Abstract
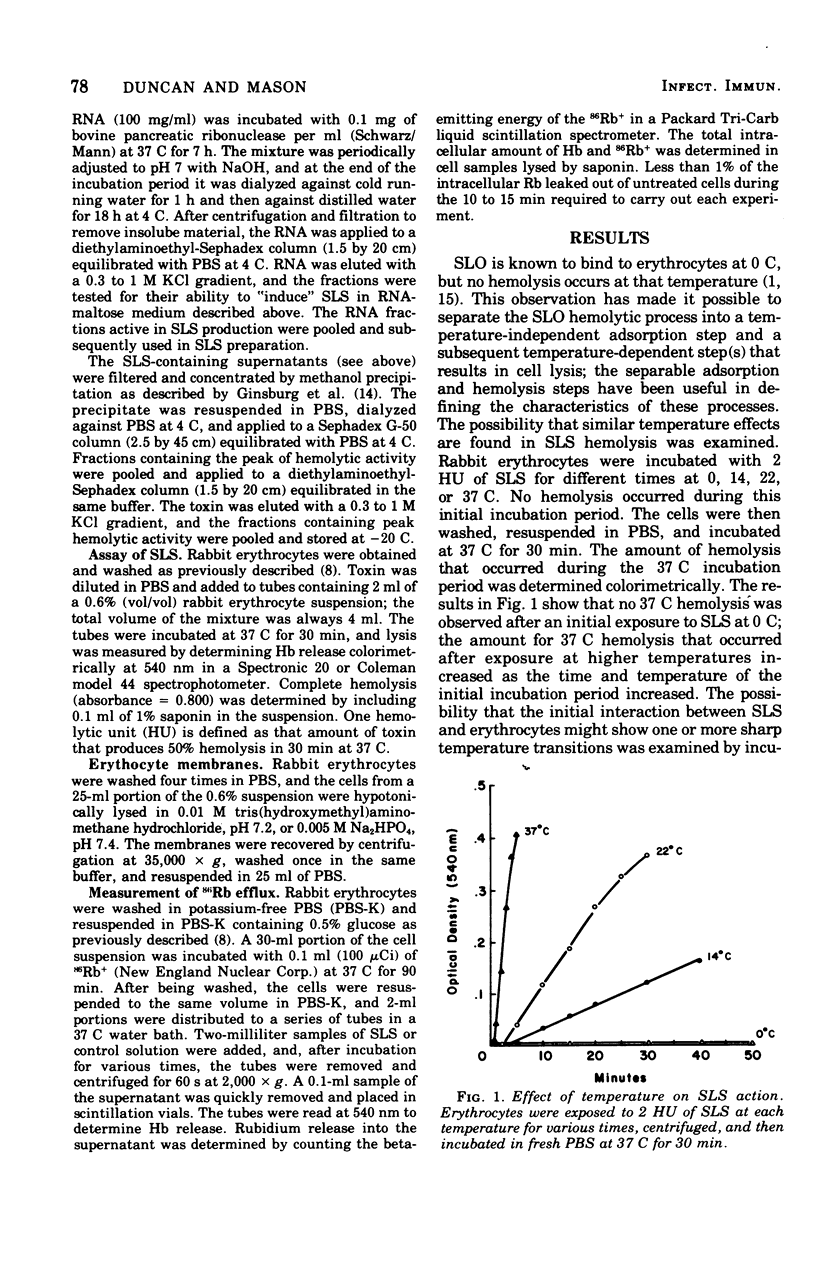
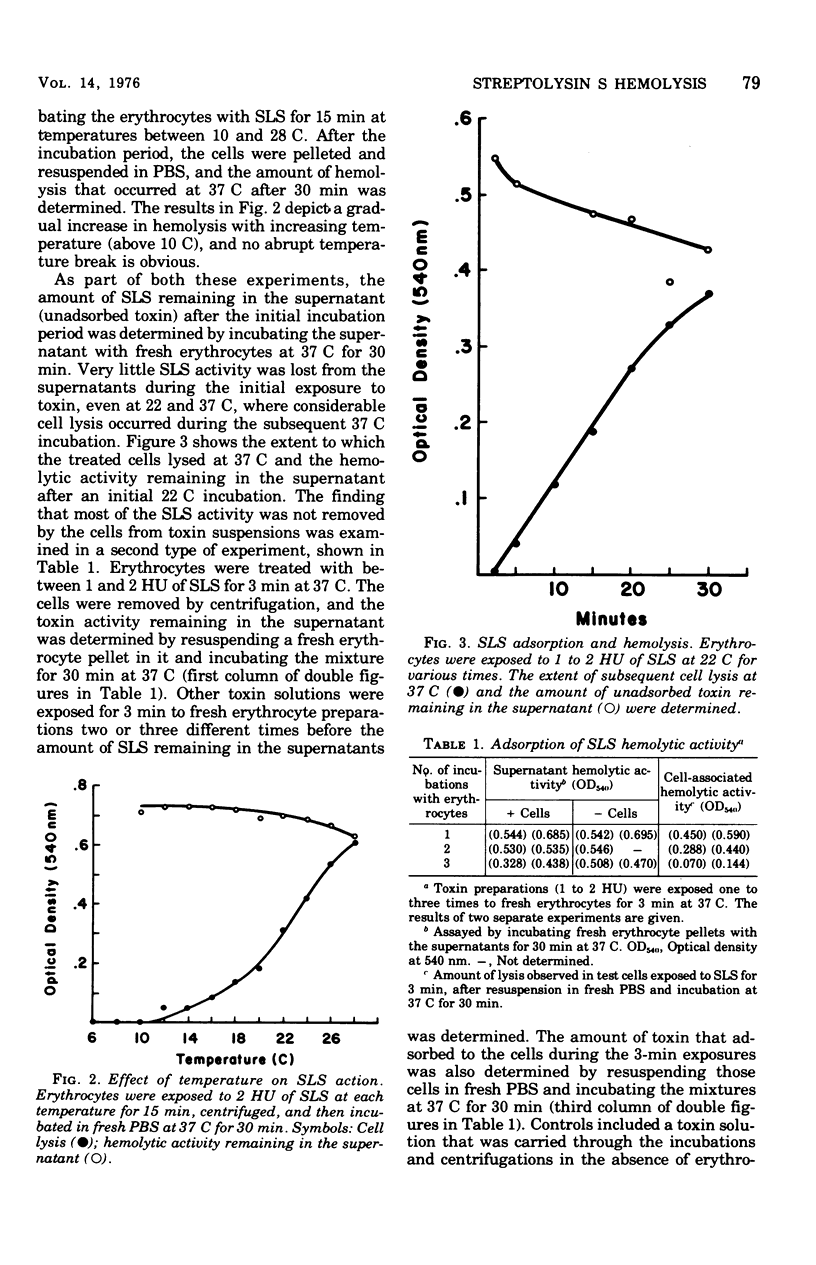
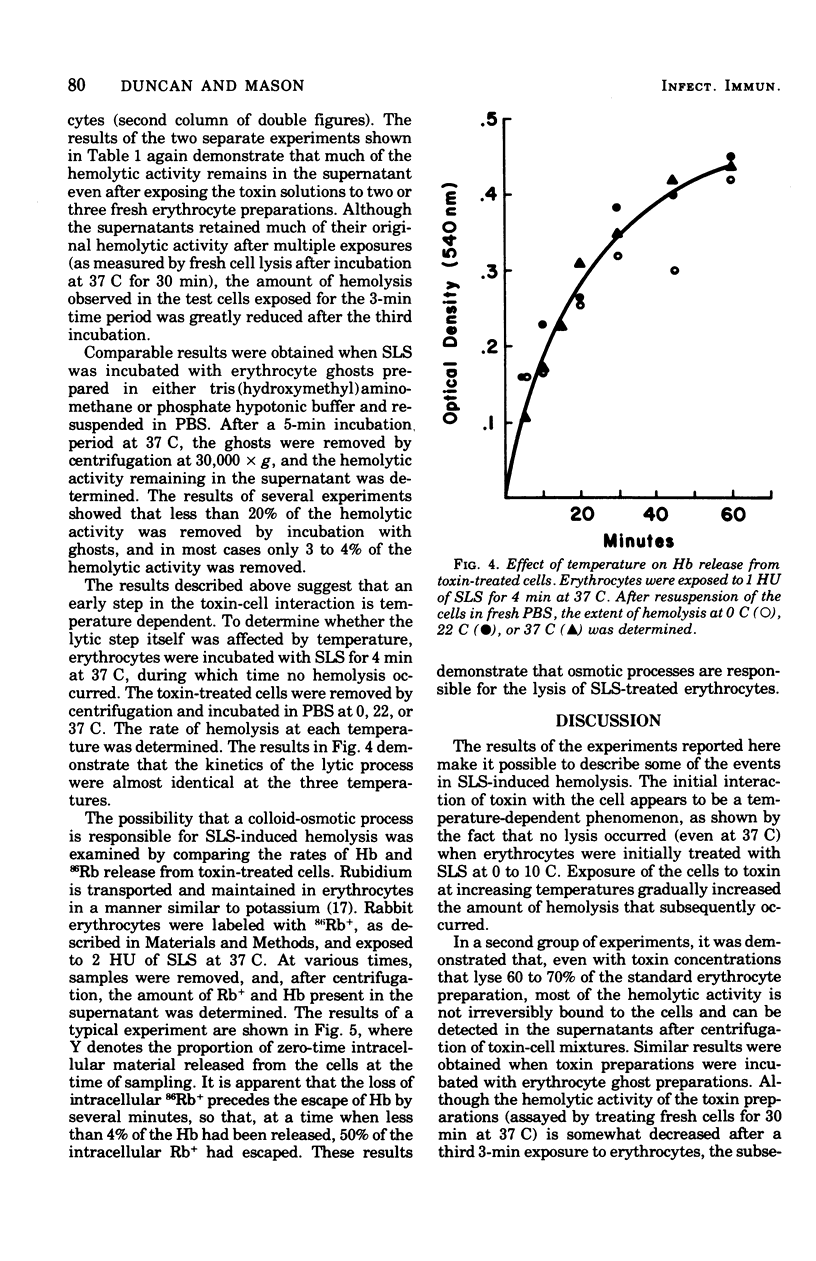
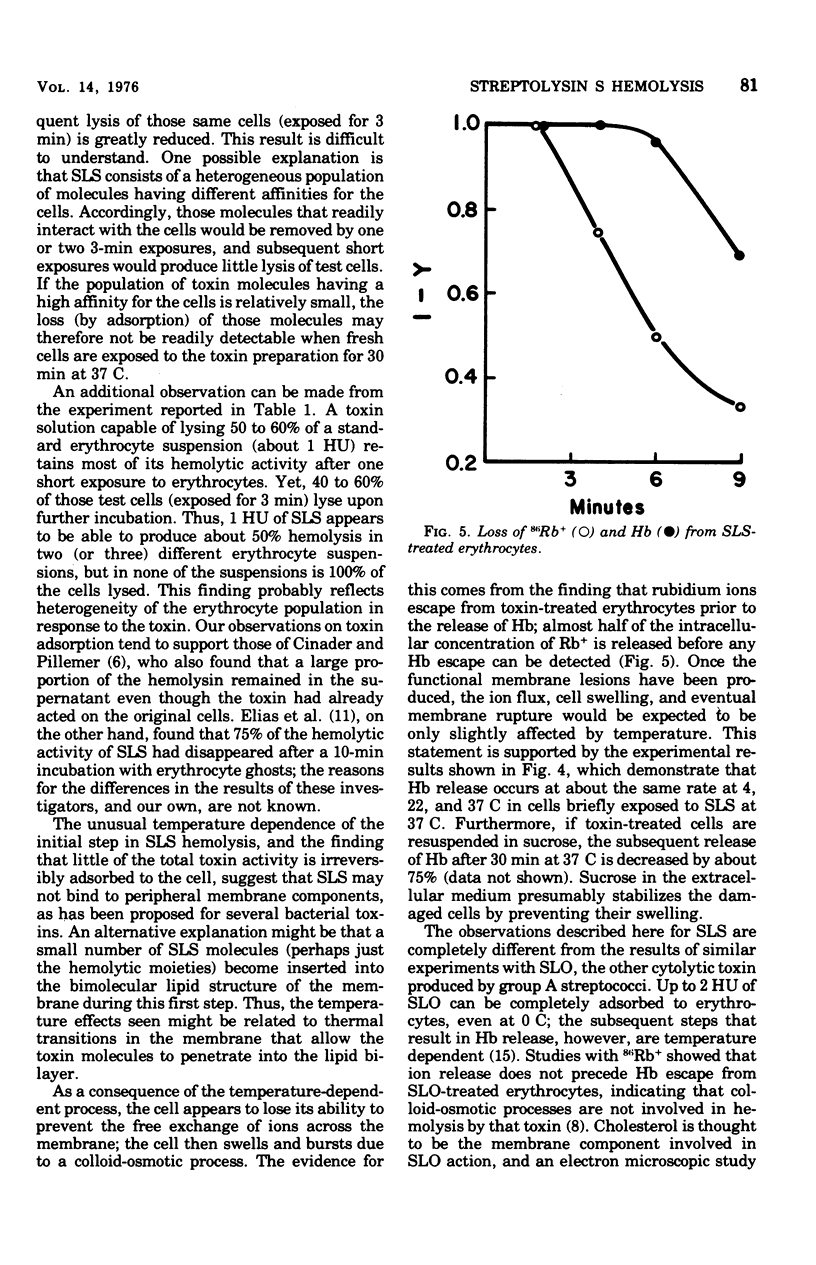
The characteristics of hemolysis produced by streptolysin S (SLS) were investigated in rabbit erythrocytes. Treatment of erythrocytes with SLS at various temperatures prior to incubation at 37 C revealed an initial temperature-dependent interaction between toxin and the cells. No subsequent hemolysis occurred when erythrocytes were exposed to SLS at 0 to 10 C; exposure to toxin at temperatures above 10 C gradually increased the amount of hemolysis that occurred at 37 C. Very little binding of toxin to erythrocytes or their ghosts, as detected by a decrease of hemolytic activity from toxin preparations, could be demonstrated at any temperature. The release of hemoglobin after the temperature-dependent process occur at virtually the same rate at 0, 22, or 37 C. The loss of intracellular rubidium-86 (Rb) and hemoglobin from SLS-treated erythrocytes was