia to postoperative mortality and morbidity. To assess the contribution of insulin in reducing such outcomes, an augmented model included DOS insulin administration. A sensitivity analysis was performed among those with glucose checks in all 3 days to account for differential risk among groups receiving insulin and those not. We also examined the impact of more effective glucose control on days 1 and 2 from best control (<130 mg/dL) to worst control (180–250 mg/dL). STATA was used for all analyses (Version 11; STATACorp, College Station, TX).
RESULTS
Of 18,278 patients in this time period, 7653 patients had glucose recorded on DOS, 8330 patients on POD 1, 5533 patients on POD 2, and 3352 patients in all 3 periods. Those who had glucose checks in all 3 periods were older (58.0 ± 14.5 years), had a higher Charlson index score (70.3% with >1 comorbidity), and were more likely to have diabetes (61.5%). In comparison, patients with glucose checks in only 1 of the 3 periods were younger (55.4 ± 15.3 years), had lower Charlson index score (48.9% with >1 comorbidity), and were less likely to have diabetes (35.3%).
A total of 11,633 patients had their glucose checked in at least 1 of the 3 periods. Of this cohort, compared with patients with glucose levels of 180 mg/dL or less (n = 8247, 70.9%), patients with glucose levels of more than 180 mg/dL at any point (n = 3383, 29.1%) were older, with higher Charlson index score, and more likely to have Medicare and Medicaid coverage, diabetes, coronary artery disease, hypertension, higher body mass index, creatinine levels more than 2 mg/dL, albumin levels less than 3 g/dL, and home oxygen treatment (Table 1).
TABLE 1.
Patient Demographics of Those Tested for Glucose and Stratified by Perioperative Hyperglycemia (Defined as >180 mg/dL at Any Point on the Day of Surgery, Postoperative Day 1, or Postoperative Day 2)
| Normal Glucose | Hyperglycemia | P | |
|---|---|---|---|
| Number | 8247 | 3383 | |
| Clinical characteristics | |||
| Age, yr | 54.3 ± 15.8 | 58.1 ± 13.6 | <0.001 |
| Sex (% female) | 5377 (65.2%) | 2268 (67.0%) | 0.06 |
| Insurance | |||
| Private | 5509 (67.1%) | 2170 (64.4%) | 0.005 |
| Medicare | 2354 (28.7%) | 1299 (38.6%) | <0.001 |
| Medicaid | 515 (6.3%) | 249 (7.4%) | 0.03 |
| Uninsured | 109 (1.3%) | 31 (0.9%) | 0.07 |
| Charlson comorbidity index | <0.001 | ||
| 0 | 5,289 (64.1%) | 771 (22.8%) | |
| 1 | 2,242 (27.2%) | 1,776 (52.5%) | |
| 2 | 603 (7.3%) | 714 (21.1%) | |
| 3+ | 115 (1.4%) | 123 (3.6%) | |
| Diabetes | 1729 (21.0%) | 2369 (70.1%) | <0.001 |
| Diabetes treatment | <0.001 | ||
| No meds | 420 (24.1%) | 231 (9.8%) | |
| Single noninsulin | 776 (44.6%) | 740 (31.2%) | |
| Multiple noninsulin | 229 (13.2%) | 437 (18.5%) | |
| Insulin | 132 (7.6%) | 370 (15.6%) | |
| Insulin plus other | 185 (10.6%) | 591 (25.0%) | |
| BMI for colorectal procedures | 27.8 ± 7.5 | 29.3 ± 7.6 | <0.001 |
| BMI for bariatric procedures | 45.8 ± 13.7 | 46.8 ± 12.6 | 0.009 |
| Tobacco use | 1287 (15.6%) | 370 (11.0%) | <0.001 |
| Creatinine >2 mg/dL | 97 (1.5%) | 71 (2.7%) | <0.001 |
| Home oxygen | 90 (1.1%) | 68 (2.0%) | <0.001 |
| Immunosuppression* | 373 (4.5%) | 181 (5.4%) | 0.06 |
| Coronary artery disease | 646 (7.8%) | 464 (13.7%) | <0.001 |
| Hypertension | 4212 (51.1%) | 2453 (72.5%) | <0.001 |
| Procedural characteristics | |||
| Procedure types | <0.001 | ||
| Bariatric | 3513 (42.6%) | 1847 (54.6%) | |
| Colorectal | 4736 (57.4%) | 1537 (45.4%) | |
| Surgical approach | <0.001 | ||
| Laparoscopic | 3,795 (46.1%) | 1,760 (52.1%) | |
| Lap converted to open | 362 (4.4%) | 152 (4.5%) | |
| Lap, hand assisted | 869 (10.6%) | 216 (6.4%) | |
| Open | 3163 (38.4%) | 1243 (36.8%) | |
| Indication for surgery | 0.9 | ||
| % Cancer | 1696 (20.6%) | 699 (20.7%) | |
| Surgery time | 145.7 ± 91.9 | 168.5 ± 101.4 | <0.001 |
| Prophylactic antibiotics† | 7462 (97.4%) | 3094 (97.4%) | 0.9 |
| Normothermia | 7,473 (95.1%) | 2,980 (95.1%) | 0.9 |
Patients on immunosuppressants preoperatively.
