Edelmann et al. compare brain morphology in long-term adult survivors of childhood acute lymphoblastic leukaemia treated with chemotherapy alone to that of individuals treated with cranial radiation, and to healthy controls. Substantial white matter abnormalities are seen in leukaemia survivors, regardless of treatment, and are associated with neurocognitive impairment.
Keywords: brain fraction, neuropsychology, neuroimaging, anisotropy, cancer
Abstract
Survivors of childhood acute lymphoblastic leukaemia are at risk for neurocognitive impairment, though little information is available on its association with brain integrity, particularly for survivors treated without cranial radiation therapy. This study compares neurocognitive function and brain morphology in long-term adult survivors of childhood acute lymphoblastic leukaemia treated with chemotherapy alone (n = 36) to those treated with cranial radiation therapy (n = 39) and to healthy control subjects (n = 23). Mean (standard deviation) age at evaluation was 24.9 (3.6) years for the chemotherapy group and 26.7 (3.4) years for the cranial radiation therapy group, while time since diagnosis was 15.0 (1.7) and 23.9 (3.1) years, respectively. Brain grey and white matter volume and diffusion tensor imaging was compared between survivor groups and to 23 healthy controls with a mean (standard deviation) age of 23.1 (2.6) years. Survivors treated with chemotherapy alone had higher fractional anisotropy in fibre tracts within the left (P < 0.05), but not in the right, hemisphere when compared to controls. Survivors of acute lymphoblastic leukaemia, regardless of treatment, had a lower ratio of white matter to intracranial volume in frontal and temporal lobes (P < 0.05) compared with control subjects. Survivors of acute lymphoblastic leukaemia treated with chemotherapy alone performed worse in processing speed (P < 0.001), verbal selective reminding (P = 0.01), and academics (P < 0.05) compared to population norms and performed better than survivors treated with cranial radiation therapy on verbal selective reminding (P = 0.02), processing speed (P = 0.05) and memory span (P = 0.009). There were significant associations between neurocognitive performance and brain imaging, particularly for frontal and temporal white and grey matter volume. Survivors of acute lymphoblastic leukaemia treated with chemotherapy alone demonstrated significant long-term differences in neurocognitive function and altered neuroanatomical integrity. These results suggest substantial region-specific white matter alterations in survivors of acute lymphoblastic leukaemia possibly resulting in restricted radial diffusion due to the compaction of neuronal fibres.
Introduction
In the 1960s, only 5% of those diagnosed with childhood acute lymphoblastic leukaemia (ALL) survived for five or more years. With the introduction of prophylactic treatment of the CNS, the 5-year survival rate now exceeds 80% (Mariotto et al., 2009; Howlander et al., 2011). Unfortunately, this improved survival comes at a cost to some children, namely through increased risk for long-term chronic health conditions. Adult survivors of childhood leukaemia have a 4-fold relative risk for having a severe or life-threatening health condition when compared to siblings of cancer survivors (Oeffinger et al., 2006). Survivors treated with cranial radiation therapy (CRT) seem to be at greatest risk of metabolic, endocrine, and neurocognitive dysfunction (Hudson et al., 2013). The side effects of ALL therapy have decreased in severity and frequency as prophylactic treatment of the CNS has evolved from CRT to intrathecal and high dose intravenous chemotherapy, though concerns for neurocognitive problems remain. In a cross-sectional study of 567 adult survivors of childhood ALL, those treated with 24 Gy of cranial radiation demonstrated impairment rates of 31% in memory and 32% in executive functioning as compared to the study’s expected rate of 2% (Krull et al., 2013). Although performance in survivors of ALL treated with chemotherapy alone was significantly better, impairment rates were still much higher than expected with 13% exhibiting impairment in memory and 16% in executive functioning. This study, however, did not provide data on direct assessment of brain integrity.
Chemotherapy treatment for childhood ALL is also associated with neuroanatomical abnormalities. MRI of children undergoing chemotherapy for ALL demonstrates that a majority have white matter abnormalities shortly after methotrexate treatment (Reddick et al., 2005). Some of these white matter abnormalities are transient whereas others are persistent with about a third of long-term survivors demonstrating brain atrophy (Hertzberg et al., 1997; Reddick et al., 2005). Some of these neuroanatomical differences have been linked to functional consequences (Reddick et al., 2006). In ageing adult survivors treated with CRT, impaired immediate memory has been correlated with temporal lobe volumes and impaired delayed memory has been related to thinner parietal and frontal cortices (Armstrong et al., 2013). Recently, adult survivors of ALL treated with chemotherapy alone were found to have reduced volume within several brain regions when compared to healthy controls (Zeller et al., 2013). However, there was also a difference in intracranial volume between survivors and controls, which complicates the interpretation of the effects of chemotherapy alone on brain region-specific volume.
Although conventional MRI is useful for determining white and grey matter loss, MRI with diffusion tensor imaging (DTI) provides a means with which to examine microstructural abnormalities within the existing white matter. DTI quantifies the magnitude and directional anisotropy of water diffusion, both of which may be affected by the structure and integrity of white matter tracts. Water diffusion in a preferential direction, such as parallel to axonal fibres, is expressed as fractional anisotropy. Previous research suggests that both decreased and increased fractional anisotropy after injury is indicative of microstructural abnormalities, whether it is demyelination/axonal degeneration or gliosis (Sidaros et al., 2008; Wilde et al., 2008; Kumar et al., 2009; Budde et al., 2011). The magnitude of water diffusion measured with DTI is expressed as the apparent diffusion coefficient (ADC), with components parallel to the fibre axis (axial diffusivity) and perpendicular to the fibre axis (radial diffusivity).
Despite the exquisite sensitivity of DTI, few studies have used this technique to help understand the microstructure in the brain of long-term survivors of childhood ALL. One small study found that 13 adult survivors of childhood ALL treated with CRT had altered fractional anisotropy within the temporal lobes when compared to controls (Dellani et al., 2008). A similar study compared fractional anisotropy among adult survivors treated with either CRT (n = 10) or chemotherapy alone (n = 10) and matched controls. Compared to controls, survivors of ALL treated with CRT had altered fractional anisotropy near the caudate nuclei, whereas survivors treated with chemotherapy alone had reduced white matter volume in the cerebellum and exhibited a trend for reduced fractional anisotropy when compared with controls (Porto et al., 2008). These studies suggest that DTI can be used to detect microstructural differences in adult survivors of ALL, however, these studies did not provide neurocognitive outcomes. The significance and impact of microstructural differences in adult survivors of ALL treated with chemotherapy alone is unclear.