studied. Rb+ release significantly preceded the escape of hemoglobin, suggesting that colloid-osmotic processes play a role in SLS hemolysis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E., Raynaud M. Action de la streptolysine O sur les membranes cellulaires. I. Fixation sur la membrane érythrocytaire. Ann Inst Pasteur (Paris) 1968 Jun;114(6):812–827. [PubMed] [Google Scholar]
- Bangham A. D., Standish M. M., Weissmann G. The action of steroids and streptolysin S on the permeability of phospholipid structures to cations. J Mol Biol. 1965 Aug;13(1):253–259. doi: 10.1016/s0022-2836(65)80094-8. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W. Disruption of wall-less bacteria by streptococcal and staphylococcal toxins. J Bacteriol. 1966 May;91(5):1677–1680. doi: 10.1128/jb.91.5.1677-1680.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CINADER B., PILLEMER L. The purification and properties of streptolysin S. J Exp Med. 1950 Sep;92(3):219–237. doi: 10.1084/jem.92.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourmashkin R. R., Rosse W. F. Morphologic changes in the membranes of red blood cells undergoing hemolysis. Am J Med. 1966 Nov;41(5):699–710. doi: 10.1016/0002-9343(66)90031-3. [DOI] [PubMed] [Google Scholar]
- Duncan J. L. Characteristics of streptolysin O hemolysis: kinetics of hemoglobin and 86rubidium release. Infect Immun. 1974 Jun;9(6):1022–1027. doi: 10.1128/iai.9.6.1022-1027.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L., Schlegel R. Effect of streptolysin O on erythrocyte membranes, liposomes, and lipid dispersions. A protein-cholesterol interaction. J Cell Biol. 1975 Oct;67(1):160–174. doi: 10.1083/jcb.67.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias N., Heller M., Ginsburg I. Binding of streptolysin S to red blood cell ghosts and ghost lipids. Isr J Med Sci. 1966 May-Jun;2(3):302–309. [PubMed] [Google Scholar]
- GINSBURG I., BENTWICH Z., HARRIS T. N. OXYGEN-STABLE HEMOLYSINS OF GROUP A STREPTOCOCCI. 3. THE RELATIONSHIP OF THE CELL-BOUND HOMOLYSIN TO STREPTOLYSIN S. J Exp Med. 1965 Apr 1;121:633–645. doi: 10.1084/jem.121.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg I. Mechanisms of cell and tissue injury induced by group A streptococci: relation to poststreptococcal sequelae. J Infect Dis. 1972 Sep;126(3):294–340. doi: 10.1093/infdis/126.3.294. [DOI] [PubMed] [Google Scholar]
- Oberley T. D., Duncan J. L. Characteristics of streptolysin O action. Infect Immun. 1971 Dec;4(6):683–687. doi: 10.1128/iai.4.6.683-687.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki H. Streptolysin S' of Streptococcus pyogenes: studies on phospholipase activity. J Biochem. 1971 Nov;70(5):867–868. doi: 10.1093/oxfordjournals.jbchem.a129704. [DOI] [PubMed] [Google Scholar]
- SOLOMON A. K. The permeability of the human erythrocyte to sodium and potassium. J Gen Physiol. 1952 May;36(1):57–110. doi: 10.1085/jgp.36.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Sessa G. The action of polyene antibiotics on phospholipid-cholesterol structures. J Biol Chem. 1967 Feb 25;242(4):616–625. [PubMed] [Google Scholar]