Preoperative antibiotics given within 60 minutes of incision.
BMI indicates body mass index.
The unadjusted rates of in-hospital mortality (1.5% vs 0.6%, P < 0.001), reoperative intervention (4.4% vs 3.1%, P < 0.001), and composite infections (6.0% vs 3.4%, P < 0.001) were higher among those who had hyperglycemia. Similar trends were seen when considering nondiabetic and diabetic patients separately (Fig. 1). Patients with hyperglycemia also had longer length of stay (6.0 ± 8.5 days vs 5.3 ± 7.4 days, P < 0.001) and were less likely to be discharged to home (91.5% vs 94.9%, P < 0.001). Significant differences in these outcomes were seen in both colorectal [in-hospital mortality (3.1% vs 1.0%, P < 0.001), reoperative intervention (5.9% vs 4.3%, P < 0.001), and composite infections (14.8% vs 9.6%, P < 0.001)] and bariatric [in-hospital mortality (0.22% vs 0.09%, P < 0.001), reoperative intervention (3.1% vs 1.6%, P < 0.001), and composite infections (2.9% vs 1.0%, P < 0.001)] procedural groups.
FIGURE 1.
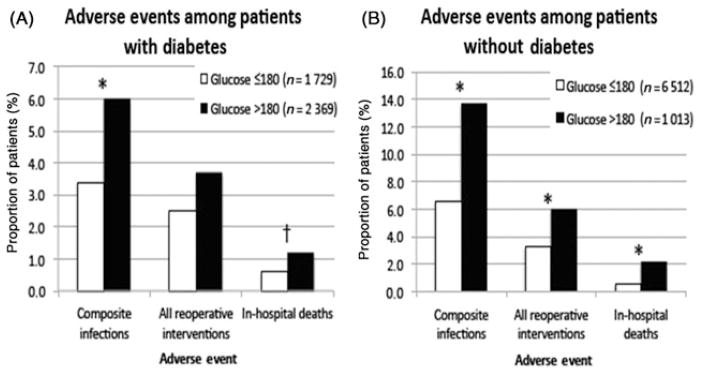
Outcomes stratified by perioperative hyperglycemia (>180 mg/dL at any point on the day of surgery, postoperative day 1, or postoperative day 2) for diabetic patients (A) and nondiabetic patients (B). *P < 0.01; †P < 0.05.
After controlling for clinical covariates (age, sex, Charlson comorbidity index, body mass index, smoking, immunosuppression, cancer, diabetes, prophylactic antibiotics, year of the operation, and type of surgical procedure), patients who had any hyperglycemia had a 2-fold higher risk of infection [odds ratio (OR) 2.0; 95% confidence interval (CI), 1.63–2.44]. They also had increased risk of death, reoperative interventions, and anastomotic failures (Table 2). These risks did not change in our sensitivity analyses controlling for hospital effects [infection (OR, 1.79; 95% CI, 1.49–2.16), death (OR, 2.63; 95% CI, 1.65–4.18), reoperative interventions (OR, 1.78; 95% CI, 1.4–2.27), and anastomotic failures (OR, 2.37; 95% CI, 1.39–4.03)]. Those with postoperative hyperglycemia had a higher risk of infection than those who were hyperglycemic only on DOS (with highest odds if hyperglycemic on both PODs) (Table 3). We found that for every 10-unit increase in blood glucose levels, there was a 7% increased odds of infection (OR, 1.07; 95% CI, 1.04–1.09). Among patients with glucose checks in all 3 periods, patients who had hyperglycemia on both POD 1 and POD 2 had the highest odds of infection (OR, 3.1; 95% CI, 1.72–5.59) compared with those with no hyperglycemia.