Therefore, this study examined brain volume and DTI measures to investigate brain integrity in long-term adult survivors of ALL treated with either chemotherapy alone or CRT, and in healthy controls. To investigate the clinical implications of potential differences in brain morphology, survivors of ALL also underwent a comprehensive neurocognitive evaluation.
Materials and methods
Participants
This study included survivors of childhood ALL who participated in the St. Jude Lifetime Cohort (SJLIFE) study, which evaluates medical and psychosocial late effects in adult survivors of childhood cancer (Hudson et al., 2011). To be eligible for SJLIFE, survivors had to have been treated at St Jude Children’s Research Hospital for childhood cancer, currently 18 years of age or older and 10 or more years from the time of diagnosis. Fifty survivors treated with chemotherapy alone were randomly identified and targeted for recruitment into the current study. Participants were excluded from the current evaluation if they had a secondary brain tumour, were not proficient in English, or had a non-cancer related neurological disorder. Of the 45 contacted, 40 agreed to participate and were scheduled for a campus visit. Four of the 40 recruited survivors withdrew during data collection, leaving 36 participants (72%) with evaluable data who were treated with chemotherapy alone. Of the 65 survivors treated with CRT that were randomly identified and contacted for recruitment into the current study, five had a secondary brain tumour and two had a subsequent neurological injury unrelated to cancer treatment, leaving 58 eligible. Six of the eligible survivors withdrew during data collection and 13 refused participation, leaving 39 participants (67%) with evaluable data who were treated with CRT. Healthy controls were recruited as part of a separate institutional protocol, though underwent the same brain imaging procedures on the same scanner, as described below. This study was approved by the Institutional Review Board at St. Jude Children’s Research Hospital and all participants provided written informed consent.
Neurocognitive testing
All survivors completed a neurocognitive evaluation with certified examiners under the general supervision of a board-certified clinical neuropsychologist. Assessed neurocognitive domains (and instruments) included: intelligence [Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999)], academics [Woodcock-Johnson-III Tests of Achievement letter-word identification and calculation subtests (Woodcock et al., 2001)], attention [the Trail Making Test A (Reitan, 1993); Conner’s Continuous Performance Test-II (Conners, 2001)], memory [California Verbal Learning Test-II (Delis et al., 2000); Wechsler Adult Intelligence Scale-III digit span forward (WAIS-III) (Wechsler, 1997); Test Of Memory And Learning-II visual and verbal selective reminding (Reynolds and Voress, 2007)]; processing speed [the Grooved Pegboard Test (Trites, 1977), WAIS-III processing speed index (Wechsler, 1997)]; and executive function [the Trail Making Test B (Reitan, 1993), Verbal Fluency Test (Benton et al., 1983), WAIS-III digit span backward (Wechsler, 1997)].
MRI
Brain imaging was conducted using a 3 T Siemens Trio MR (Siemens Medical Systems). Briefly, axial 4 mm thick contiguous T1, T2/proton density, and FLAIR imaging sets were acquired, registered to the International Consortium of Brain Mapping (ICBM) average 152 T2 atlas aligned in Talairach space, resampled, intensity corrected, and segmented using an automated hybrid neural network segmentation and classification method (Glass et al., 2006). A 3D T1-weighted MRI set acquired and processed with the FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/, Athinoula A. Martinos Centre for Biomedical Imaging) to assess cortical thickness within lobes and hippocampal volumes (Han et al., 2006). White matter and grey matter volumes were assessed for frontal, parietal, occipital, and temporal lobes. DTI was acquired with 12 non-coplanar, non-collinear diffusion gradient directions to calculate the diffusion tensor for each voxel. Voxelwise tensor calculations were performed with the DTI toolkit under SPM8 (http://www.fil.ion.ucl.ac.uk/spm/), and parameter maps of apparent diffusion coefficient, fractional anisotropy, and radial and axial diffusivity were generated. After registering the parametric maps to the atlas space, average values for each parameter within the segmented white matter regions were assessed for each lobe.
3D hippocampal shape analysis
Three-dimensional hippocampal shape analysis was performed using the SPHARM_MAT (a MATLAB based software package, http://www.nitrc.org/projects/spharm-mat/) on the segmented hippocampal volume of each subject. SPHARM_MAT uses the spherical harmonic (SPHARM) basis functions to represent a closed surface (Styner et al., 2006). In the SPHARM method, the hippocampal surface was first mapped to a unit spherical surface and then the SPHARM coefficients up to 12° were fitted as the shape descriptor. Each individual SPHARM model can be placed in a common space by aligning the first-order ellipsoids across all subjects. With aligned SPHARM models, the hippocampal shape can be sampled back into the common subject space as a triangular mesh by icosahedron division of the spherical surface. Each subject was sampled by the fourth icosahedron level which is a triangular mesh with 2562 vertices. After establishing the correspondences on each vertex across the subjects, a two-sample t-test was performed on each vertex. The calculated T-values and associated P-values were saved on an average shape model.
Tract-based spatial statistics
The quantitative DTI maps of fractional anisotropy were processed following the tract-based spatial statistics (TBSS) pipeline, part of FMRIB Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl). TBSS is more reliable compared to other techniques because it minimizes inter-subject variability. TBSS registration non-linearly transformed the fractional anisotropy images into a standard space using the FMRIB58_fractional anisotropy image as the target. After registration, the tract skeletonization process was performed using a fractional anisotropy lower threshold of 0.25. The final white matter skeleton represented the fibre bundle centers across both patients and controls. Fractional anisotropy skeletons were compared between both group of survivors and controls using the randomize permutation algorithm in FSL to perform multiple regression analyses (Nichols and Holmes, 2002). Voxel-wise tests were performed with 5000 permutations using threshold-free cluster enhancement while treating age as a covariate. Multiple comparisons were accounted for by controlling for family-wise error rates. The first analysis performed was a simple group-wise Student’s t-test of fractional anisotropy values between survivors treated with chemotherapy alone, survivors treated with CRT, and control subjects. Only fully corrected P-values < 0.01 generated by 5000 permutations were considered significant. The identified regions were then labelled anatomically using the JHU-ICBM-DTI-81 white matter atlas (Mori et al., 2008). Fibre tracts were grouped according to anatomical and functional associations with larger fibre bundles (Yu et al., 2012).
Statistical analyses
Descriptive statistics were generated for demographic and treatment characteristics. Scores on neurocognitive measures were converted into age-adjusted standard scores using national normative data. One-sample t-tests were used to compare performance on neurocognitive measures to population norms [mean = 0, standard deviation (SD) = 1]. Group comparisons were made using two-sample t-tests. Our a priori hypothesis was that those survivors treated with chemotherapy alone would have more neurocognitive problems compared to population norms, but less than those survivors treated with CRT. Given this a priori hypothesis, we used a conservative two-sided test for all analyses, though no adjustment for multiple comparisons was made. Only those neurocognitive functions that differed from the normative sample were examined for association to brain regions using Spearman correlations.