TABLE 2.
Adjusted Multivariate Logistic Regression Analysis on the Effect of Perioperative Hyperglycemia (>180 mg/dL at Any Point on the Day of Surgery, Postoperative Day 1, or Postoperative Day 2) on Outcomes Presented as Odds Ratio and 95% Confidence Intervals (Within Parenthesis)
| Composite Infections (n = 491) | Deaths (n = 48) | Reoperative Interventions (n = 257) | Anastomotic Failures (n = 43) | Myocardial Infarctions (n = 13) | |
|---|---|---|---|---|---|
| Hyperglycemia | 2.0 (1.63–2.44) | 2.71 (1.72–4.28) | 1.8 (1.41–2.3) | 2.43 (1.38–4.28) | 1.15 (0.43–3.1) |
| Age, yr | 1.01 (1.0–1.01) | 1.07 (1.04–1.09) | 1.0 (0.99–1.01) | 1.0 (0.98–1.02) | 1.08 (1.03–1.12) |
| Male sex | 1.33 (1.12–1.58) | 1.56 (1.0–2.42) | 1.63 (1.32–2.01) | 1.56 (0.94–2.57) | 1.28 (0.56–2.9) |
| Charlson comorbidity index | |||||
| 1 | 1.14 (0.9–1.45) | 2.01 (1.17–3.48) | 1.23 (0.91–1.66) | 1.75 (0.92–3.33) | 1.65 (0.4–6.88) |
| 2 | 1.69 (1.18–2.44) | 2.28 (1.04–4.98) | 1.21 (0.77–1.91) | 1.73 (0.67–4.5) | 0.42 (0.05–3.27) |
| 3+ | 2.79 (1.67–4.66) | 5.3 (2.01–13.97) | 3.02 (1.74–5.24) | 7.38 (2.6–20.94) | 0.8 (0.03–18.63) |
| Body mass index | 1.01 (1.0–1.01) | 1.01 (0.99–1.02) | 1.01 (1.0–1.01) | 1.01 (1.0–1.01) | 0.98 (0.92–1.06) |
| Smoking | 1.29 (1.04–1.6) | 1.19 (0.64–2.23) | 1.02 (0.77–1.36) | 0.88 (0.45–1.73) | 1.02 (0.32–3.27) |
| Immunosuppression* | 1.47 (1.06–2.03) | — | 1.08 (0.7–1.65) | 1.52 (0.68–3.36) | — |
| Prophylactic antibiotics† | 0.78 (0.51–1.18) | — | — | — | — |
| Cancer | 1.12 (0.92–1.35) | 0.88 (0.56–1.39) | 0.81 (0.62–1.05) | 1.1 (0.62–1.96) | — |
| Year of the operation | 0.94 (0.86–1.02) | 0.92 (0.75–1.13) | 0.82 (0.75–0.9) | 0.77 (0.63–0.95) | — |
| Surgical procedure (colorectal vs bariatric)‡ | 5.76 (4.35–7.64) | 7.17 (2.91–17.66) | 2.24 (1.68–3.0) | 2.44 (1.26–4.72) | 0.83 (0.2–3.47) |
| Diabetes§ | |||||
| Noninsulin-dependent | 0.51 (0.37–0.69) | 0.48 (0.25–0.93) | 0.63 (0.44–0.9) | 0.45 (0.21–0.99) | 0.77 (0.15–4.08) |
| Insulin-dependent | 0.52 (0.35–0.76) | 0.78 (0.36–1.68) | 0.54 (0.35–0.85) | 0.49 (0.18–1.32) | 1.66 (0.26–10.71) |
| Coronary artery disease | — | — | — | — | 4.88 (1.53–15.6) |
Each of the following odds ratio listed is adjusted for all other covariates on the left column of the table (blank space indicates that the covariate was not included in the model).
Patients on immunosuppressants preoperatively.
Preoperative antibiotics given within 60 minutes of incision.
Odds ratios in patients undergoing colorectal operations compared with bariatric operations.
Patients with diabetes who are noninsulin-dependent and those who are insulin-dependent
TABLE 3.
Risk-Adjusted Odds Ratios for Composite Infection for Hyperglycemia (>180 mg/dL) During Different Perioperative Time Periods in Patients With Glucose Checks in All 3 Time Periods (Perioperative, POD 1, and POD 2) Presented as Odds Ratio and 95% Confidence Intervals (Within parenthesis)
| Composite Infection (OR, 95% CI)—for Hyperglycemia Only on the Day of Surgery (n = 84) | Composite Infection (OR, 95% CI)— for Hyperglycemia Only During POD 1 or POD 2 (n = 162) | Composite Infection (OR, 95% CI)—for Hyperglycemia Only During POD 1 and POD 2 (n = 81) | |
|---|---|---|---|
| Hyperglycemia | 1.7 (0.98–2.94) | 2.08 (1.43–3.02) | 3.1 (1.72–5.59) |
| Age, yr | 1.02 (1.0–1.04) | 1.01 (0.99–1.03) | 1.02 (1.0–1.04) |
| Male sex | 1.35 (0.88–2.07) | 1.38 (0.99–1.94) | 1.35 (0.91–2.01) |
| Charlson comorbidity index | |||
| 1 | 1.05 (0.56–1.98) | 0.94 (0.55–1.61) | 0.9 (0.48–1.67) |
| 2 | 2.35 (1.1–5.03) | 1.54 (0.79–2.99) | 2.44 (1.19–5.01) |
| 3+ | 2.67 (0.91–7.89) | 2.68 (1.06–6.78) | 2.62 (0.79–8.62) |
| Body mass index | 1.01 (0.99–1.01) | 1.0 (0.99–1.01) | 1.0 (0.99–1.01) |
| Smoking | 1.9 (1.17–3.08) | 1.7 (1.12–2.57) | 2.19 (1.38–3.48) |
| Immunosuppression* | 1.94 (0.94–4.0) | 1.81 (1.01–3.25) | 1.77 (0.91–3.46) |
| Prophylactic antibiotics† | 2.8 (0.38–20.74) | 1.71 (0.41–7.19) | 1.65 (0.4–6.8) |
| Cancer | 1.12 (0.71–1.76) | 1.04 (0.72–1.49) | 0.99 (0.65–1.52) |
| Year of the operation | 1.3 (0.94–4.0) | 1.29 (0.98–1.68) | 1.31 (0.96–1.79) |
| Surgical procedure (colorectal vs bariatric)‡ | 5.54 (2.87–10.69) | 6.6 (3.39–12.84) | 5.67 (2.56–12.56) |
| Diabetes§ | |||
| Noninsulin-dependent | 0.58 (0.3–1.14) | 0.61 (0.35–1.07) | 0.49 (0.25–0.95) |
| Insulin-dependent | 0.46 (0.16–1.37) | 0.42 (0.2–0.88) | 0.23 (0.08–0.63) |
Each of the following odds ratio listed is adjusted for all other covariates on the left column of the table.
Patients on immunosuppressants preoperatively.
Preoperative antibiotics given within 60 minutes of incision.
Odds ratios in patients undergoing colorectal operations compared with bariatric operations.
Patients with diabetes who are noninsulin-dependent and those who are insulin-dependent.