Results
Demographic characteristics and treatment history are presented in Table 1. Gender and race did not differ between any of the three groups (all P’s > 0.05). Survivors treated with CRT were older at evaluation (26.7 years) than either the chemotherapy alone group (24.9 years; P = 0.03) or controls (23.1 years; P < 0.01). Survivors treated with CRT were younger at diagnosis and farther from diagnosis than those treated with chemotherapy alone (P < 0.01). Cumulative treatments and CNS disease status for both survivor groups are presented in Table 1.
Table 1.
Demographic and treatment characteristics
| Variable | Chemotherapy | CRT | Controls |
|---|---|---|---|
| Gender, F:M | 15:21 | 21:18 | 15:8 |
| Race, white:non-white | 33:3 | 35:4 | 19:4 |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age at evaluation (years) | 24.94 (3.58) | 26.71 (3.44) | 23.1 (2.6) |
| Age at diagnosis (years) | 9.97 (3.99) | 2.81 (1.73) | N/A |
| Time since diagnosis (years) | 14.97 (1.74) | 23.90 (3.05) | N/A |
| Cumulative treatment | |||
| Cranial radiation (Gy) | 0 (0) | 20.0 (5.7) | N/A |
| High dose methotrexate (mg/mm2) | 20453 (3159) | 5450 (3965) | N/A |
| IT methotrexate (ml) | 163.4 (58.7) | 261.1 (116.7) | N/A |
| IT hydrocortisone (ml) | 325.3 (118.3) | 492.8 (180.2) | N/A |
| IT cytarabine (ml) | 488.7 (177.7) | 740.3 (274.6) | N/A |
| Treated with glucocorticoids (%) | 100% | 100% | N/A |
| CNS disease status | n (%) | n (%) | n (%) |
| CNS1 or traumatic blast | 32 (89%) | 25 (69%) | N/A |
| CNS 2 or CNS 3 | 4 (11%) | 11 (31%) | N/A |
Note: Although not evaluated as part of this study, healthy controls had an average (SD) IQ of 111.9 (10.5) according to the Brief Intellectual Assessment from the Woodcock Johnson III. The CNS status for three survivors treated with CRT were not available. IT = intrathecal.
Macrostructure
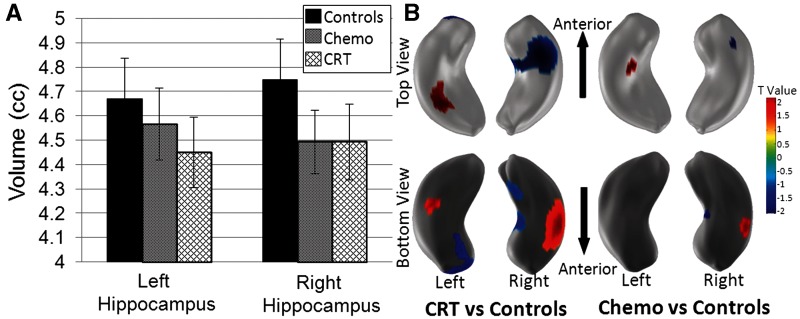
Differences in neuroanatomical structure were seen between the three groups (Table 2). The macrostructure of the left and right hemispheres were similar, therefore frontal, parietal, and temporal lobes as well as the hippocampus were examined bilaterally. The CRT group had a smaller intracranial volume than either the chemotherapy group (P = 0.02) or controls (P = 0.01). Therefore, comparisons were also made for brain fraction, which is the ratio between region-specific volume and intracranial volume. Grey matter volume was only significantly different between the chemotherapy and CRT groups in the parietal and temporal lobes, however, these comparisons were not significant when comparing grey matter brain fraction. When compared with control subjects, the chemotherapy group had larger grey matter brain fraction and smaller white matter brain fraction within the frontal and temporal lobes (P < 0.01). The chemotherapy group had larger white matter brain fraction compared to the CRT group (P < 0.05) within the frontal and temporal lobes. When compared to controls, survivors treated with CRT had smaller white matter brain fraction (P < 0.05), but larger grey matter fraction (P < 0.01) within the frontal, parietal, and temporal lobes. The chemotherapy group had smaller hippocampal brain fraction compared to the CRT group (P = 0.03). Before correcting for intracranial volume, controls had more hippocampal volume than survivors, although it did not reach statistical significance (Fig. 1A). Differences in shape between controls and survivors treated with CRT or chemotherapy are illustrated using 3D shape analyses (Fig. 1B). For survivors treated with CRT, 7.1% of the left and 14.9% of the right hippocampus was shaped significantly different from controls. For survivors treated with chemotherapy alone, 0.8% of the left and 1.8% of the right were different from control subjects. These data suggest that the right hippocampus is twice as sensitive to distortion by cancer and its treatment and that CRT has a greater effect on hippocampal shape than chemotherapy alone.
Table 2.