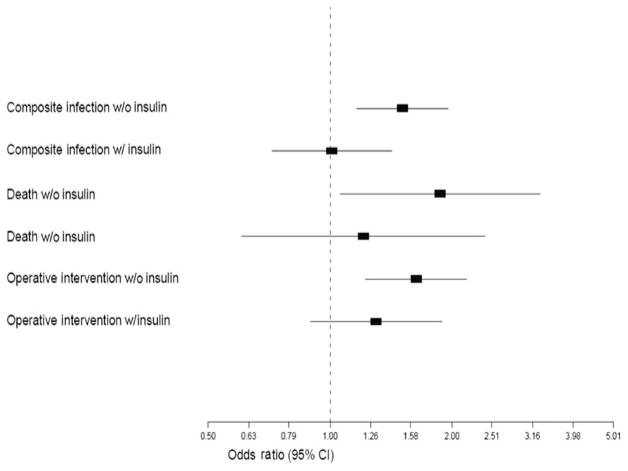
DOS hyperglycemia was associated with an increased adjusted odds of in-hospital mortality (OR, 1.87; 95% CI, 1.06–3.3), reoperative interventions (OR, 1.63; 95% CI, 1.21–2.19), and composite infections (OR, 1.51; 95% CI, 1.16–1.95) (Fig. 2). When insulin was added to the regression model, the adjusted odds ratios for all 3 outcomes were no longer significant (Fig. 2). Patients who were started on insulin included a higher proportion with insulin-dependent diabetes (66.7% among those who received insulin vs 33.3% among those who did not receive insulin), more often had higher baseline glucose levels (17.8% with glucose level >250 mg/dL in insulin group vs 1.5% in noninsulin group), and more often had hyperglycemia on POD 1 and POD 2 (62.6% insulin group vs 37.5% in noninsulin group). To evaluate the impact of insulin-related glucose control (≤ 180 mg/dL), we performed a sensitivity analysis among those with glucose checks in all 3 days. We compared composite adverse outcomes (deaths/infections/reoperations) among patients with hyperglycemia in the nonextreme range (between 180 and 250) who were and were not started on insulin perioperatively and evaluated those who achieved insulin-related glucose control (<180 on POD 1 and POD 2). We found that rates of composite adverse events were lower (5.7%) in those achieving insulin-related glucose control than those not started on insulin (10.6%) (P = 0.05). We found decreasing rates of adverse events with increasing levels of glucose control (composite adverse event rates of 0%, 5.9%, 6.5%, and 10.3% for patients with insulin and POD 1 and POD 2 glucose of <130, 130–150, 150–180, and 180–250, respectively), but the numbers in each group were small (ranging from 17 to 264). Despite this, 26.2% of patients with DOS hyperglycemia did not receive insulin.
FIGURE 2.
Multivariate logistic regression of composite infections, reoperative interventions, and in-patient mortality rates for hyperglycemia (>180 mg/dL) on the day of surgery with and without adjustment for administration of insulin.
DISCUSSION
Perioperative and postoperative hyperglycemia in general surgery patients with and without diabetes was associated with nearly 2-fold higher risk of infection, in-hospital mortality, and operative complications. Interestingly, the greatest risk of infection was among patients with no history of diabetes who experienced hyperglycemia. Although only 13.5% of nondiabetic patients had hyperglycemia compared with 58% of diabetic patients, 30% of all hyperglycemic episodes were in nondiabetic patients. Regardless of known diabetes status, insulin administration seemed to mitigate the association of hyperglycemia with adverse outcomes.
Perioperative hyperglycemia is an important marker for adverse events in surgical patients, with and without diabetes.2,9,10,17,19,25–27,31 Surgery in diabetic patients is associated with longer hospital stay,32 increased morbidity and mortality,33,34 and postoperative infection.35 Adverse effects may be worsened in diabetic patients who have acute hyperglycemia compared with chronic and sustained hyperglycemia.36 Interestingly, patients with newly diagnosed hyperglycemia have been shown to have higher mortality and lower functional outcome than those with normoglycemia or with a known history of diabetes.22 We also found that among patients with hyperglycemia, those without history of diabetes had worse outcome compared with patients with diabetes. Insulin helps avoid acute hyperglycemia in these patients. Beneficial effects of insulin may also come from its anti-inflammatory effects.37–40 Evaluating the clinical impact of insulin on outcomes using observational data is challenging. Insulin administration among patients with hyperglycemia does not seem to be a random event. This study found, patients started on insulin more often had greater diabetes severity, more extreme values of hyperglycemia, and were more likely to be hyperglycemic on POD 1 and POD 2. All of these variables were independently associated with increased risk of adverse events. Failure to account for this “confounding by indication” may lead the casual observer to think that insulin is a cause of adverse events rather than a marker for the higher risk of the patient. One of the strengths of this study is that we were able to account for severity of diabetes, degree of hyperglycemia, and also whether or not patients achieved insulin-related glucose control. Accounting for these severity “marker variables,” composite adverse event rates were significantly lower in those achieving insulin-related glucose control than those not started on insulin. We also found decreasing event rates with increasing insulin-related glucose control. This overall observation and finding of a dose-effect relationship suggest a cause-and-effect link between insulin-related glucose control and improved outcomes. This problem with confounding also highlights the limitation of observational data sets that do not account for these important variables. Our finding that the elevated risk of infection in patients with hyperglycemia improves with insulin administration is consistent with previous studies of other clinical environments.2,3,21,41
Previous studies have correlated the risk of infection with the degree of perioperative glucose elevation.3,42 We demonstrate an increasing risk of infection for every 10-unit increase in highest glucose. Previous studies have also reported an association of timing of hyper-glycemia with increased risk of adverse outcomes.26,42 Intraoperative and postoperative, but not preoperative and POD 2, hyperglycemia were associated with increased risk.26,43 In our subanalysis evaluating the timing of hyperglycemia in patients who had a glucose recorded each day for 3 days, postoperative hyperglycemia—compared with hyperglycemia on the DOS alone—had a stronger association with infection. This relationship increased when hyperglycemia was present on both PODs compared with just 1 POD.
Despite the importance of hyperglycemia, perioperative glucose levels frequently go unchecked.1 We found that only 64% of patients (55% of nondiabetic and 90% of diabetic patients) had at least 1 glucose recorded on DOS, POD 1, or POD 2. To address this, glucose checking for diabetic patients at induction of anesthesia has become a part of the SCOAP surgical checklist (www.SCOAPchecklist.org). Another barrier to effective glycemic control may be concerns about insulin administration. Among 37 academic medical centers, recommended regimens of insulin therapy were prescribed in only 45% of patients,44 and patients with hyperglycemia without a diagnosis of diabetes were less likely to be treated with insulin.22 We found that 26% of patients with hyperglycemia on the DOS (46% of these being nondiabetic) did not receive insulin. This may be due to limited awareness of the importance of perioperative hyperglycemia and the benefits of insulin in the hyperglycemic general surgery population.
Our study has limitations. A concern related to management of hyperglycemia is insulin-induced hypoglycemia.45–47 The NICE-SUGAR investigators have found increased mortality with intensive glucose control (81–108 mg/dL) compared with conventional target of less than 180 mg/dL.48 Although SCOAP added a hypoglycemia variable onto the database in 2010, it was not available for this analysis. We did not have information on the type of insulin (dose, continuous infusion vs basal bolus vs sliding scale) used. Patients with more glucose checks might have been patients who had a higher risk for complications. This is why our initial cohort was restricted to those with at least 1 glucose check, and our analysis on timing of hyperglycemia was restricted to those with all 3 glucose checks. The SCOAP database collects only highest glucose level that limited us in looking into how much glucose reduction resulted from the insulin administration on the DOS. Finally, it may be possible that the administration of insulin is a marker for better perioperative care in general and that some noninsulin benefits were conferred to patients who were given insulin. To address that we controlled for preoperative prophylactic antibiotics administration and normothermia as other markers of greater use of best practice for perioperative care to tease out some of the measured and perhaps unmeasured factors associated with better perioperative care, we performed a sensitivity analysis controlling for these and other hospital effects in hierarchical modeling which demonstrated similar results.
In summary, this is the first multi-institutional study evaluating the effect of perioperative hyperglycemia in general surgery patients using multiple endpoints and the impact of insulin administration on these endpoints. Our finding, based on the clinical records of patients from nearly the entire state of Washington, across all types of hospitals and communities, reinforces the relationship of perioperative hyperglycemia and postoperative complications and suggests that these complications are modifiable. The National Surgical Infection Prevention Program in support of the National Surgical Infection Prevention Project implemented by the Centers for Disease Control and Prevention and the Centers for Medicare & Medicaid Services has demonstrated that a bundle of interventions including glucose control in surgical patients was followed by lower rates of surgical infection and is achievable.49 We believe that the association of hyperglycemia and poor outcomes is such that patients undergoing bariatric and colorectal surgical procedures, with and without a history of diabetes, should be given consideration to have their glucose checked on the morning of surgery. Appropriate interventions and monitoring should be initiated when indicated for general surgery patients with hyperglycemia throughout the perioperative period.
Footnotes
Disclosure: SCOAP is a program of the Foundation for Healthcare Quality and is supported by a grant from Washington State’s Life Science Discovery Fund and Agency for Healthcare Research and Quality Grant Number 1 R01 HS 20025-01. There is no financial or personal conflict of interest by any of the authors.