Brain volume and DTI by brain region
| Variable | Chemotherapy Mean (SD) | Controls Mean (SD) | Chemo versus Controls P-value | CRT Mean (SD) | Chemo versus CRT P-value | Controls versus CRT P-value |
|---|---|---|---|---|---|---|
| Intracranial volume (cc) | 1114 (116.1) | 1135 (131.6) | 0.53 | 1053 (113.2) | 0.024 | 0.012 |
| Frontal lobe | ||||||
| Grey matter volume (cc) | 250.5 (26.6) | 243.1 (34.8) | 0.36 | 240.9 (28.8) | 0.14 | 0.79 |
| Grey matter brain fractiona | 0.2254 (0.0154) | 0.2138 (0.0116) | 0.003 | 0.2289 (0.0134) | 0.29 | <0.001 |
| White matter volume (cc) | 146.7 (21.1) | 160.0 (19.0) | 0.018 | 133.2 (22.0) | 0.008 | <0.001 |
| White matter brain fractiona | 0.1316 (0.0119) | 0.1414 (0.0119) | 0.003 | 0.1261 (0.0114) | 0.042 | <0.001 |
| Fractional anisotropya | 0.4429 (0.0287) | 0.4288 (0.0206) | 0.047 | 0.4538 (0.0243) | 0.08 | <0.001 |
| Radial diffusivitya | 0.5653 (0.0283) | 0.5761 (0.0235) | 0.14 | 0.5562 (0.0224) | 0.13 | 0.002 |
| Axial diffusivitya | 1.1410 (0.0305) | 1.1434 (0.0339) | 0.78 | 1.1421 (0.0300) | 0.88 | 0.88 |
| Parietal lobe | ||||||
| Grey matter volume (cc) | 160.0 (19.3) | 151.1 (21.7) | 0.11 | 149.3 (18.3) | 0.016 | 0.72 |
| Grey matter brain fractiona | 0.1439 (0.0119) | 0.1330 (0.0083) | <0.001 | 0.1421 (0.0132) | 0.54 | 0.004 |
| White matter volume (cc) | 114.0 (19.8) | 121.3 (15.6) | 0.14 | 107.0 (18.0) | 0.11 | 0.002 |
| White matter brain fractiona | 0.1022 (0.0129) | 0.1074 (0.0122) | 0.13 | 0.1013 (0.0093) | 0.72 | 0.029 |
| Fractional anisotropya | 0.4431 (0.0314) | 0.4406 (0.0217) | 0.74 | 0.4547 (0.0266) | 0.09 | 0.035 |
| Radial diffusivitya | 0.5730 (0.0303) | 0.5719 (0.0209) | 0.87 | 0.5638 (0.0242) | 0.15 | 0.19 |
| Axial diffusivitya | 1.1763 (0.0313) | 1.1750 (0.0338) | 0.88 | 1.1818 (0.0303) | 0.45 | 0.41 |
| Temporal lobe | ||||||
| Grey matter volume (cc) | 162.8 (18.5) | 159.8 (20.3) | 0.56 | 154.8 (14.3) | 0.039 | 0.26 |
| Grey matter brain fractiona | 0.1462 (0.0084) | 0.1407 (0.0057) | 0.008 | 0.1475 (0.0091) | 0.53 | 0.002 |
| White matter volume (cc) | 82.0 (13.6) | 90.5 (10.4) | 0.013 | 70.9 (12.3) | <0.001 | <0.001 |
| White matter brain fractiona | 0.0735 (0.0083) | 0.0802 (0.0088) | 0.004 | 0.0671 (0.0073) | 0.001 | <0.001 |
| Fractional anisotropya | 0.4611 (0.0312) | 0.4463 (0.0224) | 0.06 | 0.4797 (0.0276) | 0.009 | <0.001 |
| Radial diffusivitya | 0.5764 (0.0258) | 0.5890 (0.0196) | 0.05 | 0.5635 (0.0225) | 0.025 | <0.001 |
| Axial diffusivitya | 1.2146 (0.0334) | 1.2238 (0.0315) | 0.30 | 1.2280 (0.0435) | 0.15 | 0.69 |
| Hippocampus | ||||||
| Hippocampal volume (cc) | 9.1 (0.8) | 9.4 (0.8) | 0.10 | 9.0 (0.9) | 0.66 | 0.06 |
| Hippocampal brain fractiona | 0.0082 (0.0008) | 0.0083 (0.0006) | 0.38 | 0.0086 (0.0007) | 0.027 | 0.23 |
As there was a group difference in intracranial volume, group comparisons were only conducted on brain fraction.
Brain fraction = volume/intracranial volume; Chemo = survivors treated with chemotherapy alone.
aValues are unitless.
Figure 1.
Hippocampal volume and shape in survivors treated with CRT or chemotherapy alone compared to controls. (A) Mean and 95% confidence intervals for left and right hippocampal volumes by group. (B) Differences in hippocampal shape between controls and ALL survivors treated with CRT or chemotherapy alone. The T-value colour map displays positive and negative T-values representing the outward and inward shape differences.
Microstructure
Survivors treated with chemotherapy alone had higher fractional anisotropy than control subjects within the frontal lobe (P = 0.047). The chemotherapy group had smaller fractional anisotropy and higher radial diffusivity than the CRT group within the temporal lobe (P < 0.05). Survivors treated with CRT had higher fractional anisotropy and lower radial diffusivity than control subjects within the frontal and temporal lobes (P < 0.01; Table 2). Using tract-based spatial statistics, it was apparent that fractional anisotropy within fibre tracts was asymmetrical, therefore left and right fibre tracts were analysed separately (Fig. 2). Survivors treated with chemotherapy alone had higher fractional anisotropy within the left superior fronto-occipital fasciculus (P < 0.01) and left internal capsule (P = 0.04) than controls. In addition to having higher fractional anisotropy within these fibre tracts, survivors treated with CRT also had higher fractional anisotropy within the left superior longitudinal fasciculus (P = 0.02), left cingulum (P = 0.03), left external capsule (P < 0.01), left uncinate fasciculus (P = 0.04), and left sagittal stratum (P = 0.03) when compared with control subjects. Survivors treated with chemotherapy alone had higher fractional anisotropy within the left medial lemniscus when compared to those treated with CRT (P = 0.01).
Figure 2.
Comparison of fibre tract fractional anisotropy between controls and survivors treated with CRT or chemotherapy alone. Mean and 95% confidence intervals for fibre tract fractional anisotropy. Statistically significant differences between a survivor group and controls are identified by an asterisk (*P < 0.05). Statistically significant differences between survivor groups were identified by a hash symbol (#P < 0.05). Fractional anisotropy of fibre tracts primarily located in cortical (A) or subcortical (B) regions are plotted by hemisphere. Sup = superior; Fr-occip = fronto-occipital; Fasc = fasciculus; Long = longitudinal; Inf = inferior; Cereb = cerebellar; Ped = peduncle, Med = medial; Sag = sagittal; Post = posterior; Thal = thalamic; Rad = radiation; Mid = middle.
Neurocognitive performance
Neurocognitive performance for survivors of ALL is listed in Table 3. Survivors treated with chemotherapy alone performed worse than the population norm on reading (P = 0.02), math (P = 0.04), attention variability (P = 0.05), verbal selective reminding (P = 0.01), and motor processing speed (P < 0.01). In addition to performing worse on these measures (P < 0.05), survivors treated with CRT also performed worse than the population norm in vocabulary, visual selective reminding, memory span, visual-motor processing speed, cognitive flexibility, and working memory (all P’s < 0.01). The chemotherapy group performed better than the CRT group in verbal selective reminding (P = 0.01), memory span (P < 0.01), and visual-motor processing speed (P = 0.05). When comparing differences between survivor groups, the effect sizes were moderate and in the range of 0.47 to 0.62, which reflects differences of roughly half a standard deviation.
Table 3.