References
- 1.Levetan CS, Passaro M, Jablonski K, et al. Unrecognized diabetes among hospitalized patients. Diabetes Care. 1998;21:246–249. doi: 10.2337/diacare.21.2.246. [DOI] [PubMed] [Google Scholar]
- 2.Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2011;33:1783–1788. doi: 10.2337/dc10-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vriesendorp TM, Morelis QJ, Devries JH, et al. Early post-operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study. Eur J Vasc Endovasc Surg. 2004;28:520–525. doi: 10.1016/j.ejvs.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Vilar-Compte D, Alvarez de Iturbe I, Martin-Onraet A, et al. Hyperglycemia as a risk factor for surgical site infections in patients undergoing mastectomy. Am J Infect Control. 2008;36:192–198. doi: 10.1016/j.ajic.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 5.McGirt MJ, Woodworth GF, Brooke BS, et al. Hyperglycemia independently increases the risk of perioperative stroke, myocardial infarction, and death after carotid endarterectomy. Neurosurgery. 2006;58:1066–1073. doi: 10.1227/01.NEU.0000215887.59922.36. [DOI] [PubMed] [Google Scholar]
- 6.McGirt MJ, Woodworth GF, Ali M, et al. Persistent perioperative hyperglycemia as an independent predictor of poor outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;107:1080–1085. doi: 10.3171/JNS-07/12/1080. [DOI] [PubMed] [Google Scholar]
- 7.Olsen MA, Mayfield J, Lauryssen C, et al. Risk factors for surgical site infection in spinal surgery. J Neurosurg. 2003;98:149–155. [PubMed] [Google Scholar]
- 8.Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008;90:62–69. doi: 10.2106/JBJS.F.01515. [DOI] [PubMed] [Google Scholar]
- 9.Park C, Hsu C, Neelakanta G, et al. Severe intraoperative hyperglycemia is independently associated with surgical site infection after liver transplantation. Transplantation. 2009;87:1031–1036. doi: 10.1097/TP.0b013e31819cc3e6. [DOI] [PubMed] [Google Scholar]
- 10.McConnell YJ, Johnson PM, Porter GA. Surgical site infections following colorectal surgery in patients with diabetes: association with postoperative hyperglycemia. J Gastrointest Surg. 2009;13:508–515. doi: 10.1007/s11605-008-0734-1. [DOI] [PubMed] [Google Scholar]
- 11.Ambiru S, Kato A, Kimura F, et al. Poor postoperative blood glucose control increases surgical site infections after surgery for hepatobiliary-pancreatic cancer: a prospective study in a high-volume institute in Japan. J Hosp Infect. 2008;68:230–233. doi: 10.1016/j.jhin.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Chuang SC, Lee KT, Chang WT, et al. Risk factors for wound infection after cholecystectomy. J Formos Med Assoc. 2004;103:607–612. [PubMed] [Google Scholar]
- 13.McAlister FA, Man J, Bistritz L, et al. Diabetes and coronary artery bypass surgery: an examination of perioperative glycemic control and outcomes. Diabetes Care. 2003;26:1518–1524. doi: 10.2337/diacare.26.5.1518. [DOI] [PubMed] [Google Scholar]
- 14.Latham R, Lancaster AD, Covington JF, et al. The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol. 2001;22:607–612. doi: 10.1086/501830. [DOI] [PubMed] [Google Scholar]
- 15.Schmeltz LR, DeSantis AJ, Thiyagarajan V, et al. Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy. Diabetes Care. 2007;30:823–828. doi: 10.2337/dc06-2184. [DOI] [PubMed] [Google Scholar]
- 16.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 17.Bochicchio GV, Sung J, Joshi M, et al. Persistent hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. 2005;58:921–924. doi: 10.1097/01.ta.0000162141.26392.07. [DOI] [PubMed] [Google Scholar]
- 18.Laird AM, Miller PR, Kilgo PD, et al. Relationship of early hyperglycemia to mortality in trauma patients. J Trauma. 2004;56:1058–1062. doi: 10.1097/01.ta.0000123267.39011.9f. [DOI] [PubMed] [Google Scholar]
- 19.Griesdale DE, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levetan CS, Magee MF. Hospital management of diabetes. Endocrinol Metab Clin North Am. 2000;29:745–770. doi: 10.1016/s0889-8529(05)70162-6. [DOI] [PubMed] [Google Scholar]
- 21.Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery) Diabetes Care. 2011;34:256–261. doi: 10.2337/dc10-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 23.Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 24.Furnary AP, Zerr KJ, Grunkemeier GL, et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67:352–360. doi: 10.1016/s0003-4975(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 25.Finney SJ, Zekveld C, Elia A, et al. Glucose control and mortality in critically ill patients. JAMA. 2003;290:2041–2047. doi: 10.1001/jama.290.15.2041. [DOI] [PubMed] [Google Scholar]
- 26.Ramos M, Khalpey Z, Lipsitz S, et al. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg. 2008;248:585–591. doi: 10.1097/SLA.0b013e31818990d1. [DOI] [PubMed] [Google Scholar]
- 27.Ata A, Lee J, Bestle SL, et al. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg. 2010;145:858–864. doi: 10.1001/archsurg.2010.179. [DOI] [PubMed] [Google Scholar]
- 28.Flum DR, Fisher N, Thompson J, et al. Washington State’s approach to variability in surgical processes/outcomes: Surgical Clinical Outcomes Assessment Program (SCOAP) Surgery. 2005;138:821–828. doi: 10.1016/j.surg.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van den Berghe G, Wouters PJ, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. 2003;31:359–366. doi: 10.1097/01.CCM.0000045568.12881.10. [DOI] [PubMed] [Google Scholar]
- 32.Carson JL, Scholz PM, Chen AY, et al. Diabetes mellitus increases short-term mortality and morbidity in patients undergoing coronary artery bypass graft surgery. J Am Coll Cardiol. 2002;40:418–423. doi: 10.1016/s0735-1097(02)01969-1. [DOI] [PubMed] [Google Scholar]
- 33.Bower WF, Jin L, Underwood MJ, et al. Overt diabetes mellitus adversely affects surgical outcomes of noncardiovascular patients. Surgery. 2010;147:670–675. doi: 10.1016/j.surg.2009.10.070. [DOI] [PubMed] [Google Scholar]
- 34.Barone BB, Yeh HC, Snyder CF, et al. Postoperative mortality in cancer patients with preexisting diabetes: systematic review and meta-analysis. Diabetes Care. 2010;33:931–939. doi: 10.2337/dc09-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26:510–513. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 36.Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 37.Booth G, Stalker TJ, Lefer AM, et al. Elevated ambient glucose induces acute inflammatory events in the microvasculature: effects of insulin. Am J Physiol Endocrinol Metab. 2001;280:E848–E856. doi: 10.1152/ajpendo.2001.280.6.E848. [DOI] [PubMed] [Google Scholar]
- 38.Pickup JC, Chusney GD, Thomas SM, et al. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67:291–300. doi: 10.1016/s0024-3205(00)00622-6. [DOI] [PubMed] [Google Scholar]
- 39.Turina M, Fry DE, Polk HC., Jr Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005;33:1624–1633. doi: 10.1097/01.ccm.0000170106.61978.d8. [DOI] [PubMed] [Google Scholar]
- 40.Dandona P, Aljada A, Mohanty P. The anti-inflammatory and potential anti-atherogenic effect of insulin: a new paradigm. Diabetologia. 2002;45:924–930. doi: 10.1007/s00125-001-0766-5. [DOI] [PubMed] [Google Scholar]
- 41.Zerr KJ, Furnary AP, Grunkemeier GL, et al. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg. 1997;63:356–361. doi: 10.1016/s0003-4975(96)01044-2. [DOI] [PubMed] [Google Scholar]
- 42.Gandhi GY, Nuttall GA, Abel MD, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc. 2005;80:862–866. doi: 10.4065/80.7.862. [DOI] [PubMed] [Google Scholar]
- 43.Pomposelli JJ, Baxter JK, III, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22:77–81. doi: 10.1177/014860719802200277. [DOI] [PubMed] [Google Scholar]
- 44.Boord JB, Greevy RA, Braithwaite SS, et al. Evaluation of hospital glycemic control at US academic medical centers. J Hosp Med. 2009;4:35–44. doi: 10.1002/jhm.390. [DOI] [PubMed] [Google Scholar]
- 45.Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006;355:1903–1911. doi: 10.1056/NEJMcp060094. [DOI] [PubMed] [Google Scholar]
- 46.Smiley DD, Umpierrez GE. Perioperative glucose control in the diabetic or nondiabetic patient. South Med J. 2006;99:580–589. doi: 10.1097/01.smj.0000209366.91803.99. [DOI] [PubMed] [Google Scholar]
- 47.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 48.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 49.Dellinger EP, Hausmann SM, Bratzler DW, et al. Hospitals collaborate to decrease surgical site infections. Am J Surg. 2005;190:9–15. doi: 10.1016/j.amjsurg.2004.12.001. [DOI] [PubMed] [Google Scholar]