Neurocognitive function
| Task performance | Chemotherapy alone |
Cranial radiation therapy |
Chemo versus CRT P | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Population P | Impairment (%) | Mean (SD) | Population P | Impairment (%) | |||
| Intelligence | ||||||||
| Vocabulary | −0.34 (1.19) | 0.09 | 31 | −0.73 (1.08) | <0.001 | 36 | 0.15 | |
| Matrices | 0.19 (0.82) | 0.17 | 6 | −0.04 (0.86) | 0.78 | 10 | 0.24 | |
| Academics | ||||||||
| Reading | −0.36 (0.86) | 0.016 | 14 | −0.48 (0.50) | <0.001 | 15 | 0.49 | |
| Math | −0.33 (0.92) | 0.041 | 28 | −0.69 (0.87) | <0.001 | 38 | 0.08 | |
| Attention | ||||||||
| Focused attention | −0.10 (1.26) | 0.64 | 19 | −0.13 (1.05) | 0.45 | 18 | 0.91 | |
| Sustained attention | −0.53 (1.84) | 0.09 | 19 | −0.36 (1.79) | 0.23 | 10 | 0.68 | |
| Variability | −0.40 (1.18) | 0.049 | 25 | −0.32 (0.96) | 0.046 | 18 | 0.75 | |
| Memory | ||||||||
| New learning | 0.06 (1.16) | 0.76 | 11 | −0.29 (1.26) | 0.16 | 28 | 0.22 | |
| Short term recall | 0.22 (0.97) | 0.18 | 6 | −0.12 (1.27) | 0.57 | 18 | 0.20 | |
| Long term recall | 0.00 (0.94) | 1.00 | 8 | −0.25 (1.25) | 0.22 | 15 | 0.34 | |
| Verbal selective reminding immediate | −0.42 (0.94) | 0.012 | 28 | −0.97 (0.96) | <0.001 | 33 | 0.014 | |
| Verbal selective reminding delay | −0.04 (0.85) | 0.79 | 11 | −0.25 (1.03) | 0.14 | 15 | 0.33 | |
| Visual selective reminding immediate | −0.21 (1.05) | 0.23 | 17 | −0.67 (0.98) | <0.001 | 28 | 0.06 | |
| Memory span | −0.02 (0.93) | 0.91 | 19 | −0.62 (1.00) | <0.001 | 44 | 0.009 | |
| Processing speed | ||||||||
| Motor | −1.28 (1.57) | <0.001 | 39 | −1.04 (1.16) | <0.001 | 38 | 0.46 | |
| Visual | 0.15 (1.14) | 0.44 | 8 | −0.16 (0.80) | 0.21 | 5 | 0.17 | |
| Visual-motor | 0.06 (1.18) | 0.74 | 19 | −0.39 (0.73) | 0.002 | 8 | 0.045 | |
| Executive function | ||||||||
| Cognitive flexibility | −0.40 (1.73) | 0.17 | 28 | −0.89 (1.39) | <0.001 | 33 | 0.18 | |
| Cognitive fluency | −0.31 (0.95) | 0.05 | 22 | −0.21 (0.86) | 0.13 | 18 | 0.63 | |
| Working memory | −0.11 (0.82) | 0.44 | 14 | −0.35 (0.74) | 0.005 | 15 | 0.18 | |
Impairment, <−1 SD; expected impairment rate = 15.9%. Chemo = chemotherapy alone.
Neuroanatomy and neurocognitive performance
Neurocognitive tasks in which survivors performed worse than the population norm were correlated to brain morphology. Correlations were only conducted for the fibre tract fractional anisotropies that were different between groups (Table 4). Frontal and temporal lobe volumes were associated with vocabulary, reading, math and memory span. Parietal lobe volume was associated with math and memory span (P < 0.05). The left longitudinal and uncinate fasciculi fractional anisotropies were inversely associated with immediate visual selective reminding (rs = −0.24, P = 0.04; rs = −0.30, P = 0.01, respectively), a measure of memory and learning. The left sagittal stratum was positively correlated with variability (rs = 0.30, P = 0.01), a sustained attention measure.
Table 4.
Spearman’s rho for correlations conducted between neurocognitive measures and brain morphology among survivors
| Vocab | Reading | Math | Variability | Verbal SRIm | Visual SRIm | Memory span | Motor speed | Vis-Motor Speed | Flexibility | Working memory | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal WM volume | 0.33 | 0.22 | 0.31 | 0.19 | 0.04 | 0.14 | 0.29 | −0.05 | 0.00 | 0.23 | 0.17 |
| Frontal WM brain function | 0.21 | 0.08 | 0.18 | 0.16 | 0.05 | 0.05 | 0.19 | −0.09 | −0.02 | 0.19 | 0.18 |
| Frontal GM volume | 0.24 | 0.25 | 0.24 | 0.07 | 0.04 | 0.19 | 0.09 | 0.00 | −0.03 | 0.13 | 0.06 |
| Frontal GM brain function | −0.11 | −0.05 | −0.09 | −0.12 | 0.04 | 0.05 | −0.24 | −0.01 | −0.16 | −0.12 | −0.15 |
| Parietal WM volume | 0.21 | 0.09 | 0.24 | 0.16 | −0.03 | 0.08 | 0.31 | −0.05 | −0.04 | 0.16 | 0.16 |
| Parietal WM brain function | 0.00 | −0.08 | 0.07 | 0.13 | −0.09 | −0.02 | 0.26 | −0.09 | −0.08 | 0.09 | 0.17 |
| Parietal GM volume | 0.11 | 0.20 | 0.12 | 0.02 | −0.03 | −0.02 | 0.09 | 0.06 | −0.02 | 0.12 | 0.02 |
| Parietal GM brain function | −0.23 | −0.04 | −0.19 | −0.12 | −0.05 | 0.22 | −0.14 | 0.01 | −0.13 | −0.12 | −0.15 |
| Temporal WM volume | 0.27 | 0.23 | 0.28 | 0.12 | −0.01 | 0.14 | 0.31 | −0.09 | −0.01 | 0.18 | 0.16 |
| Temporal WM brain function | 0.15 | 0.12 | 0.13 | 0.09 | −0.03 | 0.09 | 0.25 | −0.1 | 0.02 | 0.11 | 0.11 |
| Temporal GM volume | 0.27 | 0.26 | 0.23 | 0.07 | 0.01 | 0.18 | 0.11 | 0.05 | 0.05 | 0.13 | 0.02 |
| Temporal GM brain function | −0.07 | 0.01 | −0.10 | −0.15 | 0.00 | 0.02 | −0.19 | 0.07 | 0.05 | −0.11 | −0.17 |
| Left sup. fr-occip Fasc. | 0.21 | 0.18 | −0.04 | 0.10 | −0.02 | 0.09 | 0.27 | 0.04 | −0.02 | 0.08 | 0.11 |
| Left long fasciculus | 0.11 | 0.13 | 0.01 | −0.00 | 0.05 | −0.24 | 0.00 | 0.14 | −0.19 | 0.05 | 0.05 |
| Left cingulum | 0.00 | 0.03 | −0.03 | −0.03 | −0.05 | −0.17 | 0.13 | 0.20 | 0.02 | 0.06 | 0.16 |
| Left external capsule | 0.21 | 0.13 | −0.04 | 0.14 | 0.02 | −0.18 | 0.02 | 0.06 | −0.13 | 0.05 | −0.04 |
| Left internal capsule | 0.17 | 0.13 | −0.03 | 0.16 | 0.12 | −0.04 | 0.15 | −0.00 | −0.11 | 0.15 | 0.03 |
| Left medial lemniscus | 0.07 | 0.16 | −0.00 | 0.12 | 0.06 | 0.22 | 0.15 | −0.05 | −0.09 | 0.02 | 0.14 |
| Left uncinate fasciculus | 0.09 | 0.07 | 0.11 | −0.00 | 0.04 | −0.30 | −0.14 | 0.00 | −0.04 | −0.02 | 0.02 |
| Left sagittal stratum | 0.13 | 0.18 | 0.07 | 0.30 | 0.04 | −0.01 | 0.04 | 0.12 | −0.10 | 0.03 | 0.11 |
WM = white matter; GM = grey matter; Fr = frontal; Occip = occipital; Vocab = vocabulary; SRIm = selective reminding immediate.
Values in bold where P < 0.05.
Discussion
To the best of our knowledge, this is the first study to examine macrostructure, microstructure, and neurocognitive function in long-term adult survivors of childhood ALL treated with either CRT or chemotherapy alone. Survivors treated at least 15 years ago for childhood ALL had reduced white and grey matter brain fraction when compared with control subjects. As expected from previous literature, survivors treated with CRT had a larger reduction in brain fraction than survivors treated with chemotherapy alone. Adult survivors of ALL, regardless of treatment, had higher fractional anisotropy in fibre tracts within the left, but not the right, hemisphere when compared with control subjects. Survivors treated with chemotherapy alone performed worse than the population norm in academic learning, attention, memory, and processing speed. Interestingly, the chemotherapy alone group performed better than survivors treated with CRT in only 3 of 20 neurocognitive measures. Taken together, these data suggest substantial region-specific white matter alterations in survivors of ALL regardless of treatment that seem to contribute to difficulties in neurocognitive function.
Although correlations between brain morphology and neurocognitive measures are limited due to the complexity of the neurocognitive tasks and the relatively large brain regions of interest examined, there appears to be functional consequences of brain integrity differences seen among survivors of ALL. Several measures of brain morphology correlated with neurocognitive performance. Frontal and temporal lobe volumes correlated with vocabulary and academic ability. This correlation is similar to a previous study that found that the volume of the frontal gyrus was positively correlated with vocabulary and math in nine long-term survivors of ALL and 14 healthy controls (Carey et al., 2008). The current study also found that frontal, parietal, and temporal white matter volumes were associated with memory. This finding is supported by a recent study, which found that better memory performance was associated with more temporal white matter, as well as thicker regions of frontal and parietal cortices in long-term adult survivors treated with CRT (Armstrong et al., 2013). Fractional anisotropy in the left longitudinal fasciculus and left uncinate fasciculus were inversely correlated with performance on visual selective reminding, a measure of memory and learning. Studies have shown associations between fractional anisotropy in the superior longitudinal fasciculus and uncinate fasciculus with memory function in typically developing children and healthy older adults (Metzler-Baddeley et al., 2011; Vestergaard et al., 2011). The current study found a positive correlation between fractional anisotropy in the left sagittal stratum and sustained attention. Previously, fractional anisotropy in the left sagittal stratum was associated with attention deficit hyperactivity disorder symptom severity (Peterson et al., 2011).
Our initial hypothesis was that survivors of ALL would have reduced brain volume and lower fractional anisotropy when compared to controls. Indeed, even when correcting for intracranial volume, survivors treated with CRT or chemotherapy alone had less white matter than controls in frontal and temporal lobes. Unexpectedly, survivors of ALL, regardless of treatment, had higher fractional anisotropy than controls in multiple fibre tracts. Few studies have reported on brain microstructure in adult survivors of ALL treated with either CRT or chemotherapy alone. When differences between survivors and controls have been reported, survivors of ALL had lower fractional anisotropy (Khong et al., 2006; Dellani et al., 2008; Porto et al., 2008; Schuitema et al., 2013). The largest of these studies found that survivors of ALL treated with CRT had significantly lower fractional anisotropy in the cingulum, superior longitudinal fasciculus, and uncinate fasciculus. In survivors treated with chemotherapy alone, there was a trend for lower fractional anisotropy in frontal white matter tracts (Schuitema et al., 2013). Conversely, a recent study found a trend for higher fractional anisotropy in survivors of childhood ALL treated only with chemotherapy when compared to age-matched healthy controls (Genschaft et al., 2013). Although our results are seemingly contradictory to the majority of previously published literature, we used two techniques, region of interest analyses and tract-based spatial statistics, to determine fractional anisotropy in our study. Furthermore, no other previously published study randomly recruited participants suggesting that our study may be more representative of the general patient population. Additional research will be required to identify medical (e.g. chemotherapy), demographic (e.g. time since treatment), or technical factors (e.g. regions analysed, slice thickness) that can explain these differences.
Several mechanisms may account for our observation that poorer neurocognitive function was associated with high fractional anisotropy in survivors of ALL, including glial scarring, white matter compaction, and compensatory myelination. Increased fractional anisotropy has been observed in humans after brain injury (Sidaros et al., 2008; Wilde et al., 2008). Individuals with mild traumatic brain injury had increased fractional anisotropy and decreased radial diffusivity within the corpus callosum compared to age- and gender-matched controls (Wilde et al., 2008), and increased fractional anisotropy in the cortex after brain injury was linked to reactive astrocytic gliosis in rats (Budde et al., 2011). Alternatively, white matter in survivors might be more compact, possibly due to a loss of glia cells. White matter compaction is supported by the observed decrease in white matter volume. As white matter compacts, radial diffusion is restricted more than axial diffusion, leading to a higher fractional anisotropy measurement. A study examining compaction of white matter due to hydrocephalus found a similar pattern of reduced radial diffusivity and an overall increase in fractional anisotropy (Assaf et al., 2006). Although this compaction was due to hydrocephalus, compaction due to a developing brain within a small fixed skull size could have the same phenotypical expression. Whether by reactive gliosis or white matter compaction, our findings of increased fractional anisotropy are contrary to the common interpretation that higher fractional anisotropy is indicative of better axonal or myelin integrity. Indeed, during normal development fractional anisotropy increases with myelin maturation (Huppi and Dubois, 2006) and myelin degradation and/or axonal degeneration have been associated with decreased fractional anisotropy (Kochunov et al., 2007; Sidaros et al., 2008). Therefore, another alternative explanation is that compensatory myelination occurs for intact white matter to improve conduction efficiency in the context of brain injury and overall white matter deterioration. However, a compensatory mechanism is inconsistent with the observed associations between fractional anisotropy and neurocognitive function. We found a negative correlation between memory function and fractional anisotropy in the left longitudinal and uncinate fasciculi as well as a positive correlation between sustained attention and fractional anisotropy in the left sagittal stratum. These associations are in the opposite direction of previously observed correlations in typically developing and/or ageing individuals (Metzler-Baddeley et al., 2011; Peterson et al., 2011; Vestergaard et al., 2011). For example, Peterson et al. (2011) found lower fractional anisotropy in the left sagittal stratum of healthy children when compared to children with attention deficit hyperactivity disorder. As the association between fractional anisotropy and neurocognitive performance in our investigation is in the opposite direction of what is expected during typical development, it suggests that the increased fractional anisotropy in our study is an indication of poorer functioning. This lends support to the concept of glial scarring and/or white matter compaction, but not compensatory myelination. Future research is needed to clarify the relationships among brain injury, brain function, and the diffusion properties of white matter in ALL survivors
The strengths of this study include correcting for intracranial volume and using multiple techniques to examine brain microstructure. Correcting for intracranial volume is critical for interpretation as CRT influences bone growth and smaller intracranial volume is known to be a risk factor for those who receive CRT. Thus, when comparing brain volumes it is unclear whether the results are primarily due to a direct effect on skull growth or brain cells. In contrast, brain fraction differences are more clearly associated with differences in brain development or maturation. For example, grey matter volume, but not grey matter brain fraction, differed between the chemotherapy and CRT groups in the parietal and temporal lobes suggesting that that the differences in grey matter volume are driven by differences in intracranial volume and probably reflect differences in skull growth. Although regional differences in brain fraction were found in both white and grey matter, differences in brain volume were only found in white matter. This suggests that white matter is more susceptible than grey matter to cancer treatment. When interpreting the findings, study limitations should be considered. Neurocognitive data were not collected from the control group as these individuals were initially recruited for a different study focused on neuroimaging, however, all neurocognitive measures used have established normative data that allowed neurocognitive performance of survivors to be compared to the general population. Survivors treated with CRT were on average 7 years younger at diagnosis and were evaluated farther from diagnosis and treatment. These differences are primarily a result of the evolution of ALL treatment as therapy in the modern era rarely uses CRT and more children diagnosed during adolescence, typically associated with high-risk status, are surviving into adulthood. Recent reports demonstrate that younger age at diagnosis increases risk for impairment only in those treated with CRT (Krull et al., 2013). Children treated with chemotherapy only do not demonstrate this age vulnerability. Also, as late-effects are typically more clearly detected in survivors further from diagnosis (Oeffinger et al., 2006) and a younger age at diagnosis is associated with increased risk for neurocognitive deficits (Peterson et al., 2008), the differences found between the CRT and chemotherapy groups could be biased away from the null. Several large studies have established that adult survivors of childhood ALL, especially those treated with CRT, have deficits in multiple neurocognitive domains (Krull et al., 2013). The older age at diagnosis and shorter time to follow-up may underestimate the neurocognitive challenges facing adult survivors of ALL treated only with chemotherapy. Another limitation is that although on average each group was evaluated in their mid-20s, the age at evaluation did statistically differ between the three groups. Research has found that several fibre tracts show exponential increases in fractional anisotropy with a plateau where the microstructure reaches 90% of maturation during the late teens or early 20s, including the superior fronto-occipital fasciculus, superior longitudinal fasciculus, internal capsule, and external capsule. In contrast, the cingulum and uncinate fasciculus continue to mature throughout the third decade of life (Lebel et al., 2008). The developmental time course of microstructure maturation suggests that the age difference seen among groups would have minimal effects on fractional anisotropy values, although the difference observed within the cingulum and uncinate fasciculus should be interpreted more cautiously.
In conclusion, these results suggest that long-term adult survivors of childhood ALL, treated with or without cranial radiation, are at risk for altered brain structure and diminished neurocognitive function. Specifically, survivors of ALL have reduced white matter fraction in frontal and temporal lobes and higher fractional anisotropy in multiple fibre tracts when compared to controls. Although differences were more severe in long-term survivors of ALL treated with CRT, differences in neurocognitive function and neuroanatomical integrity were significant for survivors treated with chemotherapy alone. Overall, these data demonstrate substantial region-specific white matter alterations in survivors of ALL, regardless of treatment, which seem to contribute to difficulties in neurocognitive function. Longitudinal follow-up in ALL survivors is needed to determine whether the white matter continues to degenerate, whether patient characteristics (such as age at diagnosis and CNS diseases status) influence changes in white matter, how changes in brain imaging metrics are related to cognitive function, and how changes in brain structure and cognitive function in ageing ALL survivors compare with changes associated with normal ageing.
Funding
This work was supported by the Cancer Centre Support (CORE) grant CA21765 from the National Cancer Institute, grant HD049888 from the National Institute for Child Health and Human Development, grant RR029005 from the National Centre for Research Resources, and by ALSAC.
Glossary
Abbreviations
- ALL
acute lymphoblastic leukaemia
- CRT
cranial radiation therapy
- DTI
diffusion tensor imaging
References
- Assaf Y, Ben-Sira L, Constantini S, Chang LC, Beni-Adani L. Diffusion tensor imaging in hydrocephalus: initial experience. Am J Neuroradiol. 2006;27:1717–24. [PMC free article] [PubMed] [Google Scholar]
- Armstrong GT, Reddick WE, Petersen RC, Santucci A, Zhang N, Srivastava D, et al. Evaluation of memory impairment in aging adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiotherapy. J Natl Cancer Inst. 2013;105:899–907. doi: 10.1093/jnci/djt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton A, Hamsher K, Sivan A. Multilingual aphasi examination, 3rd edn., Iowa City, IA: AJA Associates; 1983. [Google Scholar]
- Budde MD, Janes L, Gold E, Turtzo LC, Frank JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011;134:2248–60. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey ME, Haut MW, Reminger SL, Hutter JJ, Theilmann R, Kaemingk KL. Reduced frontal white matter volume in long-term childhood leukemia survivors: a voxel-based morphometry study. AJNR Am J Neuroradiol. 2008;29:792–7. doi: 10.3174/ajnr.A0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C. Conners' continuous performance test II. North Tonawanda, NY: Multi-Health Systems; 2001. [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California verbal learning test. 2nd edn. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Dellani PR, Eder S, Gawehn J, Vucurevic G, Fellgiebel A, Muller MJ, et al. Late structural alterations of cerebral white matter in long-term survivors of childhood leukemia. J Magn Reson Imaging. 2008;27:1250–5. doi: 10.1002/jmri.21364. [DOI] [PubMed] [Google Scholar]
- Genschaft M, Huebner T, Plessow F, Ikonomidou VN, Abolmaali N, Krone F, et al. Impact of chemotherapy for childhood leukemia on brain morphology and function. PLoS One. 2013;8:e78599. doi: 10.1371/journal.pone.0078599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JO, Reddick WE, Li CS, Laningham FH, Helton KJ, Pui CH. Computer-aided detection of therapy-induced leukoencephalopathy in pediatric acute lymphoblastic leukemia patients treated with intravenous high-dose methotrexate. Magn Reson Imaging. 2006;24:785–91. doi: 10.1016/j.mri.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–94. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hertzberg H, Huk WJ, Ueberall MA, Langer T, Meier W, Dopfer R, et al. CNS late effects after ALL therapy in childhood. Part I: neuroradiological findings in long-term survivors of childhood ALL–an evaluation of the interferences between morphology and neuropsychological performance. The German Late Effects Working Group. Med Pediatr Oncol. 1997;28:387–400. doi: 10.1002/(sici)1096-911x(199706)28:6<387::aid-mpo1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Howlander N, Noone A, Krapcho M, Neyman N, Aminou R, Altekruse S, et al. SEER Cancer Statistics Review, 1975–2008. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–81. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson MM, Ness KK, Nolan VG, Armstrong GT, Green DM, Morris EB, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56:825–36. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med. 2006;11:489–97. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Khong PL, Leung LH, Fung AS, Fong DY, Qiu D, Kwong DL, et al. White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. J Clin Oncol. 2006;24:884–90. doi: 10.1200/JCO.2005.02.4505. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, et al. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage. 2007;35:478–87. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Krull KR, Brinkman TM, Li C, Armstrong GT, Ness KK, Srivastava DK, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St. Jude Lifetime Cohort Study. J Clin Oncol. 2013;35:4407–15. doi: 10.1200/JCO.2012.48.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Husain M, Gupta RK, Hasan KM, Haris M, Agarwal AK, et al. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J Neurotrauma. 2009;26:481–95. doi: 10.1089/neu.2008.0461. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–55. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Mariotto AB, Rowland JH, Yabroff KR, Scoppa S, Hachey M, Ries L, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1033–40. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Jones DK, Belaroussi B, Aggleton JP, O'Sullivan MJ. Frontotemporal connections in episodic memory and aging: a diffusion MRI tractography study. J Neurosci. 2011;31:13236–45. doi: 10.1523/JNEUROSCI.2317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–82. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- Peterson CC, Johnson CE, Ramirez LY, Huestis S, Pai AL, Demaree HA, et al. A meta-analysis of the neuropsychological sequelae of chemotherapy-only treatment for pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;51:99–104. doi: 10.1002/pbc.21544. [DOI] [PubMed] [Google Scholar]
- Peterson DJ, Ryan M, Rimrodt SL, Cutting LE, Denckla MB, Kaufmann WE, et al. Increased regional fractional anisotropy in highly screened attention-deficit hyperactivity disorder (ADHD) J Child Neurol. 2011;26:1296–302. doi: 10.1177/0883073811405662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto L, Preibisch C, Hattingen E, Bartels M, Lehrnbecher T, Dewitz R, et al. Voxel-based morphometry and diffusion-tensor MR imaging of the brain in long-term survivors of childhood leukemia. Eur Radiol. 2008;18:2691–700. doi: 10.1007/s00330-008-1038-2. [DOI] [PubMed] [Google Scholar]
- Reddick WE, Glass JO, Helton KJ, Langston JW, Xiong X, Wu S, et al. Prevalence of leukoencephalopathy in children treated for acute lymphoblastic leukemia with high-dose methotrexate. AJNR Am J Neuroradiol. 2005;26:1263–9. [PMC free article] [PubMed] [Google Scholar]
- Reddick WE, Shan ZY, Glass JO, Helton S, Xiong X, Wu S, et al. Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer. 2006;106:941–9. doi: 10.1002/cncr.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. 2nd edn. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- Reynolds CR, Voress JK. Test of memory and learning. 2nd edn. Austin, TX: PRO-ED; 2007. [Google Scholar]
- Schuitema I, Deprez S, Van HW, Daams M, Uyttebroeck A, Sunaert S, et al. Accelerated aging, decreased white matter integrity, and associated neuropsychological dysfunction 25 years after pediatric lymphoid malignancies. J Clin Oncol. 2013;31:3378–88. doi: 10.1200/JCO.2012.46.7050. [DOI] [PubMed] [Google Scholar]
- Sidaros A, Engberg AW, Sidaros K, Liptrot MG, Herning M, Petersen P, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131:559–72. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- Styner M, Oguz I, Xu S, Brechbuhler C, Pantazis D, Levitt JJ, et al. Framework for the statistical shape analysis of brain structures using SPHARM-PDM. Insight J. 2006:242–50. [PMC free article] [PubMed] [Google Scholar]
- Trites R. Neuropsychological test manual. Ottawa, ON: Royal Ottawa Hospital; 1977. [Google Scholar]
- Vestergaard M, Madsen KS, Baare WF, Skimminge A, Ejersbo LR, Ramsoy TZ, et al. White matter microstructure in superior longitudinal fasciculus associated with spatial working memory performance in children. J Cogn Neurosci. 2011;23:2135–46. doi: 10.1162/jocn.2010.21592. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3rd edn. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–55. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III: tests of achievement. Itasca, IL: Riverside; 2001. [Google Scholar]
- Yu HJ, Christodoulou C, Bhise V, Greenblatt D, Patel Y, Serafin D, et al. Multiple white matter tract abnormalities underlie cognitive impairment in RRMS. Neuroimage. 2012;59:3713–22. doi: 10.1016/j.neuroimage.2011.10.053. [DOI] [PubMed] [Google Scholar]
- Zeller B, Tamnes CK, Kanellopoulos A, Amlien IK, Andersson S, Due-Tonnessen P, et al. Reduced neuroanatomic volumes in long-term survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2013;31:2078–85. doi: 10.1200/JCO.2012.47.4031. [DOI] [PubMed] [Google Scholar]